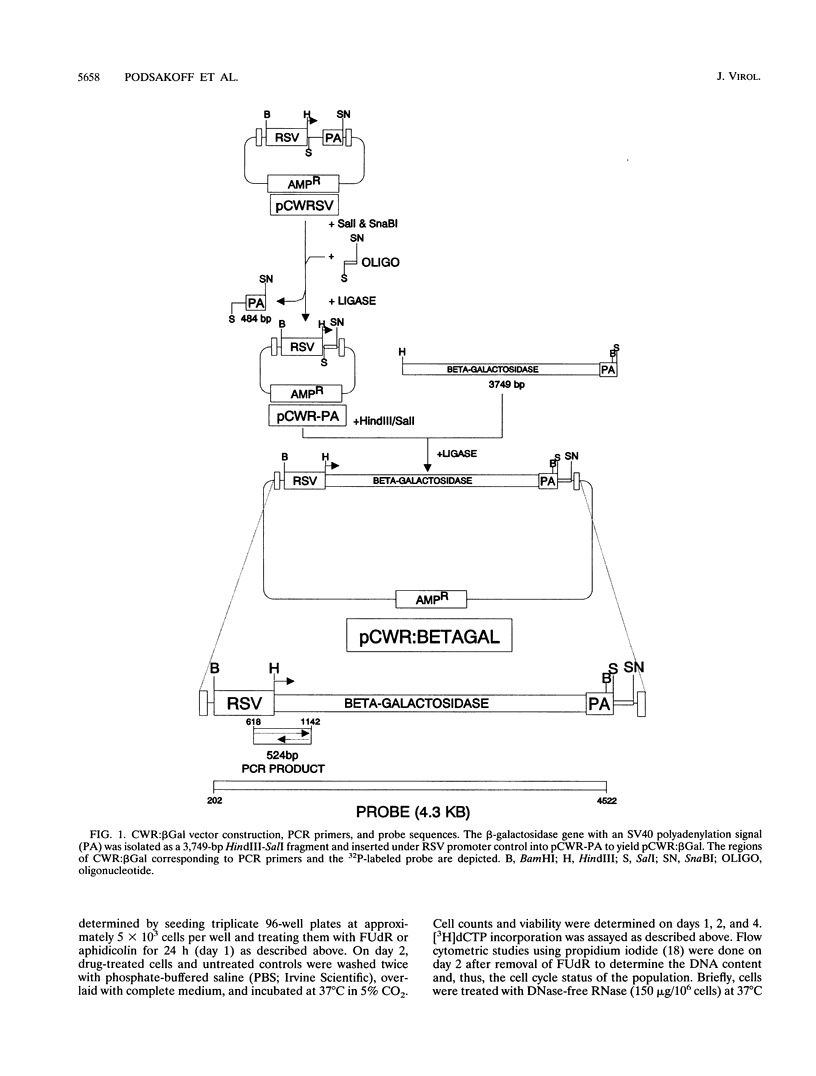
Abstract
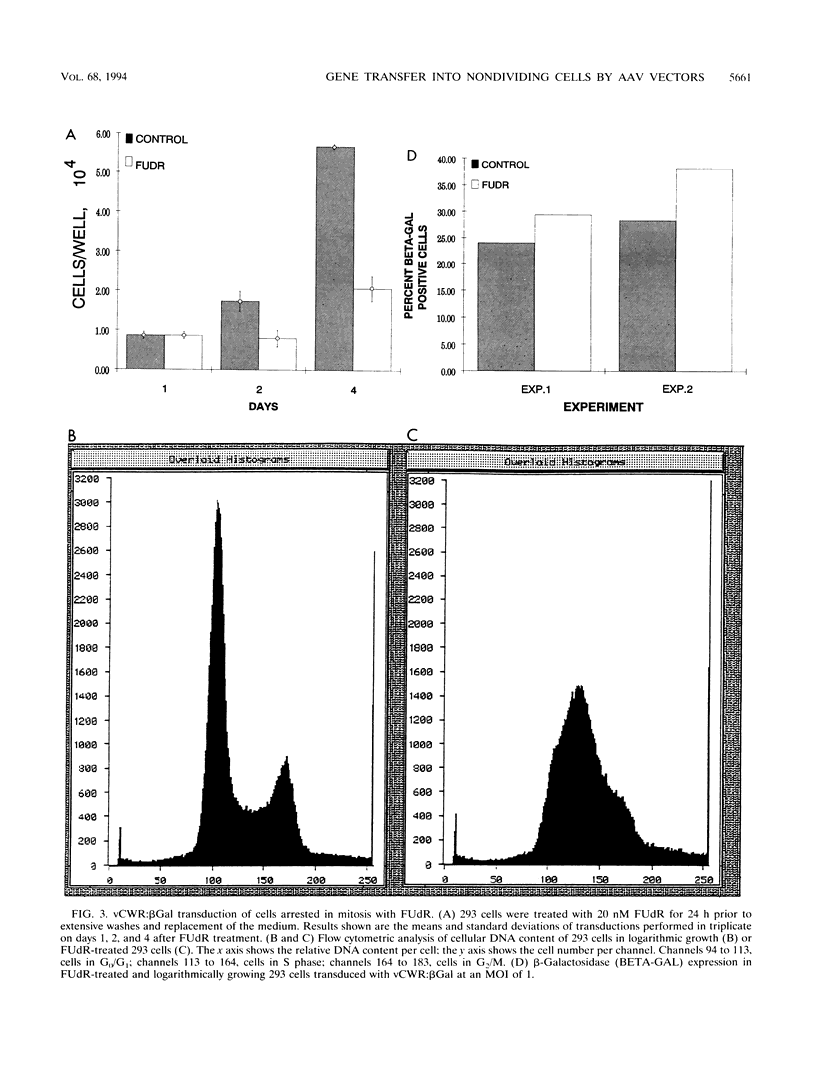
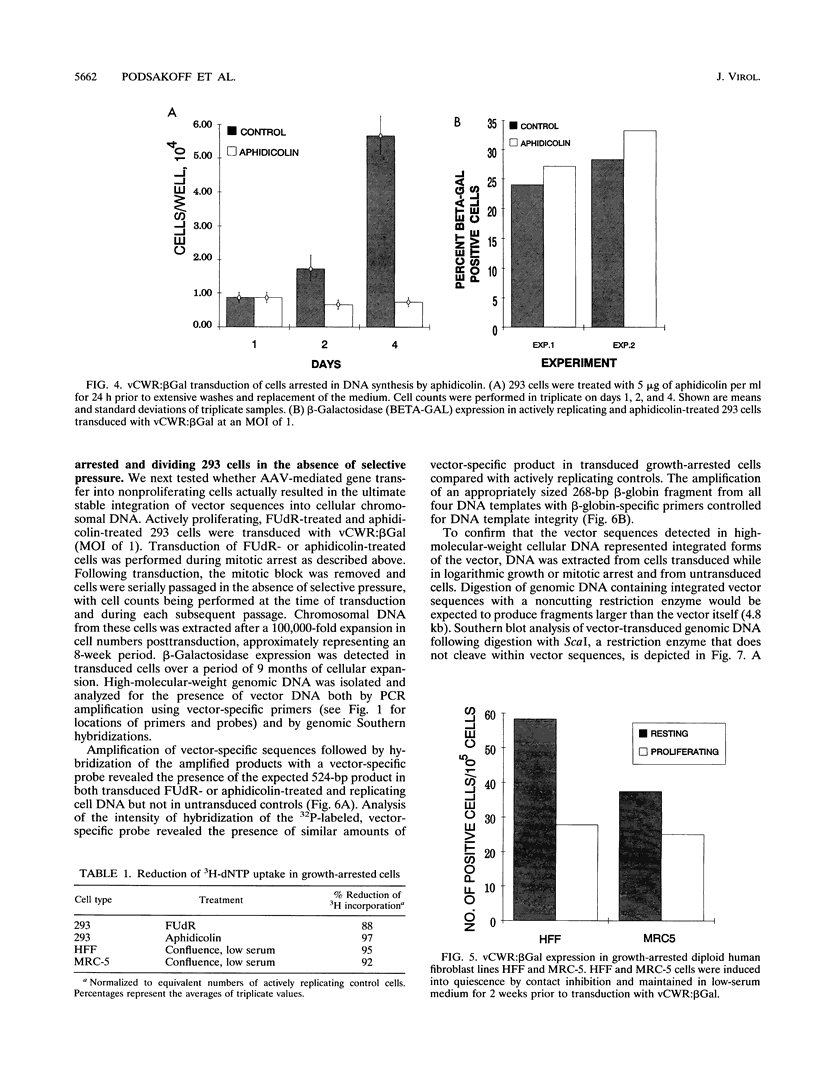
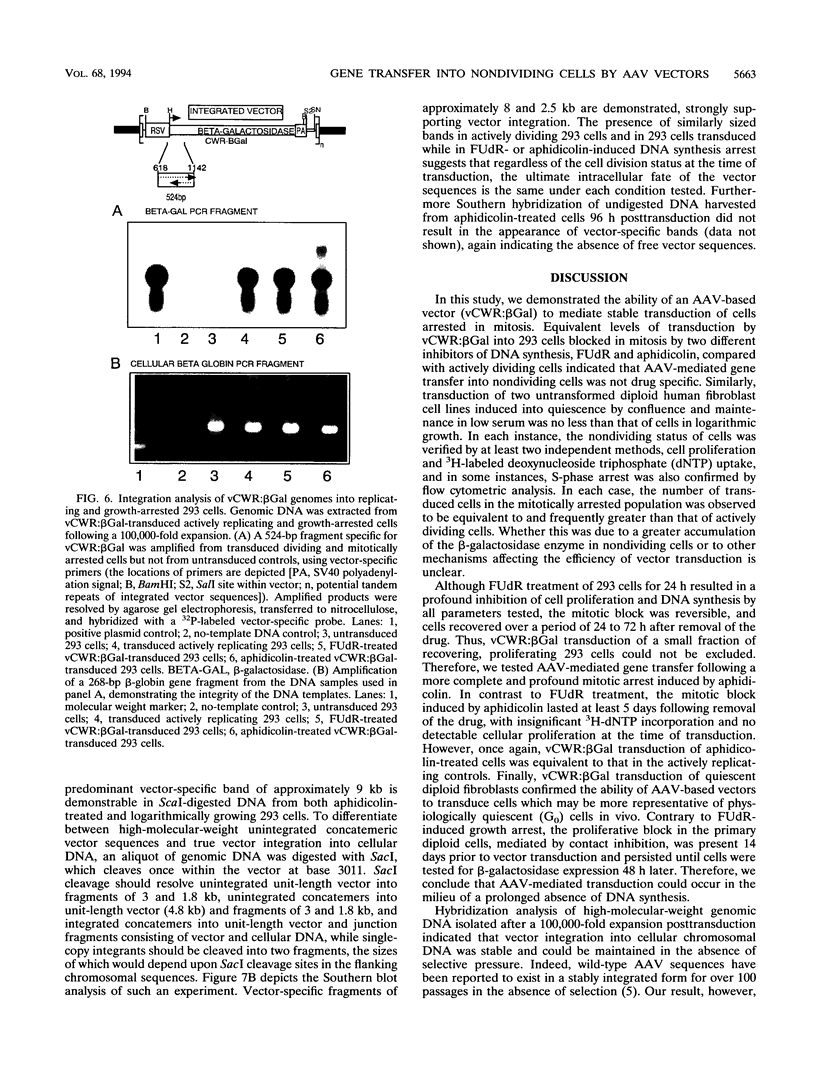
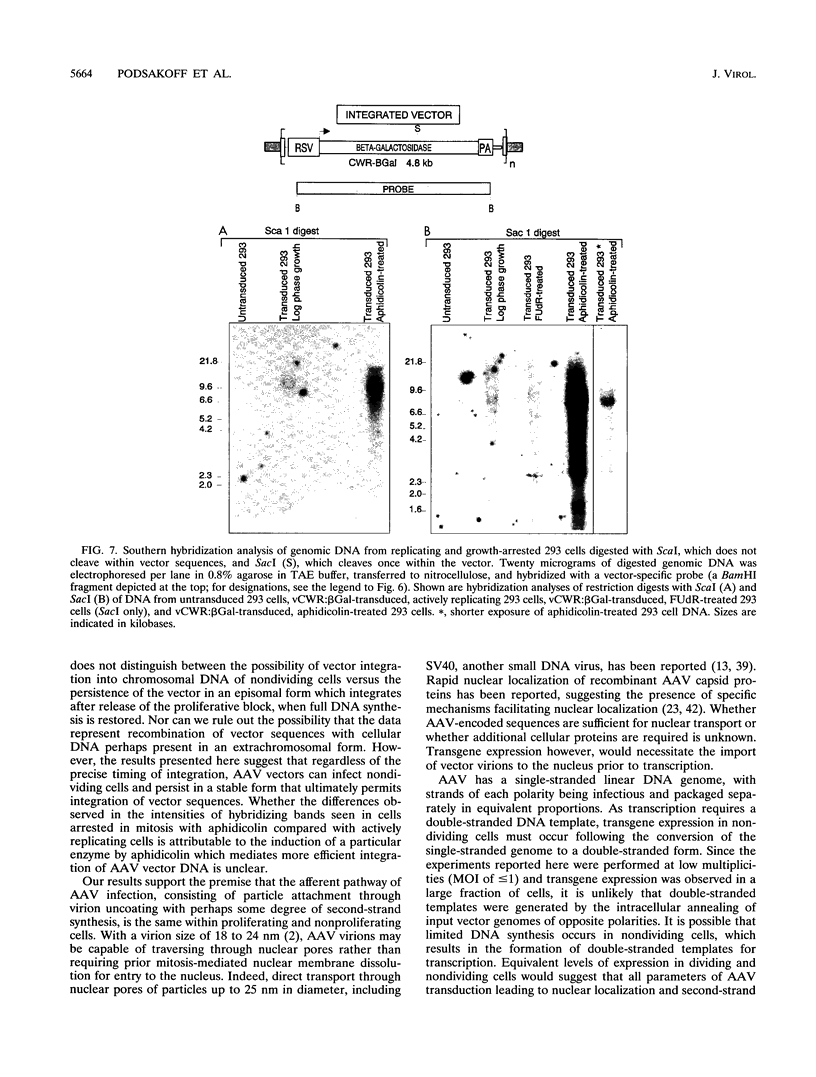
Gene transfer vectors based on adeno-associated virus (AAV) are emerging as highly promising for use in human gene therapy by virtue of their characteristics of wide host range, high transduction efficiencies, and lack of cytopathogenicity. To better define the biology of AAV-mediated gene transfer, we tested the ability of an AAV vector to efficiently introduce transgenes into nonproliferating cell populations. Cells were induced into a nonproliferative state by treatment with the DNA synthesis inhibitors fluorodeoxyuridine and aphidicolin or by contact inhibition induced by confluence and serum starvation. Cells in logarithmic growth or DNA synthesis arrest were transduced with vCWR:beta gal, an AAV-based vector encoding beta-galactosidase under Rous sarcoma virus long terminal repeat promoter control. Under each condition tested, vCWR:beta Gal expression in nondividing cells was at least equivalent to that in actively proliferating cells, suggesting that mechanisms for virus attachment, nuclear transport, virion uncoating, and perhaps some limited second-strand synthesis of AAV vectors were present in nondividing cells. Southern hybridization analysis of vector sequences from cells transduced while in DNA synthetic arrest and expanded after release of the block confirmed ultimate integration of the vector genome into cellular chromosomal DNA. These findings may provide the basis for the use of AAV-based vectors for gene transfer into quiescent cell populations such as totipotent hematopoietic stem cells.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATCHISON R. W., CASTO B. C., HAMMON W. M. ADENOVIRUS-ASSOCIATED DEFECTIVE VIRUS PARTICLES. Science. 1965 Aug 13;149(3685):754–756. doi: 10.1126/science.149.3685.754. [DOI] [PubMed] [Google Scholar]
- Anderson W. F. Human gene therapy. Science. 1992 May 8;256(5058):808–813. doi: 10.1126/science.1589762. [DOI] [PubMed] [Google Scholar]
- Bender M. A., Miller A. D., Gelinas R. E. Expression of the human beta-globin gene after retroviral transfer into murine erythroleukemia cells and human BFU-E cells. Mol Cell Biol. 1988 Apr;8(4):1725–1735. doi: 10.1128/mcb.8.4.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns K. I., Bohenzky R. A. Adeno-associated viruses: an update. Adv Virus Res. 1987;32:243–306. doi: 10.1016/s0065-3527(08)60479-0. [DOI] [PubMed] [Google Scholar]
- Berns K. I., Pinkerton T. C., Thomas G. F., Hoggan M. D. Detection of adeno-associated virus (AAV)-specific nucleotide sequences in DNA isolated from latently infected Detroit 6 cells. Virology. 1975 Dec;68(2):556–560. doi: 10.1016/0042-6822(75)90298-6. [DOI] [PubMed] [Google Scholar]
- Blaese R. M. Development of gene therapy for immunodeficiency: adenosine deaminase deficiency. Pediatr Res. 1993 Jan;33(1 Suppl):S49–S55. doi: 10.1203/00006450-199305001-00278. [DOI] [PubMed] [Google Scholar]
- Bordignon C., Yu S. F., Smith C. A., Hantzopoulos P., Ungers G. E., Keever C. A., O'Reilly R. J., Gilboa E. Retroviral vector-mediated high-efficiency expression of adenosine deaminase (ADA) in hematopoietic long-term cultures of ADA-deficient marrow cells. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6748–6752. doi: 10.1073/pnas.86.17.6748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner M. K., Rill D. R., Moen R. C., Krance R. A., Mirro J., Jr, Anderson W. F., Ihle J. N. Gene-marking to trace origin of relapse after autologous bone-marrow transplantation. Lancet. 1993 Jan 9;341(8837):85–86. doi: 10.1016/0140-6736(93)92560-g. [DOI] [PubMed] [Google Scholar]
- Buller R. M., Janik J. E., Sebring E. D., Rose J. A. Herpes simplex virus types 1 and 2 completely help adenovirus-associated virus replication. J Virol. 1981 Oct;40(1):241–247. doi: 10.1128/jvi.40.1.241-247.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S., Johnson P. R., Wong K. K., Jr Dual-target inhibition of HIV-1 in vitro by means of an adeno-associated virus antisense vector. Science. 1992 Nov 27;258(5087):1485–1488. doi: 10.1126/science.1359646. [DOI] [PubMed] [Google Scholar]
- Clever J., Yamada M., Kasamatsu H. Import of simian virus 40 virions through nuclear pore complexes. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7333–7337. doi: 10.1073/pnas.88.16.7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree G. R. Contingent genetic regulatory events in T lymphocyte activation. Science. 1989 Jan 20;243(4889):355–361. doi: 10.1126/science.2783497. [DOI] [PubMed] [Google Scholar]
- Culver K. W., Ram Z., Wallbridge S., Ishii H., Oldfield E. H., Blaese R. M. In vivo gene transfer with retroviral vector-producer cells for treatment of experimental brain tumors. Science. 1992 Jun 12;256(5063):1550–1552. doi: 10.1126/science.1317968. [DOI] [PubMed] [Google Scholar]
- Fritsch E. F., Temin H. M. Inhibition of viral DNA synthesis in stationary chicken embryo fibroblasts infected with avian retroviruses. J Virol. 1977 Nov;24(2):461–469. doi: 10.1128/jvi.24.2.461-469.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Gray J. W., Coffino P. Cell cycle analysis by flow cytometry. Methods Enzymol. 1979;58:233–248. doi: 10.1016/s0076-6879(79)58140-3. [DOI] [PubMed] [Google Scholar]
- Harel J., Rassart E., Jolicoeur P. Cell cycle dependence of synthesis of unintegrated viral DNA in mouse cells newly infected with murine leukemia virus. Virology. 1981 Apr 15;110(1):202–207. doi: 10.1016/0042-6822(81)90022-2. [DOI] [PubMed] [Google Scholar]
- Hermonat P. L., Muzyczka N. Use of adeno-associated virus as a mammalian DNA cloning vector: transduction of neomycin resistance into mammalian tissue culture cells. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6466–6470. doi: 10.1073/pnas.81.20.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H. J., Yee J. K., Shew J. Y., Chen P. L., Bookstein R., Friedmann T., Lee E. Y., Lee W. H. Suppression of the neoplastic phenotype by replacement of the RB gene in human cancer cells. Science. 1988 Dec 16;242(4885):1563–1566. doi: 10.1126/science.3201247. [DOI] [PubMed] [Google Scholar]
- Hunter L. A., Samulski R. J. Colocalization of adeno-associated virus Rep and capsid proteins in the nuclei of infected cells. J Virol. 1992 Jan;66(1):317–324. doi: 10.1128/jvi.66.1.317-324.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J. P., Jones C. M., Baille J. P. Characteristics of a human diploid cell designated MRC-5. Nature. 1970 Jul 11;227(5254):168–170. doi: 10.1038/227168a0. [DOI] [PubMed] [Google Scholar]
- Janik J. E., Huston M. M., Rose J. A. Locations of adenovirus genes required for the replication of adenovirus-associated virus. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1925–1929. doi: 10.1073/pnas.78.3.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotin R. M., Linden R. M., Berns K. I. Characterization of a preferred site on human chromosome 19q for integration of adeno-associated virus DNA by non-homologous recombination. EMBO J. 1992 Dec;11(13):5071–5078. doi: 10.1002/j.1460-2075.1992.tb05614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotin R. M., Siniscalco M., Samulski R. J., Zhu X. D., Hunter L., Laughlin C. A., McLaughlin S., Muzyczka N., Rocchi M., Berns K. I. Site-specific integration by adeno-associated virus. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2211–2215. doi: 10.1073/pnas.87.6.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFace D., Hermonat P., Wakeland E., Peck A. Gene transfer into hematopoietic progenitor cells mediated by an adeno-associated virus vector. Virology. 1988 Feb;162(2):483–486. doi: 10.1016/0042-6822(88)90491-6. [DOI] [PubMed] [Google Scholar]
- Lebkowski J. S., McNally M. M., Okarma T. B., Lerch L. B. Adeno-associated virus: a vector system for efficient introduction and integration of DNA into a variety of mammalian cell types. Mol Cell Biol. 1988 Oct;8(10):3988–3996. doi: 10.1128/mcb.8.10.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis P., Hensel M., Emerman M. Human immunodeficiency virus infection of cells arrested in the cell cycle. EMBO J. 1992 Aug;11(8):3053–3058. doi: 10.1002/j.1460-2075.1992.tb05376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
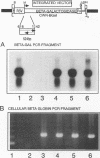
- Li G., Simm M., Potash M. J., Volsky D. J. Human immunodeficiency virus type 1 DNA synthesis, integration, and efficient viral replication in growth-arrested T cells. J Virol. 1993 Jul;67(7):3969–3977. doi: 10.1128/jvi.67.7.3969-3977.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S. K., Collis P., Hermonat P. L., Muzyczka N. Adeno-associated virus general transduction vectors: analysis of proviral structures. J Virol. 1988 Jun;62(6):1963–1973. doi: 10.1128/jvi.62.6.1963-1973.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D. Human gene therapy comes of age. Nature. 1992 Jun 11;357(6378):455–460. doi: 10.1038/357455a0. [DOI] [PubMed] [Google Scholar]
- Miller D. G., Adam M. A., Miller A. D. Gene transfer by retrovirus vectors occurs only in cells that are actively replicating at the time of infection. Mol Cell Biol. 1990 Aug;10(8):4239–4242. doi: 10.1128/mcb.10.8.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan R. C. The basic science of gene therapy. Science. 1993 May 14;260(5110):926–932. doi: 10.1126/science.8493530. [DOI] [PubMed] [Google Scholar]
- Muzyczka N. Use of adeno-associated virus as a general transduction vector for mammalian cells. Curr Top Microbiol Immunol. 1992;158:97–129. doi: 10.1007/978-3-642-75608-5_5. [DOI] [PubMed] [Google Scholar]
- Neckers L., Whitesell L., Rosolen A., Geselowitz D. A. Antisense inhibition of oncogene expression. Crit Rev Oncog. 1992;3(1-2):175–231. [PubMed] [Google Scholar]
- Nienhuis A. W., McDonagh K. T., Bodine D. M. Gene transfer into hematopoietic stem cells. Cancer. 1991 May 15;67(10 Suppl):2700–2704. doi: 10.1002/1097-0142(19910515)67:10+<2700::aid-cncr2820671705>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Nigg E. A., Baeuerle P. A., Lührmann R. Nuclear import-export: in search of signals and mechanisms. Cell. 1991 Jul 12;66(1):15–22. doi: 10.1016/0092-8674(91)90135-l. [DOI] [PubMed] [Google Scholar]
- Ogawa M. Differentiation and proliferation of hematopoietic stem cells. Blood. 1993 Jun 1;81(11):2844–2853. [PubMed] [Google Scholar]
- Roe T., Reynolds T. C., Yu G., Brown P. O. Integration of murine leukemia virus DNA depends on mitosis. EMBO J. 1993 May;12(5):2099–2108. doi: 10.1002/j.1460-2075.1993.tb05858.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruffing M., Zentgraf H., Kleinschmidt J. A. Assembly of viruslike particles by recombinant structural proteins of adeno-associated virus type 2 in insect cells. J Virol. 1992 Dec;66(12):6922–6930. doi: 10.1128/jvi.66.12.6922-6930.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samulski R. J., Zhu X., Xiao X., Brook J. D., Housman D. E., Epstein N., Hunter L. A. Targeted integration of adeno-associated virus (AAV) into human chromosome 19. EMBO J. 1991 Dec;10(12):3941–3950. doi: 10.1002/j.1460-2075.1991.tb04964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheaff R., Ilsley D., Kuchta R. Mechanism of DNA polymerase alpha inhibition by aphidicolin. Biochemistry. 1991 Sep 3;30(35):8590–8597. doi: 10.1021/bi00099a014. [DOI] [PubMed] [Google Scholar]
- Snyder D. S., Wu Y., Wang J. L., Rossi J. J., Swiderski P., Kaplan B. E., Forman S. J. Ribozyme-mediated inhibition of bcr-abl gene expression in a Philadelphia chromosome-positive cell line. Blood. 1993 Jul 15;82(2):600–605. [PubMed] [Google Scholar]
- Springett G. M., Moen R. C., Anderson S., Blaese R. M., Anderson W. F. Infection efficiency of T lymphocytes with amphotropic retroviral vectors is cell cycle dependent. J Virol. 1989 Sep;63(9):3865–3869. doi: 10.1128/jvi.63.9.3865-3869.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullenger B. A., Gallardo H. F., Ungers G. E., Gilboa E. Overexpression of TAR sequences renders cells resistant to human immunodeficiency virus replication. Cell. 1990 Nov 2;63(3):601–608. doi: 10.1016/0092-8674(90)90455-n. [DOI] [PubMed] [Google Scholar]
- Tratschin J. D., Miller I. L., Smith M. G., Carter B. J. Adeno-associated virus vector for high-frequency integration, expression, and rescue of genes in mammalian cells. Mol Cell Biol. 1985 Nov;5(11):3251–3260. doi: 10.1128/mcb.5.11.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida N., Fleming W. H., Alpern E. J., Weissman I. L. Heterogeneity of hematopoietic stem cells. Curr Opin Immunol. 1993 Apr;5(2):177–184. doi: 10.1016/0952-7915(93)90002-a. [DOI] [PubMed] [Google Scholar]
- Walsh C. E., Liu J. M., Xiao X., Young N. S., Nienhuis A. W., Samulski R. J. Regulated high level expression of a human gamma-globin gene introduced into erythroid cells by an adeno-associated virus vector. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):7257–7261. doi: 10.1073/pnas.89.15.7257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K. K., Jr, Chatterjee S. Controlling herpes simplex virus infections: is intracellular immunization the way of the future? Curr Top Microbiol Immunol. 1992;179:159–174. doi: 10.1007/978-3-642-77247-4_10. [DOI] [PubMed] [Google Scholar]
- Zaia J. A., Chatterjee S., Wong K. K., Elkins D., Taylor N. R., Rossi J. J. Status of ribozyme and antisense-based developmental approaches for anti-HIV-1 therapy. Ann N Y Acad Sci. 1992 Oct 28;660:95–106. doi: 10.1111/j.1749-6632.1992.tb21062.x. [DOI] [PubMed] [Google Scholar]
- Zaia J. A., Oxman M. N. Antibody to varicella-zoster virus-induced membrane antigen: immunofluorescence assay using monodisperse glutaraldehyde-fixed target cells. J Infect Dis. 1977 Oct;136(4):519–530. doi: 10.1093/infdis/136.4.519. [DOI] [PubMed] [Google Scholar]
- Zhou S. Z., Broxmeyer H. E., Cooper S., Harrington M. A., Srivastava A. Adeno-associated virus 2-mediated gene transfer in murine hematopoietic progenitor cells. Exp Hematol. 1993 Jul;21(7):928–933. [PubMed] [Google Scholar]
- von Rüden T., Gilboa E. Inhibition of human T-cell leukemia virus type I replication in primary human T cells that express antisense RNA. J Virol. 1989 Feb;63(2):677–682. doi: 10.1128/jvi.63.2.677-682.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]