Abstract
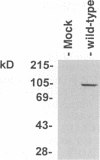
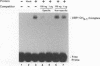
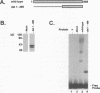
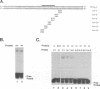
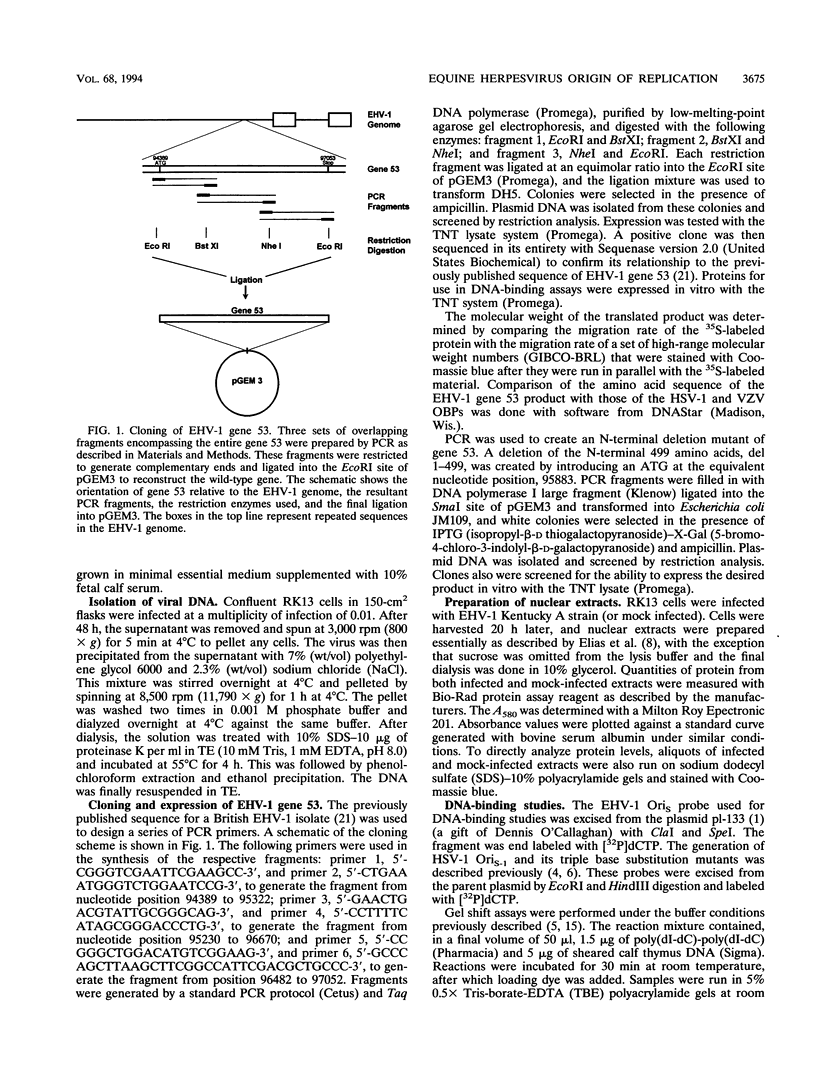
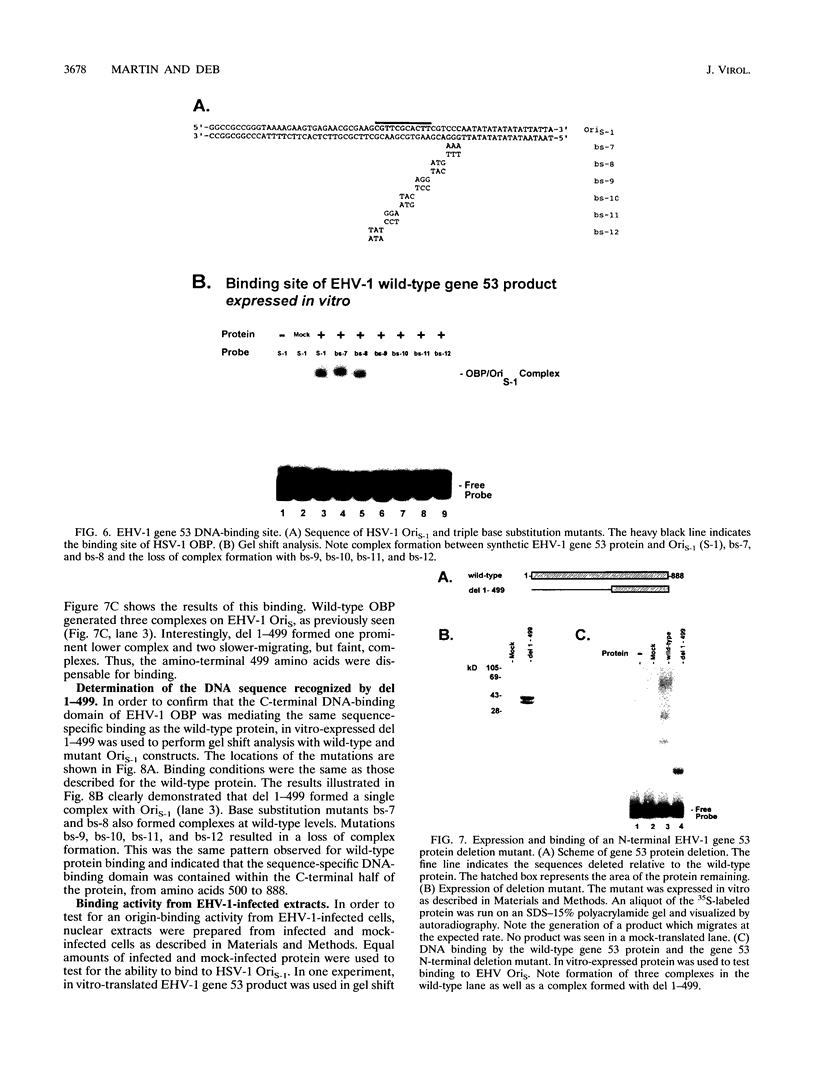
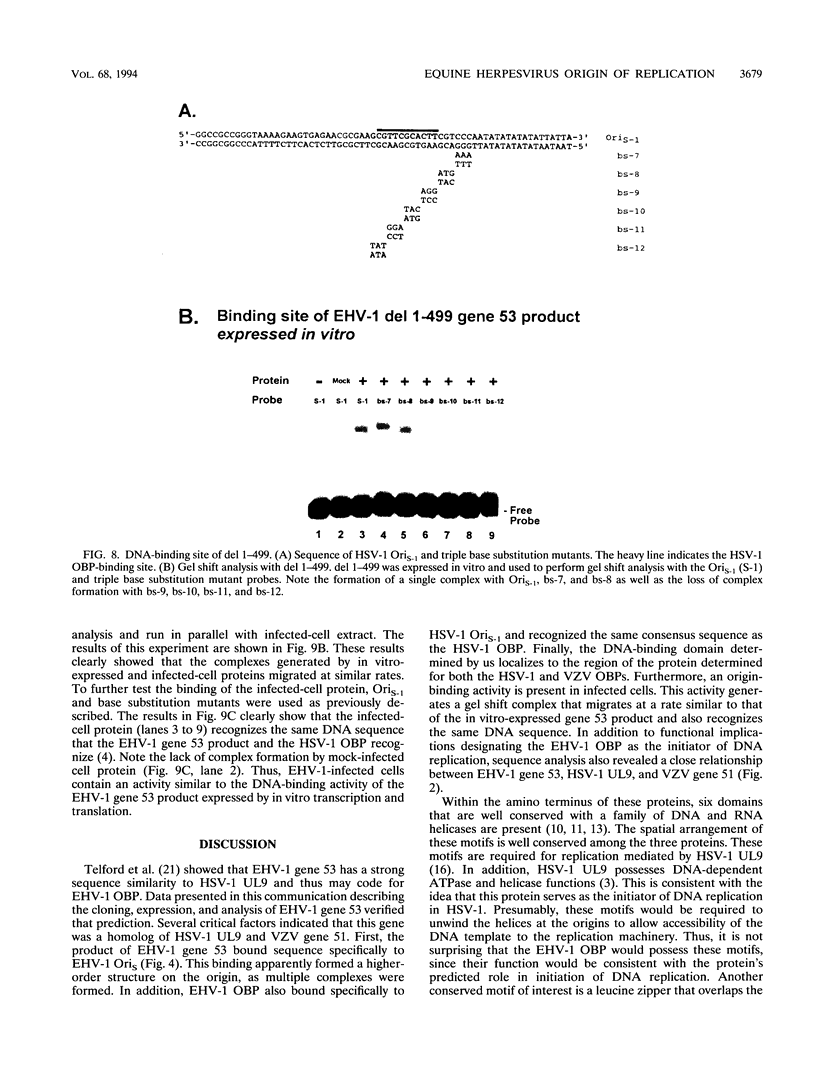
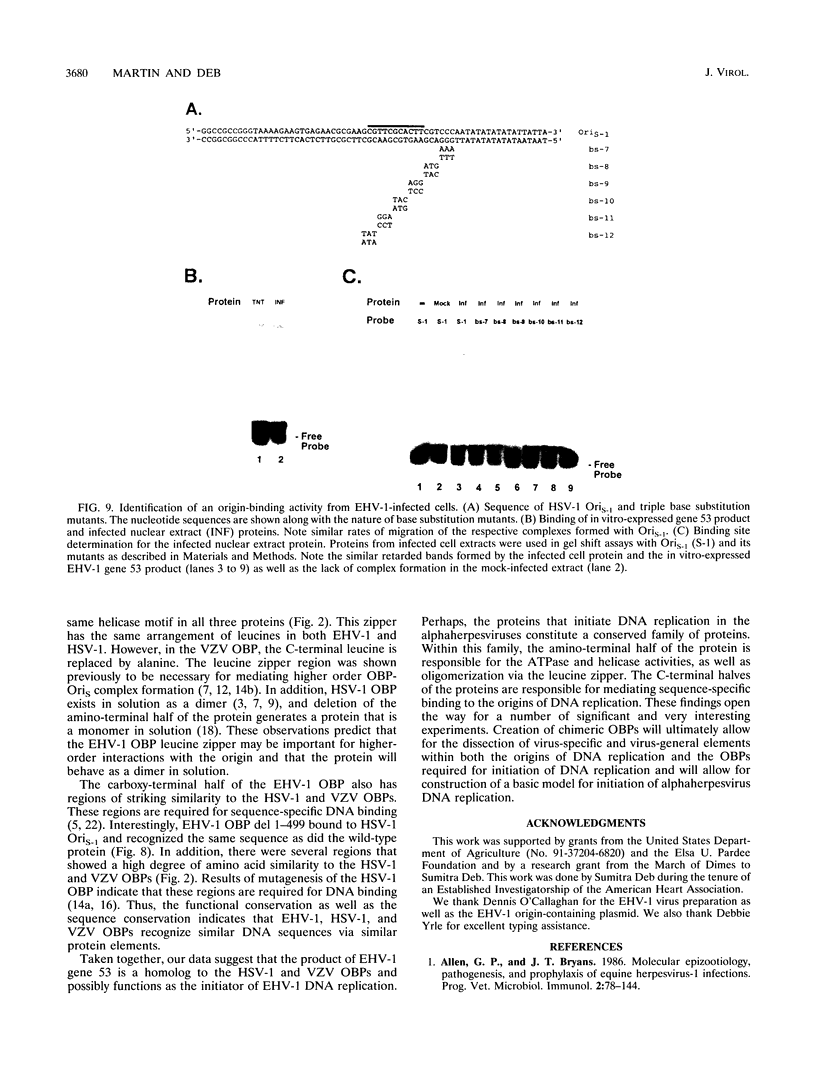
Equine herpesvirus 1 (EHV-1) is an important pathogen of horses and is closely related to several important human pathogens, herpes simplex virus types 1 and 2 (HSV-1 and HSV-2) and varicella-zoster virus. The EHV-1 genome contains open reading frames similar in sequence to the HSV-1 replication genes. PCR was used to clone EHV-1 gene 53, which is similar in sequence to the HSV-1 UL9 gene. The gene 53 product has regions of striking similarity to the HSV-1 UL9 and VZV gene 51 products. In vitro transcription and translation of this gene generated a protein of 87 kDa as measured by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Further characterization of this protein was accomplished through the use of gel shift analysis. The in vitro-synthesized protein bound sequence specifically to EHV-1 OriS as well as HSV-1 OriS. A site was used in gel shift analysis to show that the EHV-1 origin-binding protein bound to the same consensus site as the HSV-1 origin-binding protein, 5'-CGTTCGCACTT-3'. Using a nuclear extract of EHV-1-infected RK13 cells, we have identified an activity that interacts similarly with this consensus site. In gel shift assays, the retarded band arising from the nuclear extract migrated similarly to the retarded band arising from in vitro-translated EHV-1 gene 53. An N-terminal deletion of EHV-1 gene 53 was also created, expressed in vitro, and used in gel shift assays to localize the DNA-binding domain. Results of these experiments indicated that amino acids 1 to 499 were dispensable for binding and that the C-terminal fragment (amino acids 500 to 888) recognized the same consensus site as did the wild-type protein. Thus, the product of EHV-1 gene 53 is an origin-binding protein with a high degree of similarity to the HSV-1 and varicella-zoster virus origin-binding proteins and possibly serves as the initiator of DNA replication in EHV-1.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen G. P., Bryans J. T. Molecular epizootiology, pathogenesis, and prophylaxis of equine herpesvirus-1 infections. Prog Vet Microbiol Immunol. 1986;2:78–144. [PubMed] [Google Scholar]
- Baumann R. P., Yalamanchili V. R., O'Callaghan D. J. Functional mapping and DNA sequence of an equine herpesvirus 1 origin of replication. J Virol. 1989 Mar;63(3):1275–1283. doi: 10.1128/jvi.63.3.1275-1283.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruckner R. C., Crute J. J., Dodson M. S., Lehman I. R. The herpes simplex virus 1 origin binding protein: a DNA helicase. J Biol Chem. 1991 Feb 5;266(4):2669–2674. [PubMed] [Google Scholar]
- Deb S., Deb S. P. A 269-amino-acid segment with a pseudo-leucine zipper and a helix-turn-helix motif codes for the sequence-specific DNA-binding domain of herpes simplex virus type 1 origin-binding protein. J Virol. 1991 Jun;65(6):2829–2838. doi: 10.1128/jvi.65.6.2829-2838.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deb S., Deb S. P. Analysis of Ori-S sequence of HSV-1: identification of one functional DNA binding domain. Nucleic Acids Res. 1989 Apr 11;17(7):2733–2752. doi: 10.1093/nar/17.7.2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deb S., Doelberg M. A 67-base-pair segment from the Ori-S region of herpes simplex virus type 1 encodes origin function. J Virol. 1988 Jul;62(7):2516–2519. doi: 10.1128/jvi.62.7.2516-2519.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias P., Gustafsson C. M., Hammarsten O., Stow N. D. Structural elements required for the cooperative binding of the herpes simplex virus origin binding protein to oriS reside in the N-terminal part of the protein. J Biol Chem. 1992 Aug 25;267(24):17424–17429. [PubMed] [Google Scholar]
- Elias P., O'Donnell M. E., Mocarski E. S., Lehman I. R. A DNA binding protein specific for an origin of replication of herpes simplex virus type 1. Proc Natl Acad Sci U S A. 1986 Sep;83(17):6322–6326. doi: 10.1073/pnas.83.17.6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fierer D. S., Challberg M. D. Purification and characterization of UL9, the herpes simplex virus type 1 origin-binding protein. J Virol. 1992 Jul;66(7):3986–3995. doi: 10.1128/jvi.66.7.3986-3995.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A. E., Koonin E. V., Donchenko A. P., Blinov V. M. A novel superfamily of nucleoside triphosphate-binding motif containing proteins which are probably involved in duplex unwinding in DNA and RNA replication and recombination. FEBS Lett. 1988 Aug 1;235(1-2):16–24. doi: 10.1016/0014-5793(88)81226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A. E., Koonin E. V., Donchenko A. P., Blinov V. M. Two related superfamilies of putative helicases involved in replication, recombination, repair and expression of DNA and RNA genomes. Nucleic Acids Res. 1989 Jun 26;17(12):4713–4730. doi: 10.1093/nar/17.12.4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazuda D. J., Perry H. C., McClements W. L. Cooperative interactions between replication origin-bound molecules of herpes simplex virus origin-binding protein are mediated via the amino terminus of the protein. J Biol Chem. 1992 Jul 15;267(20):14309–14315. [PubMed] [Google Scholar]
- Hodgman T. C. A new superfamily of replicative proteins. Nature. 1988 May 5;333(6168):22–23. doi: 10.1038/333022b0. [DOI] [PubMed] [Google Scholar]
- Koff A., Tegtmeyer P. Characterization of major recognition sequences for a herpes simplex virus type 1 origin-binding protein. J Virol. 1988 Nov;62(11):4096–4103. doi: 10.1128/jvi.62.11.4096-4103.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin D. W., Deb S. P., Klauer J. S., Deb S. Analysis of the herpes simplex virus type 1 OriS sequence: mapping of functional domains. J Virol. 1991 Aug;65(8):4359–4369. doi: 10.1128/jvi.65.8.4359-4369.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez R., Shao L., Weller S. K. The conserved helicase motifs of the herpes simplex virus type 1 origin-binding protein UL9 are important for function. J Virol. 1992 Nov;66(11):6735–6746. doi: 10.1128/jvi.66.11.6735-6746.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D. J., Dalrymple M. A., Davison A. J., Dolan A., Frame M. C., McNab D., Perry L. J., Scott J. E., Taylor P. The complete DNA sequence of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988 Jul;69(Pt 7):1531–1574. doi: 10.1099/0022-1317-69-7-1531. [DOI] [PubMed] [Google Scholar]
- Robertson G. R., Scott N. A., Miller J. M., Sabine M., Zheng M., Bell C. W., Whalley J. M. Sequence characteristics of a gene in equine herpesvirus 1 homologous to glycoprotein H of herpes simplex virus. DNA Seq. 1991;1(4):241–249. doi: 10.3109/10425179109020779. [DOI] [PubMed] [Google Scholar]
- Telford E. A., Watson M. S., McBride K., Davison A. J. The DNA sequence of equine herpesvirus-1. Virology. 1992 Jul;189(1):304–316. doi: 10.1016/0042-6822(92)90706-u. [DOI] [PubMed] [Google Scholar]
- Weir H. M., Calder J. M., Stow N. D. Binding of the herpes simplex virus type 1 UL9 gene product to an origin of viral DNA replication. Nucleic Acids Res. 1989 Feb 25;17(4):1409–1425. doi: 10.1093/nar/17.4.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir H. M., Stow N. D. Two binding sites for the herpes simplex virus type 1 UL9 protein are required for efficient activity of the oriS replication origin. J Gen Virol. 1990 Jun;71(Pt 6):1379–1385. doi: 10.1099/0022-1317-71-6-1379. [DOI] [PubMed] [Google Scholar]