Abstract
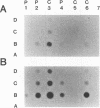
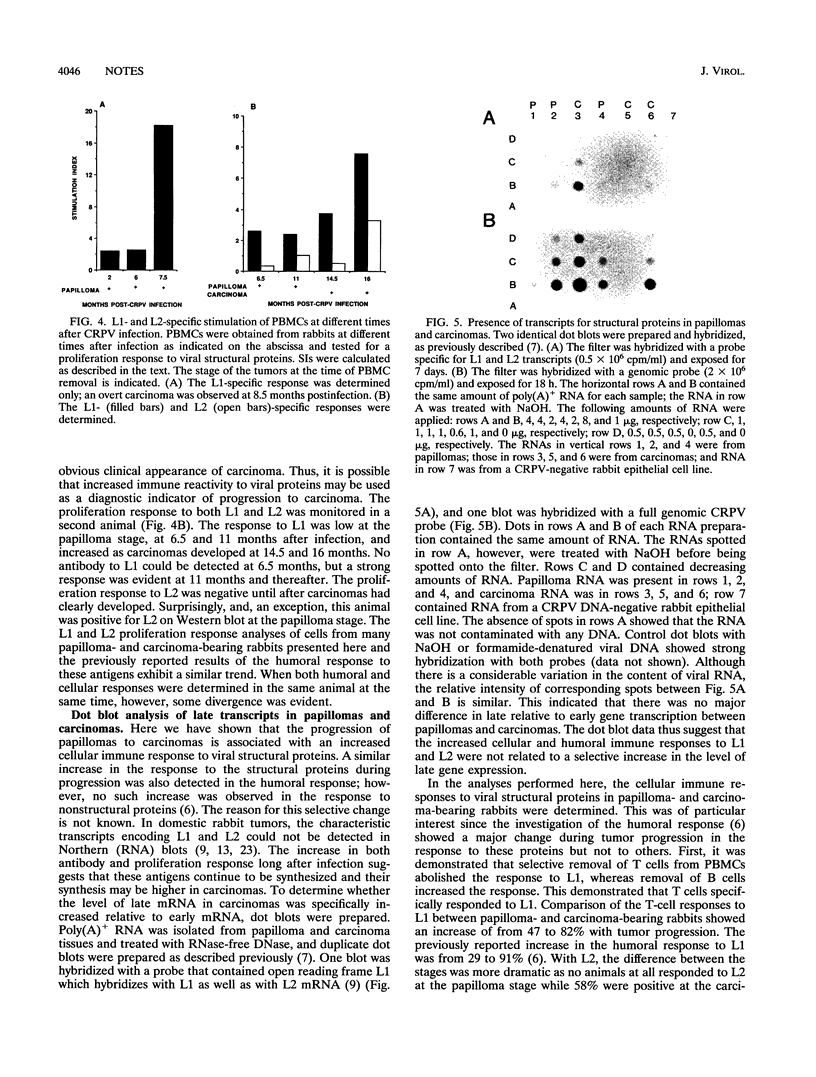
Cottontail rabbit papillomavirus (CRPV)-induced papillomas progress at a high frequency to carcinomas and thus can serve as a model for high-cancer-risk human papillomavirus infection. Previously, we have shown that antibodies to nonstructural and structural proteins are detected in only a fraction of papilloma-bearing animals. However, the antibody response to structural proteins drastically increases as papillomas progress to carcinoma (Y.-L. Lin, L. A. Borenstein, R. Selvakumar, R. Ahmed, and F. O. Wettstein, J. Virol. 67:382-389, 1993). Here we have monitored the cellular immune response to viral proteins during the course of infection and particularly during progression from papilloma to carcinoma. This was done by measuring the in vitro proliferation response of peripheral blood mononuclear cells (PBMCs) to CRPV structural proteins L1 and L2. The proliferating cells were identified as T cells by selective removal of B or T cells. In general, the T-cell response was low for rabbits at the papilloma stage and none responded to L2. Lymphocytes from animals with carcinomas more frequently and more strongly responded to L1, and more than half also responded to L2. In addition to stimulation of PBMCs, L1- and L2-specific proliferation could also be demonstrated with lymph node and spleen cells. Overall, our data show that progression of papilloma to carcinoma is associated with an increased T-cell response to CRPV structural proteins in addition to an increased humoral response. This greater immune reactivity, however, was not associated with a selectively increased expression of structural proteins, since RNA isolated from papillomas and carcinomas contained similar relative levels of late and early RNA as shown by dot blot analysis. Thus, the heightened immune reactivity seen in carcinoma-bearing rabbits most likely reflects greater stimulation of the immune system owing to dissemination of the tumor. These findings suggest that increased immune responses to papillomavirus proteins may be prognostic of progression to carcinoma and particularly of the development of metastases.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bleul C., Müller M., Frank R., Gausepohl H., Koldovsky U., Mgaya H. N., Luande J., Pawlita M., ter Meulen J., Viscidi R. Human papillomavirus type 18 E6 and E7 antibodies in human sera: increased anti-E7 prevalence in cervical cancer patients. J Clin Microbiol. 1991 Aug;29(8):1579–1588. doi: 10.1128/jcm.29.8.1579-1588.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubie H. A., Norval M., Crawford L., Banks L., Crook T. Lymphoproliferative response to fusion proteins of human papillomaviruses in patients with cervical intraepithelial neoplasia. Epidemiol Infect. 1989 Dec;103(3):625–632. doi: 10.1017/s0950268800031022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han R., Breitburd F., Marche P. N., Orth G. Linkage of regression and malignant conversion of rabbit viral papillomas to MHC class II genes. Nature. 1992 Mar 5;356(6364):66–68. doi: 10.1038/356066a0. [DOI] [PubMed] [Google Scholar]
- Jochmus-Kudielka I., Schneider A., Braun R., Kimmig R., Koldovsky U., Schneweis K. E., Seedorf K., Gissmann L. Antibodies against the human papillomavirus type 16 early proteins in human sera: correlation of anti-E7 reactivity with cervical cancer. J Natl Cancer Inst. 1989 Nov 15;81(22):1698–1704. doi: 10.1093/jnci/81.22.1698. [DOI] [PubMed] [Google Scholar]
- Lin Y. L., Borenstein L. A., Selvakumar R., Ahmed R., Wettstein F. O. Effective vaccination against papilloma development by immunization with L1 or L2 structural protein of cottontail rabbit papillomavirus. Virology. 1992 Apr;187(2):612–619. doi: 10.1016/0042-6822(92)90463-y. [DOI] [PubMed] [Google Scholar]
- Lin Y. L., Borenstein L. A., Selvakumar R., Ahmed R., Wettstein F. O. Progression from papilloma to carcinoma is accompanied by changes in antibody response to papillomavirus proteins. J Virol. 1993 Jan;67(1):382–389. doi: 10.1128/jvi.67.1.382-389.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers C., Harry J., Lin Y. L., Wettstein F. O. Identification of three transforming proteins encoded by cottontail rabbit papillomavirus. J Virol. 1992 Mar;66(3):1655–1664. doi: 10.1128/jvi.66.3.1655-1664.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morison W. L. Viral warts, herpes simplex and herpes zoster in patients with secondary immune deficiencies and neoplasms. Br J Dermatol. 1975 Jun;92(6):625–630. doi: 10.1111/j.1365-2133.1975.tb03141.x. [DOI] [PubMed] [Google Scholar]
- Nasseri M., Wettstein F. O. Cottontail rabbit papillomavirus-specific transcripts in transplantable tumors with integrated DNA. Virology. 1984 Oct 30;138(2):362–367. doi: 10.1016/0042-6822(84)90362-3. [DOI] [PubMed] [Google Scholar]
- Okabayashi M., Angell M. G., Budgeon L. R., Kreider J. W. Shope papilloma cell and leukocyte proliferation in regressing and progressing lesions. Am J Pathol. 1993 Feb;142(2):489–496. [PMC free article] [PubMed] [Google Scholar]
- Okabayashi M., Angell M. G., Christensen N. D., Kreider J. W. Morphometric analysis and identification of infiltrating leucocytes in regressing and progressing Shope rabbit papillomas. Int J Cancer. 1991 Dec 2;49(6):919–923. doi: 10.1002/ijc.2910490620. [DOI] [PubMed] [Google Scholar]
- Phelps W. C., Leary S. L., Faras A. J. Shope papillomavirus transcription in benign and malignant rabbit tumors. Virology. 1985 Oct 15;146(1):120–129. doi: 10.1016/0042-6822(85)90058-3. [DOI] [PubMed] [Google Scholar]
- SYVERTON J. T. The pathogenesis of the rabbit papilloma-to-carcinoma sequence. Ann N Y Acad Sci. 1952 Jul 10;54(6):1126–1140. doi: 10.1111/j.1749-6632.1952.tb39983.x. [DOI] [PubMed] [Google Scholar]
- Steele J. C., Stankovic T., Gallimore P. H. Production and characterization of human proliferative T-cell clones specific for human papillomavirus type 1 E4 protein. J Virol. 1993 May;67(5):2799–2806. doi: 10.1128/jvi.67.5.2799-2806.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steger G., Olszewsky M., Stockfleth E., Pfister H. Prevalence of antibodies to human papillomavirus type 8 in human sera. J Virol. 1990 Sep;64(9):4399–4406. doi: 10.1128/jvi.64.9.4399-4406.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J. G., Wettstein F. O. Multiple copies of Shope virus DNA are present in cells of benign and malignant non-virus-producing neoplasms. J Virol. 1979 Jun;30(3):891–898. doi: 10.1128/jvi.30.3.891-898.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara K., Fujinaga K., Yamashita T., Ito Y. Integration and methylation of shope papilloma virus DNA in the transplantable Vx2 and Vx7 rabbit carcinomas. Virology. 1983 Nov;131(1):88–99. doi: 10.1016/0042-6822(83)90536-6. [DOI] [PubMed] [Google Scholar]
- Watts S. L., Ostrow R. S., Phelps W. C., Prince J. T., Faras A. J. Free cottontail rabbit papillomavirus DNA persists in warts and carcinomas of infected rabbits and in cells in culture transformed with virus or viral DNA. Virology. 1983 Feb;125(1):127–138. doi: 10.1016/0042-6822(83)90069-7. [DOI] [PubMed] [Google Scholar]
- Wettstein F. O., Barbosa M. S., Nasseri M. Identification of the major cottontail rabbit papillomavirus late RNA cap site and mapping and quantitation of an E2 and minor E6 coding mRNA in papillomas and carcinomas. Virology. 1987 Aug;159(2):321–328. doi: 10.1016/0042-6822(87)90470-3. [DOI] [PubMed] [Google Scholar]
- Zeltner R., Borenstein L. A., Wettstein F. O., Iftner T. Changes in RNA expression pattern during the malignant progression of cottontail rabbit papillomavirus-induced tumors in rabbits. J Virol. 1994 Jun;68(6):3620–3630. doi: 10.1128/jvi.68.6.3620-3630.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Hausen H. Human papillomaviruses in the pathogenesis of anogenital cancer. Virology. 1991 Sep;184(1):9–13. doi: 10.1016/0042-6822(91)90816-t. [DOI] [PubMed] [Google Scholar]