Abstract
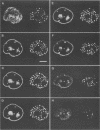
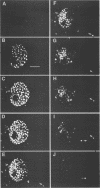
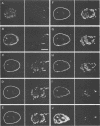
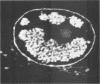
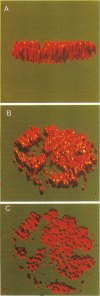
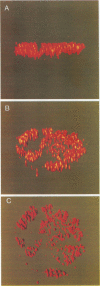
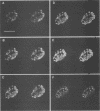
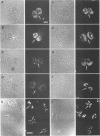
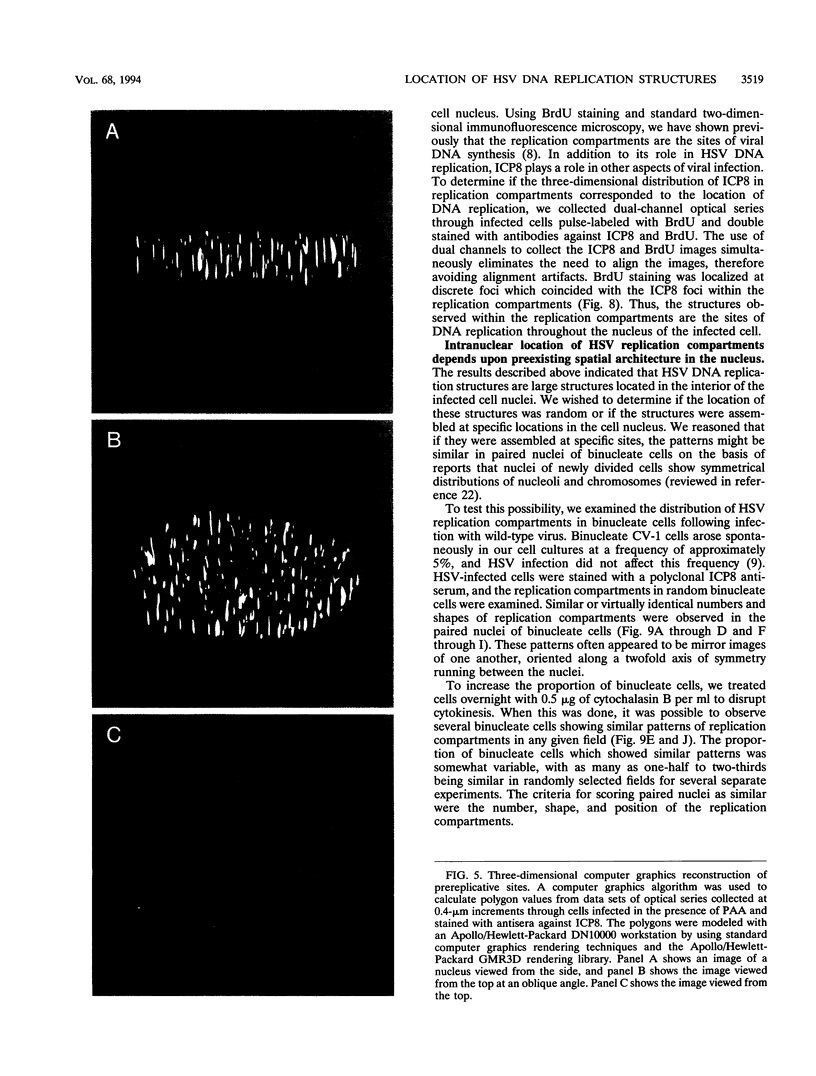
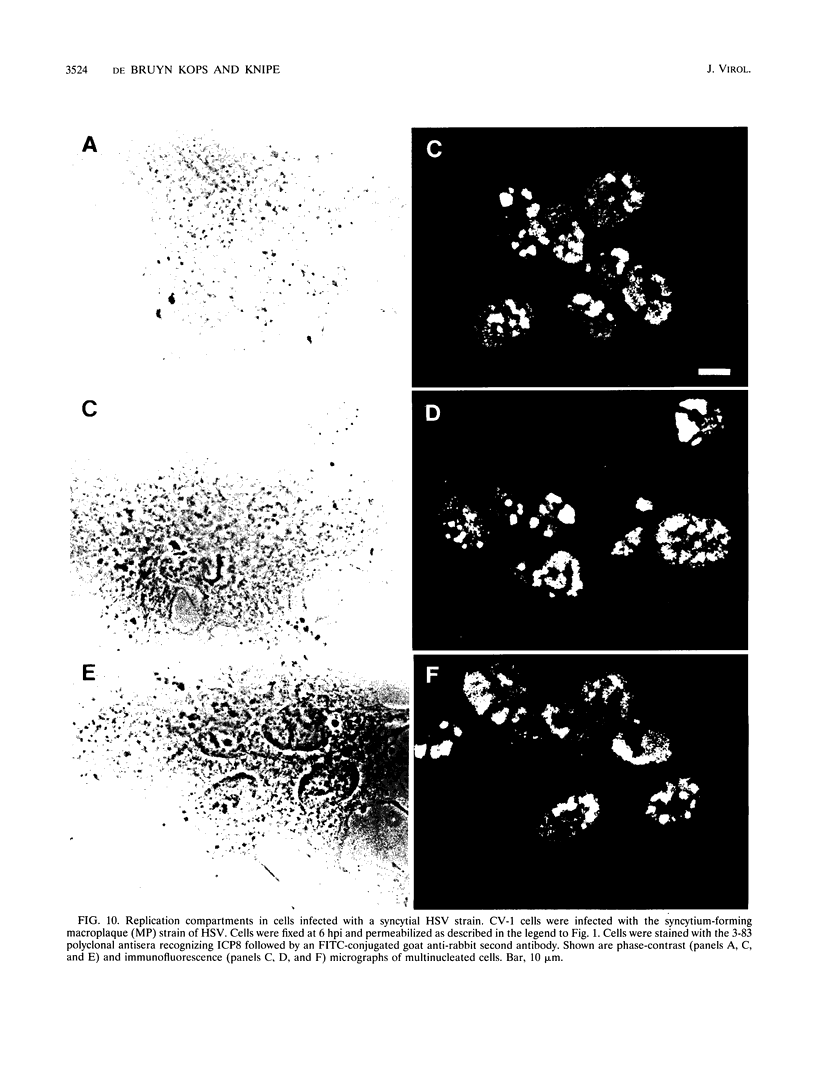
Herpes simplex virus DNA replication proteins localize in characteristic patterns corresponding to viral DNA replication structures in the infected cell nucleus. The intranuclear spatial organization of the HSV DNA replication structures and the factors regulating their nuclear location remain to be defined. We have used the HSV ICP8 DNA-binding protein and bromodeoxyuridine labeling as markers for sites of herpesviral DNA synthesis to examine the spatial organization of these structures within the cell nucleus. Confocal microscopy and three-dimensional computer graphics reconstruction of optical series through infected cells indicated that viral DNA replication structures extend through the interior of the cell nucleus and appear to be spatially separate from the nuclear lamina. Examination of viral DNA replication structures in infected, binucleate cells showed similar or virtually identical patterns of DNA replication structures oriented along a twofold axis of symmetry between many of the sister nuclei. These results demonstrate that HSV DNA replication structures are organized in the interior of the nucleus and that their location is defined by preexisting host cell nuclear architecture, probably the internal nuclear matrix.
Full text
PDF
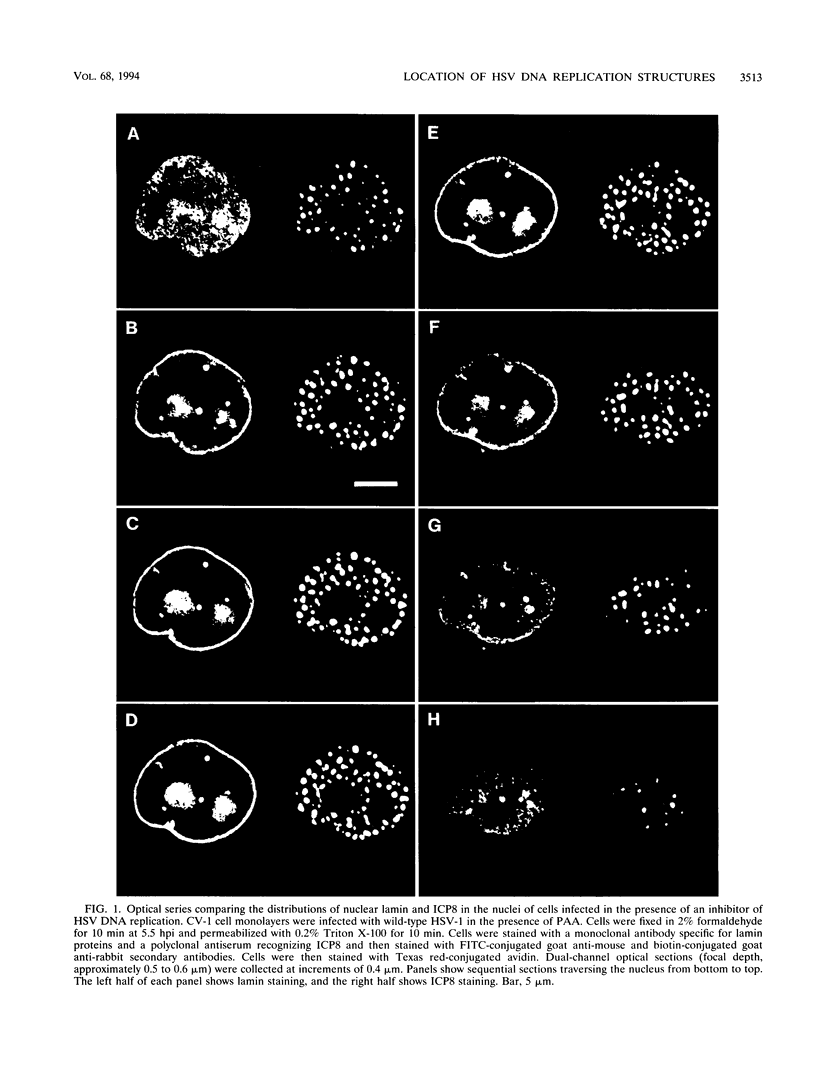

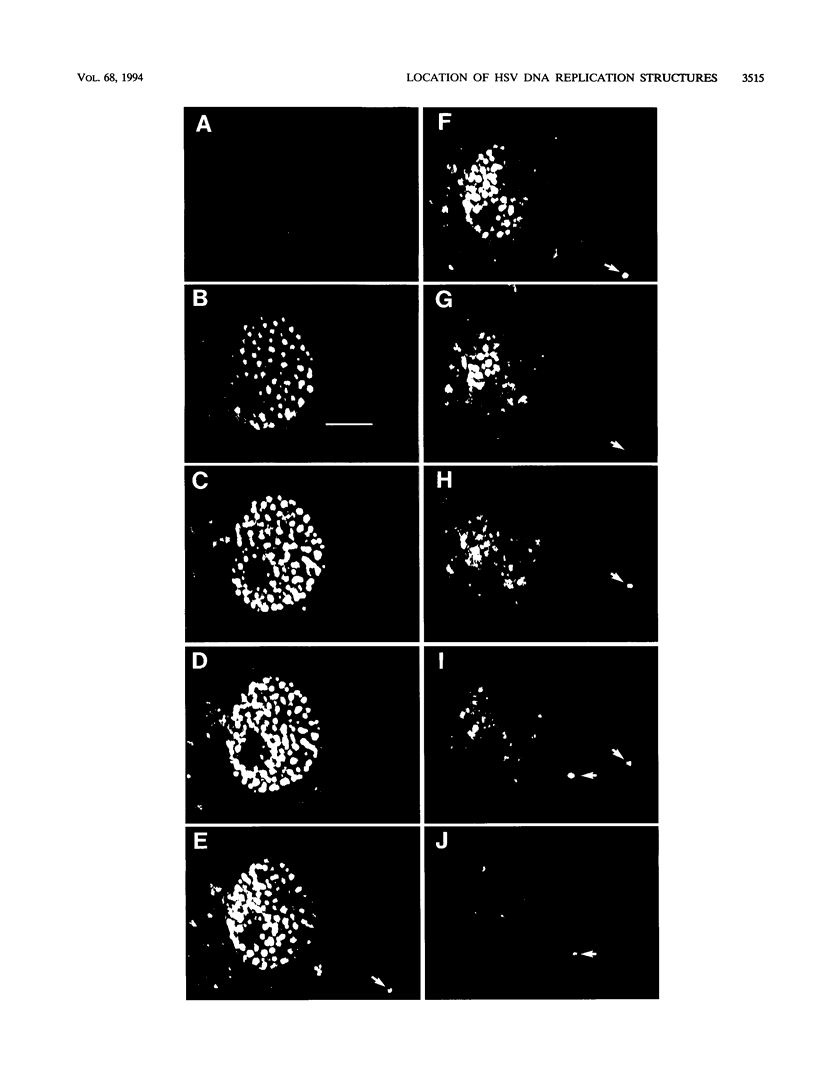

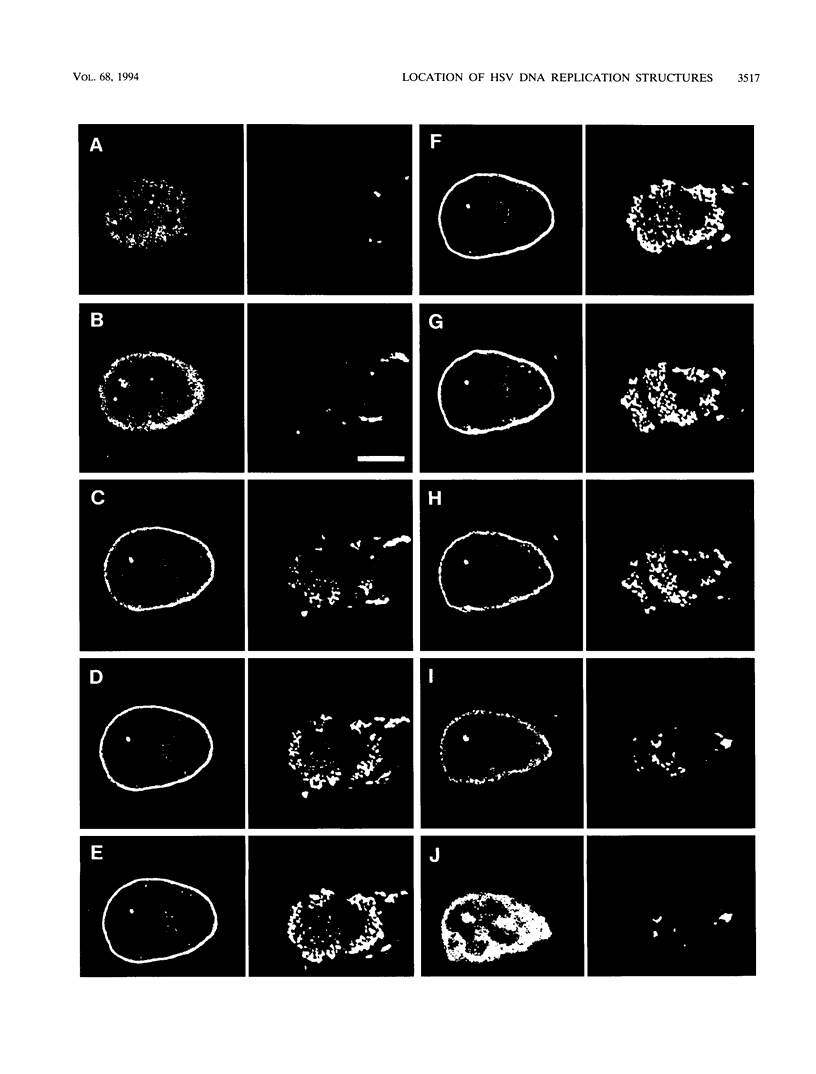

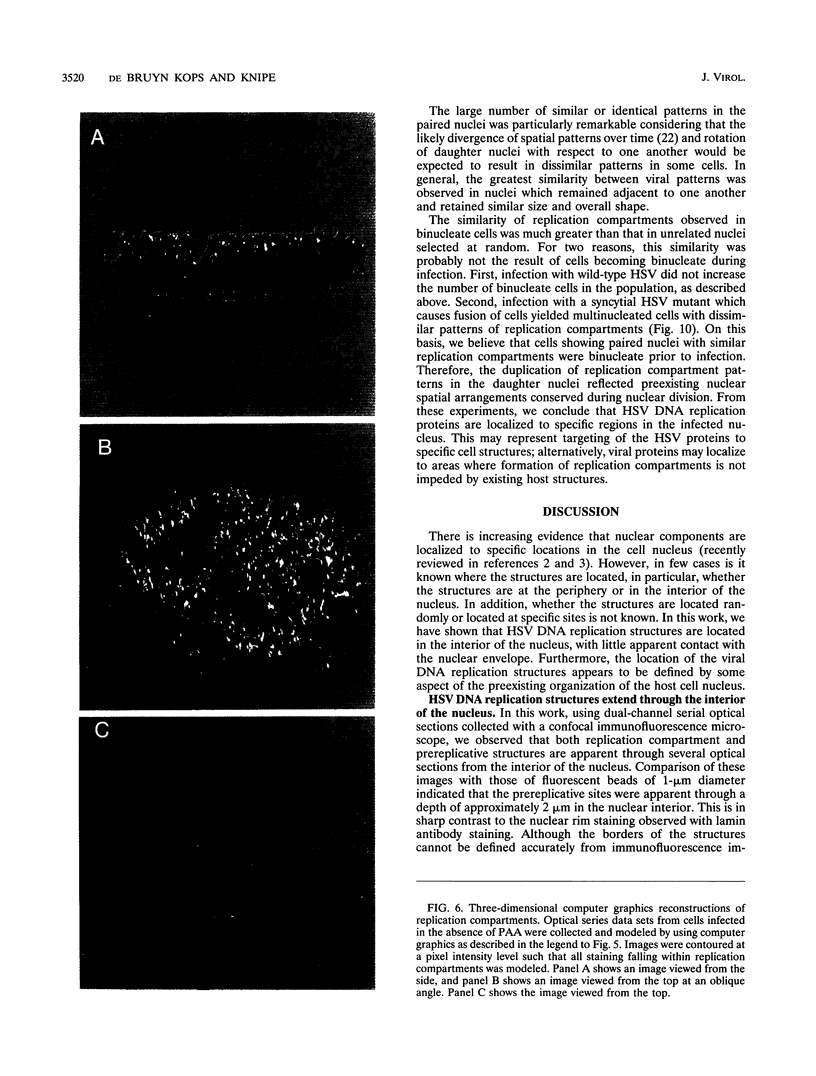
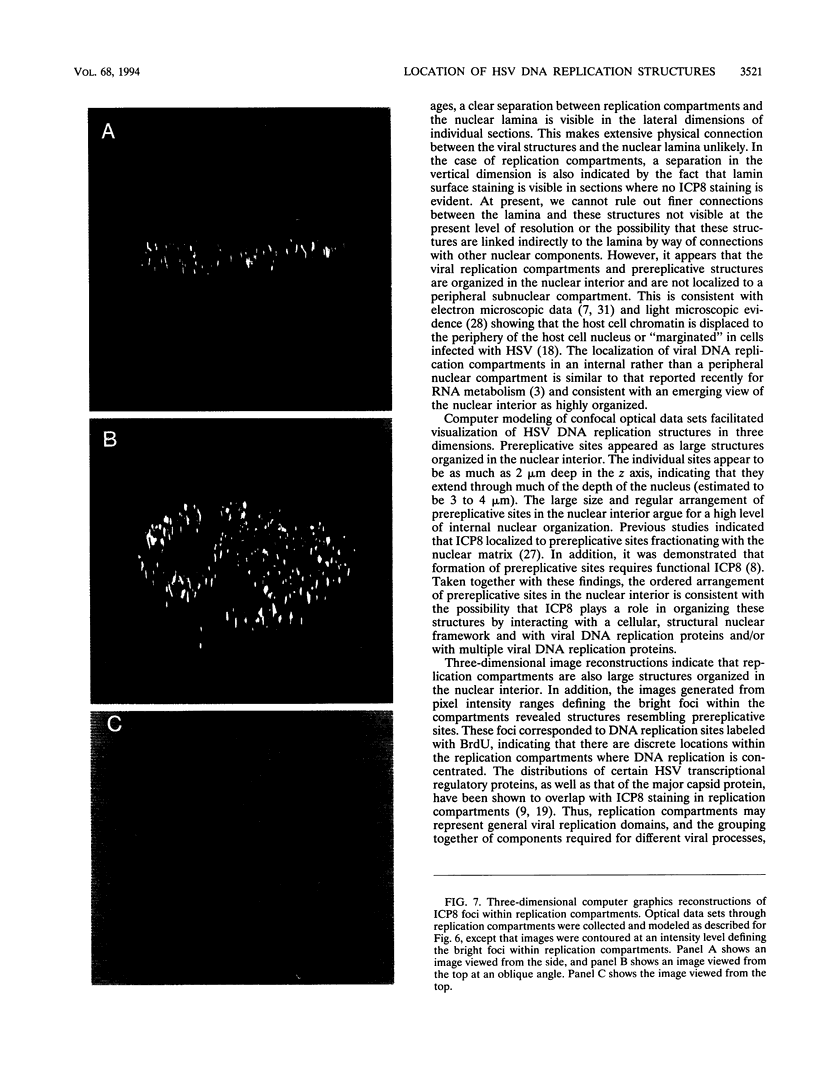
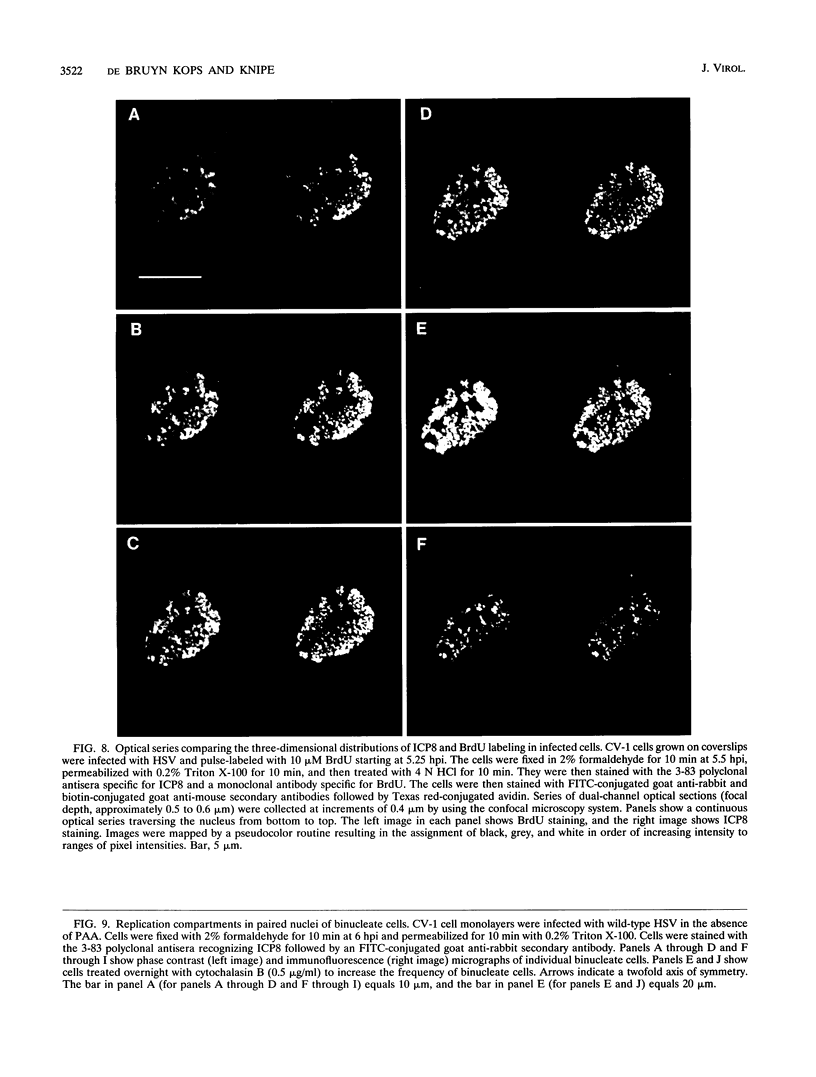
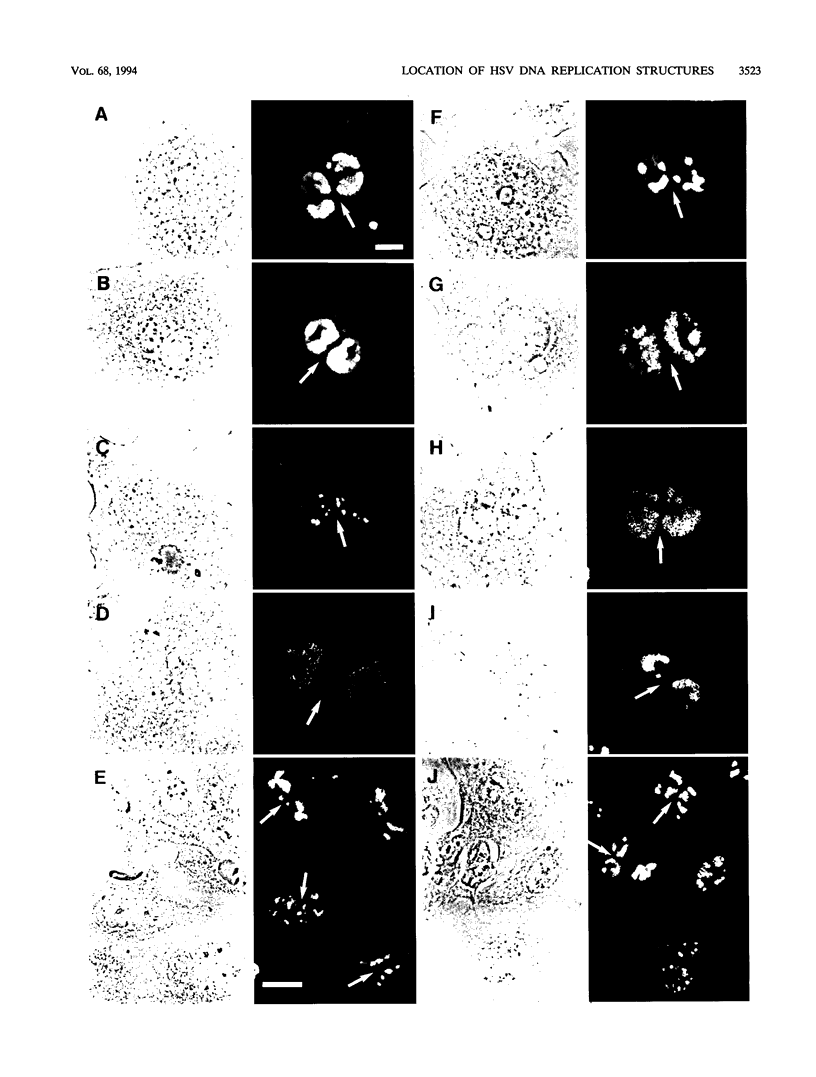


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bush M., Yager D. R., Gao M., Weisshart K., Marcy A. I., Coen D. M., Knipe D. M. Correct intranuclear localization of herpes simplex virus DNA polymerase requires the viral ICP8 DNA-binding protein. J Virol. 1991 Mar;65(3):1082–1089. doi: 10.1128/jvi.65.3.1082-1089.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso M. C., Leonhardt H., Nadal-Ginard B. Reversal of terminal differentiation and control of DNA replication: cyclin A and Cdk2 specifically localize at subnuclear sites of DNA replication. Cell. 1993 Sep 24;74(6):979–992. doi: 10.1016/0092-8674(93)90721-2. [DOI] [PubMed] [Google Scholar]
- Carter K. C., Bowman D., Carrington W., Fogarty K., McNeil J. A., Fay F. S., Lawrence J. B. A three-dimensional view of precursor messenger RNA metabolism within the mammalian nucleus. Science. 1993 Feb 26;259(5099):1330–1335. doi: 10.1126/science.8446902. [DOI] [PubMed] [Google Scholar]
- Challberg M. D. A method for identifying the viral genes required for herpesvirus DNA replication. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9094–9098. doi: 10.1073/pnas.83.23.9094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challberg M. D., Kelly T. J. Animal virus DNA replication. Annu Rev Biochem. 1989;58:671–717. doi: 10.1146/annurev.bi.58.070189.003323. [DOI] [PubMed] [Google Scholar]
- Darlington R. W., James C. Biological and morphological aspects of the growth of equine abortion virus. J Bacteriol. 1966 Jul;92(1):250–257. doi: 10.1128/jb.92.1.250-257.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M., Knipe D. M. Distal protein sequences can affect the function of a nuclear localization signal. Mol Cell Biol. 1992 Mar;12(3):1330–1339. doi: 10.1128/mcb.12.3.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich L. D., Schaffer P. A., Dorsky D. I., Crumpacker C. S., Parris D. S. Localization of the herpes simplex virus type 1 65-kilodalton DNA-binding protein and DNA polymerase in the presence and absence of viral DNA synthesis. J Virol. 1990 Dec;64(12):5738–5749. doi: 10.1128/jvi.64.12.5738-5749.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOGGAN M. D., ROIZMAN B. The isolation and properties of a variant of Herpes simplex producing multinucleated giant cells in monolayer cultures in the presence of antibody. Am J Hyg. 1959 Sep;70:208–219. doi: 10.1093/oxfordjournals.aje.a120071. [DOI] [PubMed] [Google Scholar]
- Huberman J. A., Tsai A., Deich R. A. DNA replication sites within nuclei of mammalian cells. Nature. 1973 Jan 5;241(5384):32–36. doi: 10.1038/241032a0. [DOI] [PubMed] [Google Scholar]
- Hughes R. G., Jr, Munyon W. H. Temperature-sensitive mutants of herpes simplex virus type 1 defective in lysis but not in transformation. J Virol. 1975 Aug;16(2):275–283. doi: 10.1128/jvi.16.2.275-283.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe D. M., Senechek D., Rice S. A., Smith J. L. Stages in the nuclear association of the herpes simplex virus transcriptional activator protein ICP4. J Virol. 1987 Feb;61(2):276–284. doi: 10.1128/jvi.61.2.276-284.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe D. M., Spang A. E. Definition of a series of stages in the association of two herpesviral proteins with the cell nucleus. J Virol. 1982 Jul;43(1):314–324. doi: 10.1128/jvi.43.1.314-324.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe D. M. The role of viral and cellular nuclear proteins in herpes simplex virus replication. Adv Virus Res. 1989;37:85–123. doi: 10.1016/s0065-3527(08)60833-7. [DOI] [PubMed] [Google Scholar]
- Lehner C. F., Kurer V., Eppenberger H. M., Nigg E. A. The nuclear lamin protein family in higher vertebrates. Identification of quantitatively minor lamin proteins by monoclonal antibodies. J Biol Chem. 1986 Oct 5;261(28):13293–13301. [PubMed] [Google Scholar]
- Mao J. C., Robishaw E. E. Mode of inhibition of herpes simplex virus DNA polymerase by phosphonoacetate. Biochemistry. 1975 Dec 16;14(25):5475–5479. doi: 10.1021/bi00696a015. [DOI] [PubMed] [Google Scholar]
- Moen P. T., Jr, Fox E., Bodnar J. W. Adenovirus and minute virus of mice DNAs are localized at the nuclear periphery. Nucleic Acids Res. 1990 Feb 11;18(3):513–520. doi: 10.1093/nar/18.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivo P. D., Nelson N. J., Challberg M. D. Herpes simplex virus type 1 gene products required for DNA replication: identification and overexpression. J Virol. 1989 Jan;63(1):196–204. doi: 10.1128/jvi.63.1.196-204.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan M. P., Chen L. B., Knipe D. M. The intranuclear location of a herpes simplex virus DNA-binding protein is determined by the status of viral DNA replication. Cell. 1984 Apr;36(4):857–868. doi: 10.1016/0092-8674(84)90035-7. [DOI] [PubMed] [Google Scholar]
- Randall R. E., Dinwoodie N. Intranuclear localization of herpes simplex virus immediate-early and delayed-early proteins: evidence that ICP 4 is associated with progeny virus DNA. J Gen Virol. 1986 Oct;67(Pt 10):2163–2177. doi: 10.1099/0022-1317-67-10-2163. [DOI] [PubMed] [Google Scholar]
- Rixon F. J., Atkinson M. A., Hay J. Intranuclear distribution of herpes simplex virus type 2 DNA synthesis: examination by light and electron microscopy. J Gen Virol. 1983 Sep;64(Pt 9):2087–2092. doi: 10.1099/0022-1317-64-9-2087. [DOI] [PubMed] [Google Scholar]
- Schwartz J., Roizman B. Similarities and Differences in the Development of Laboratory Strains and Freshly Isolated Strains of Herpes Simplex Virus in HEp-2 Cells: Electron Microscopy. J Virol. 1969 Dec;4(6):879–889. doi: 10.1128/jvi.4.6.879-889.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears A. E., Roizman B. Amplification by host cell factors of a sequence contained within the herpes simplex virus 1 genome. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9441–9444. doi: 10.1073/pnas.87.23.9441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuman H., Murray J. M., DiLullo C. Confocal microscopy: an overview. Biotechniques. 1989 Feb;7(2):154–163. [PubMed] [Google Scholar]
- Voelkerding K., Klessig D. F. Identification of two nuclear subclasses of the adenovirus type 5-encoded DNA-binding protein. J Virol. 1986 Nov;60(2):353–362. doi: 10.1128/jvi.60.2.353-362.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcock D., Lane D. P. Localization of p53, retinoblastoma and host replication proteins at sites of viral replication in herpes-infected cells. Nature. 1991 Jan 31;349(6308):429–431. doi: 10.1038/349429a0. [DOI] [PubMed] [Google Scholar]
- Wu C. A., Nelson N. J., McGeoch D. J., Challberg M. D. Identification of herpes simplex virus type 1 genes required for origin-dependent DNA synthesis. J Virol. 1988 Feb;62(2):435–443. doi: 10.1128/jvi.62.2.435-443.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruyn Kops A., Knipe D. M. Formation of DNA replication structures in herpes virus-infected cells requires a viral DNA binding protein. Cell. 1988 Dec 2;55(5):857–868. doi: 10.1016/0092-8674(88)90141-9. [DOI] [PubMed] [Google Scholar]