Abstract
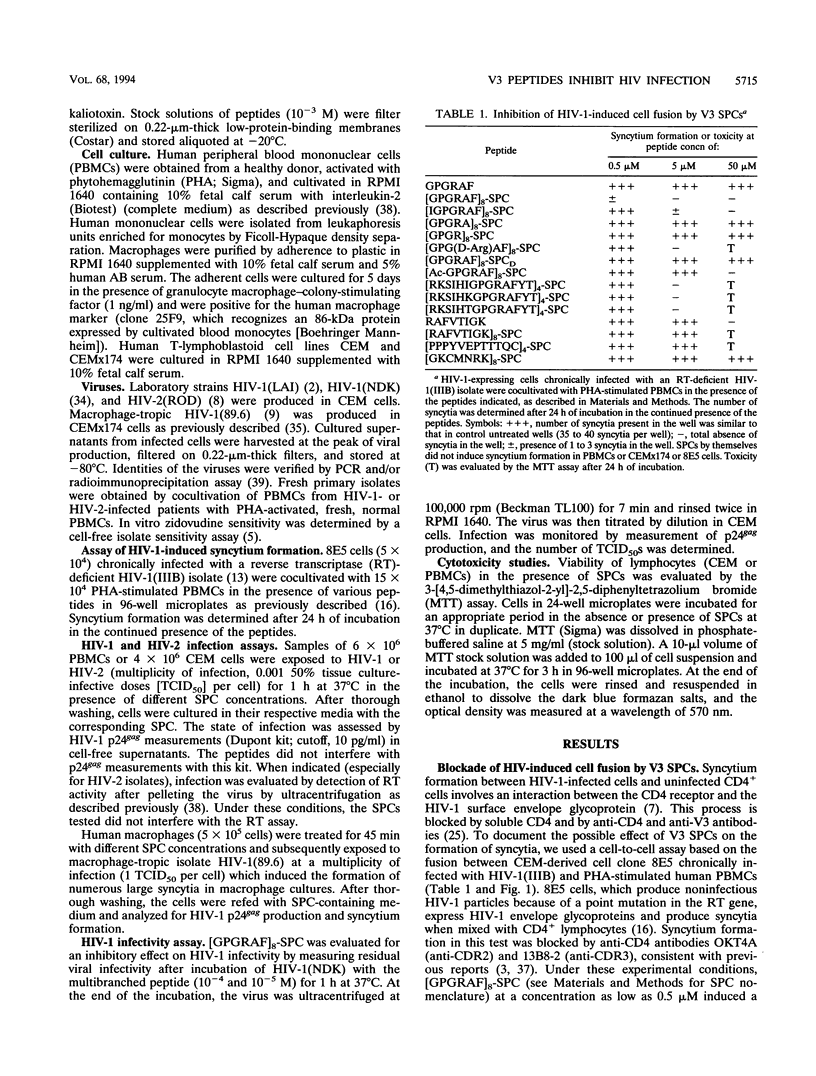
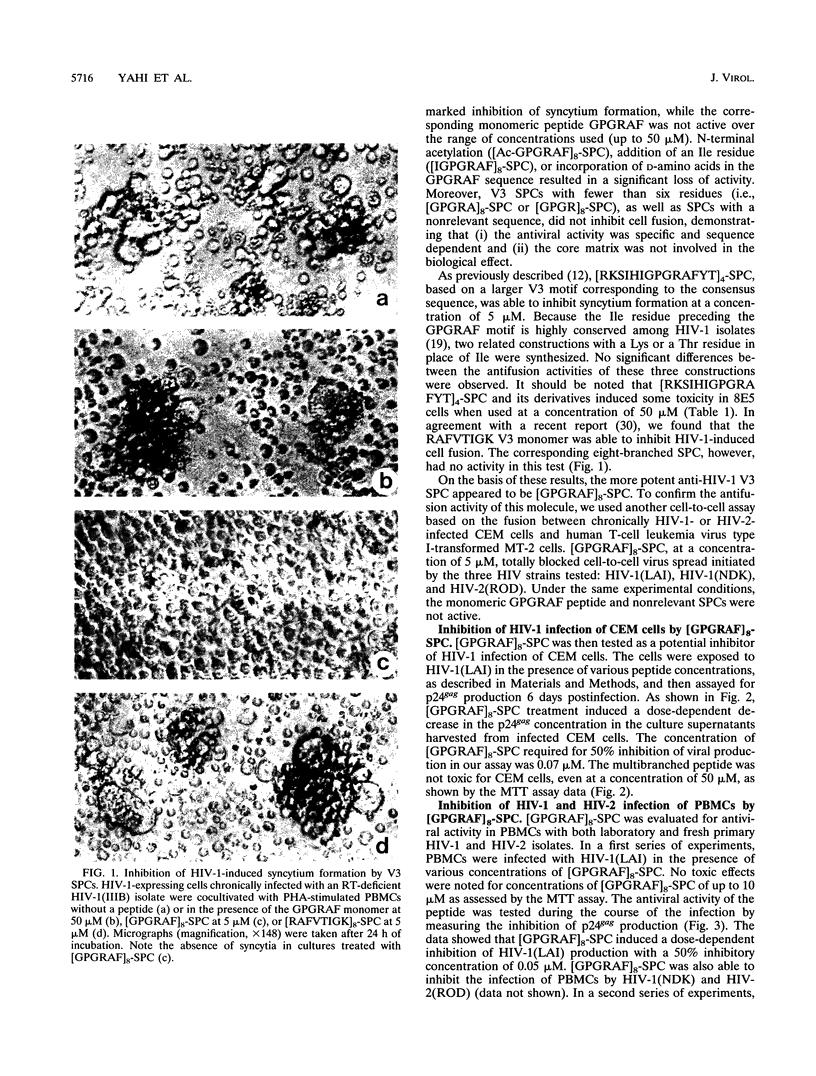
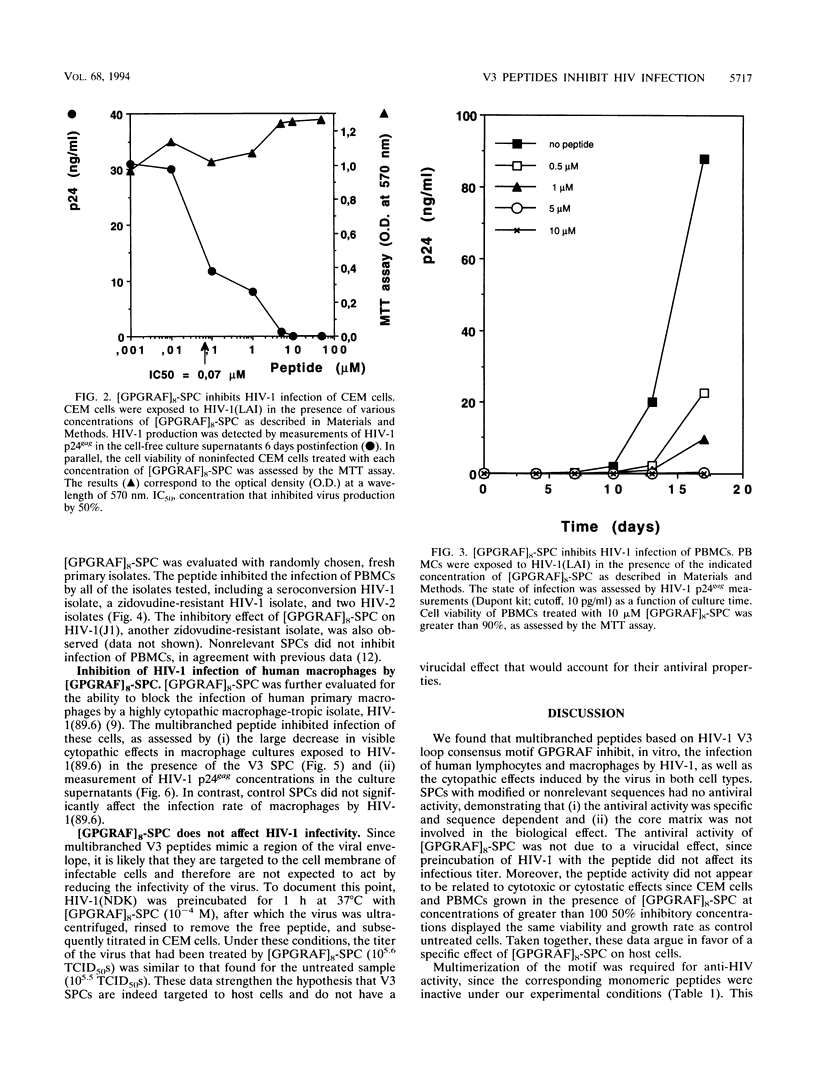
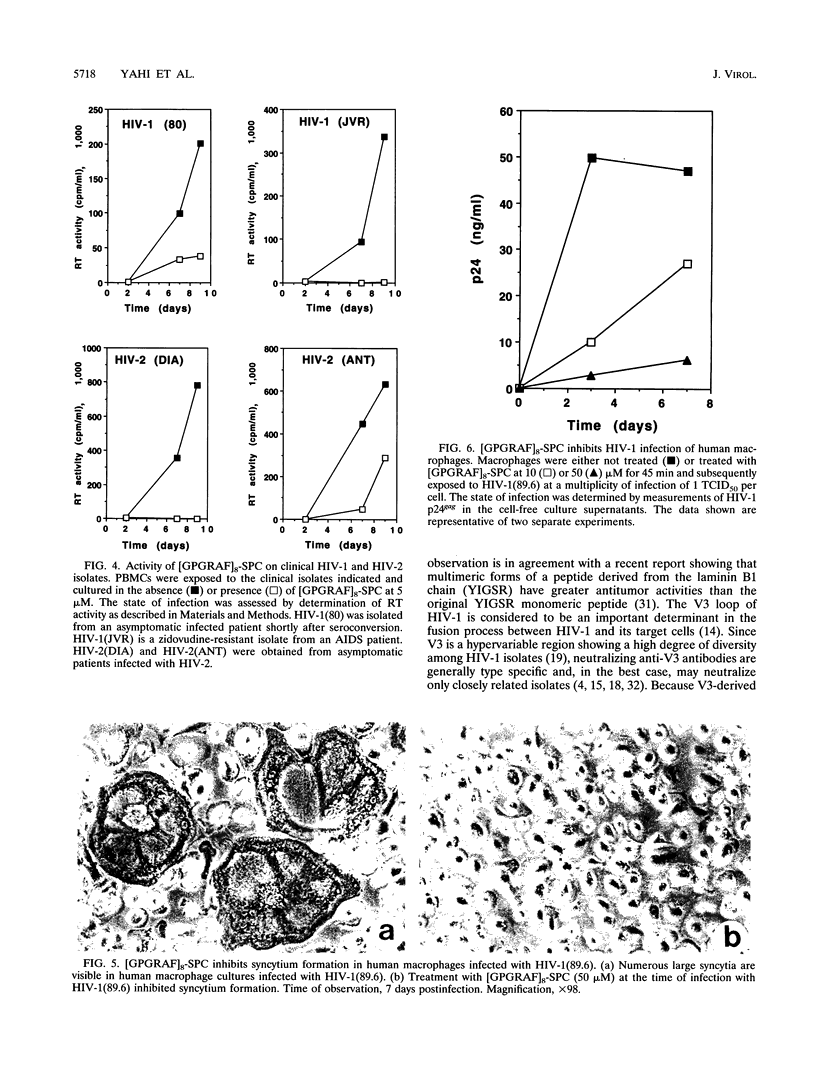
Synthetic polymeric constructions (SPCs) including the consensus sequence of the human immunodeficiency virus type 1 (HIV-1) surface envelope glycoprotein gp120 V3 loop (GPGRAF) blocked the fusion between HIV-1- and HIV-2-infected cells and CD4+ uninfected cells. A structure-activity relationship study using V3 SPC analogs showed that the most efficient inhibitor of cell fusion was an eight-branched SPC with the hexapeptide motif GPGRAF (i.e., [GPGRAF]8-SPC). N-terminal acetylation or incorporation of D-amino acids in the GPGRAF sequence of this SPC resulted in significant loss of activity. Analogs with fewer than six residues in the motif (i.e., GPGRA or GPGR), as well as SPCs with a nonrelevant sequence, did not inhibit cell fusion, demonstrating the high specificity of the antifusion activity. [GPGRAF]8-SPC, which was not toxic to CEM cells at concentrations of up to 50 microM, inhibited 50% of HIV-1(LAI) replication in these cells at a concentration of 0.07 microM. Moreover, [GPGRAF]8-SPC inhibited the infection of human peripheral blood mononuclear cells by several HIV-1 and HIV-2 isolates, including laboratory strains [HIV-1(LAI), HIV-1(NDK), and HIV-2(ROD)], and fresh primary isolates, including two zidovudine-resistant HIV-1 isolates and two HIV-2 isolates obtained from infected individuals. The multibranched peptide also inhibited infection of human primary macrophages by the highly cytopathic macrophage-tropic isolate HIV-1(89.6). The antiviral activity of [GPGRAF]8-SPC was not related to a virucidal effect, since preincubation of HIV-1 with the peptide did not affect its infectious titer. This result is in agreement with the concept that the multibranched peptide mimics a part of the V3 loop and thus interacts with the host cell. The therapeutic properties of synthetic multibranched peptides based on the V3 loop consensus motif should be evaluated in HIV-infected patients.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Autiero M., Abrescia P., Dettin M., Di Bello C., Guardiola J. Binding to CD4 of synthetic peptides patterned on the principal neutralizing domain of the HIV-1 envelope protein. Virology. 1991 Dec;185(2):820–828. doi: 10.1016/0042-6822(91)90553-n. [DOI] [PubMed] [Google Scholar]
- Barré-Sinoussi F., Chermann J. C., Rey F., Nugeyre M. T., Chamaret S., Gruest J., Dauguet C., Axler-Blin C., Vézinet-Brun F., Rouzioux C. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science. 1983 May 20;220(4599):868–871. doi: 10.1126/science.6189183. [DOI] [PubMed] [Google Scholar]
- Benkirane M., Corbeau P., Housset V., Devaux C. An antibody that binds the immunoglobulin CDR3-like region of the CD4 molecule inhibits provirus transcription in HIV-infected T cells. EMBO J. 1993 Dec 15;12(13):4909–4921. doi: 10.1002/j.1460-2075.1993.tb06185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolognesi D. P. Human immunodeficiency virus vaccines. Adv Virus Res. 1993;42:103–148. doi: 10.1016/s0065-3527(08)60084-6. [DOI] [PubMed] [Google Scholar]
- Brun-Vézinet F., Ingrand D., Deforges L., Gochi K., Ferchal F., Schmitt M. P., Jung M., Masquelier B., Aubert J., Buffet-Janvresse C. HIV-1 sensitivity to zidovudine: a consensus culture technique validated by genotypic analysis of the reverse transcriptase. J Virol Methods. 1992 May;37(2):177–188. doi: 10.1016/0166-0934(92)90045-f. [DOI] [PubMed] [Google Scholar]
- Callebaut C., Krust B., Jacotot E., Hovanessian A. G. T cell activation antigen, CD26, as a cofactor for entry of HIV in CD4+ cells. Science. 1993 Dec 24;262(5142):2045–2050. doi: 10.1126/science.7903479. [DOI] [PubMed] [Google Scholar]
- Camerini D., Seed B. A CD4 domain important for HIV-mediated syncytium formation lies outside the virus binding site. Cell. 1990 Mar 9;60(5):747–754. doi: 10.1016/0092-8674(90)90089-w. [DOI] [PubMed] [Google Scholar]
- Clavel F., Guétard D., Brun-Vézinet F., Chamaret S., Rey M. A., Santos-Ferreira M. O., Laurent A. G., Dauguet C., Katlama C., Rouzioux C. Isolation of a new human retrovirus from West African patients with AIDS. Science. 1986 Jul 18;233(4761):343–346. doi: 10.1126/science.2425430. [DOI] [PubMed] [Google Scholar]
- Collman R., Balliet J. W., Gregory S. A., Friedman H., Kolson D. L., Nathanson N., Srinivasan A. An infectious molecular clone of an unusual macrophage-tropic and highly cytopathic strain of human immunodeficiency virus type 1. J Virol. 1992 Dec;66(12):7517–7521. doi: 10.1128/jvi.66.12.7517-7521.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rossi A., Pasti M., Mammano F., Panozzo M., Dettin M., Di Bello C., Chieco-Bianchi L. Synthetic peptides from the principal neutralizing domain of human immunodeficiency virus type 1 (HIV-1) enhance HIV-1 infection through a CD4-dependent mechanism. Virology. 1991 Sep;184(1):187–196. doi: 10.1016/0042-6822(91)90835-y. [DOI] [PubMed] [Google Scholar]
- Ebenbichler C., Westervelt P., Carrillo A., Henkel T., Johnson D., Ratner L. Structure-function relationships of the HIV-1 envelope V3 loop tropism determinant: evidence for two distinct conformations. AIDS. 1993 May;7(5):639–646. [PubMed] [Google Scholar]
- Fantini J., Yahi N., Mabrouk K., Van Rietschoten J., Rochat H., Sabatier J. M. Multi-branched peptides based on the HIV-1 V3 loop consensus motif inhibit HIV-1 and HIV-2 infection in CD4+ and CD4- cells. C R Acad Sci III. 1993 Nov;316(11):1381–1387. [PubMed] [Google Scholar]
- Folks T., Benn S., Rabson A., Theodore T., Hoggan M. D., Martin M., Lightfoote M., Sell K. Characterization of a continuous T-cell line susceptible to the cytopathic effects of the acquired immunodeficiency syndrome (AIDS)-associated retrovirus. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4539–4543. doi: 10.1073/pnas.82.13.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed E. O., Myers D. J., Risser R. Identification of the principal neutralizing determinant of human immunodeficiency virus type 1 as a fusion domain. J Virol. 1991 Jan;65(1):190–194. doi: 10.1128/jvi.65.1.190-194.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudsmit J., Debouck C., Meloen R. H., Smit L., Bakker M., Asher D. M., Wolff A. V., Gibbs C. J., Jr, Gajdusek D. C. Human immunodeficiency virus type 1 neutralization epitope with conserved architecture elicits early type-specific antibodies in experimentally infected chimpanzees. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4478–4482. doi: 10.1073/pnas.85.12.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildreth J. E., Orentas R. J. Involvement of a leukocyte adhesion receptor (LFA-1) in HIV-induced syncytium formation. Science. 1989 Jun 2;244(4908):1075–1078. doi: 10.1126/science.2543075. [DOI] [PubMed] [Google Scholar]
- Hwang S. S., Boyle T. J., Lyerly H. K., Cullen B. R. Identification of the envelope V3 loop as the primary determinant of cell tropism in HIV-1. Science. 1991 Jul 5;253(5015):71–74. doi: 10.1126/science.1905842. [DOI] [PubMed] [Google Scholar]
- Javaherian K., Langlois A. J., LaRosa G. J., Profy A. T., Bolognesi D. P., Herlihy W. C., Putney S. D., Matthews T. J. Broadly neutralizing antibodies elicited by the hypervariable neutralizing determinant of HIV-1. Science. 1990 Dec 14;250(4987):1590–1593. doi: 10.1126/science.1703322. [DOI] [PubMed] [Google Scholar]
- LaRosa G. J., Davide J. P., Weinhold K., Waterbury J. A., Profy A. T., Lewis J. A., Langlois A. J., Dreesman G. R., Boswell R. N., Shadduck P. Conserved sequence and structural elements in the HIV-1 principal neutralizing determinant. Science. 1990 Aug 24;249(4971):932–935. doi: 10.1126/science.2392685. [DOI] [PubMed] [Google Scholar]
- Levy J. A. Pathogenesis of human immunodeficiency virus infection. Microbiol Rev. 1993 Mar;57(1):183–289. doi: 10.1128/mr.57.1.183-289.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifson J. D., Hwang K. M., Nara P. L., Fraser B., Padgett M., Dunlop N. M., Eiden L. E. Synthetic CD4 peptide derivatives that inhibit HIV infection and cytopathicity. Science. 1988 Aug 5;241(4866):712–716. doi: 10.1126/science.2969619. [DOI] [PubMed] [Google Scholar]
- Lifson J. D., Rausch D. M., Kalyanaraman V. S., Hwang K. M., Eiden L. E. Synthetic peptides allow discrimination of structural features of CD4(81-92) important for HIV-1 infection versus HIV-1-induced syncytium formation. AIDS Res Hum Retroviruses. 1991 Jun;7(6):521–527. doi: 10.1089/aid.1991.7.521. [DOI] [PubMed] [Google Scholar]
- Merrifield B. Solid phase synthesis. Science. 1986 Apr 18;232(4748):341–347. doi: 10.1126/science.3961484. [DOI] [PubMed] [Google Scholar]
- Moore J. P., Nara P. L. The role of the V3 loop of gp120 in HIV infection. AIDS. 1991;5 (Suppl 2):S21–S33. doi: 10.1097/00002030-199101001-00004. [DOI] [PubMed] [Google Scholar]
- Murakami T., Hattori T., Takatsuki K. A principal neutralizing domain of human immunodeficiency virus type 1 interacts with proteinase-like molecule(s) at the surface of Molt-4 clone 8 cells. Biochim Biophys Acta. 1991 Sep 20;1079(3):279–284. doi: 10.1016/0167-4838(91)90070-g. [DOI] [PubMed] [Google Scholar]
- Nara P. L., Garrity R. R., Goudsmit J. Neutralization of HIV-1: a paradox of humoral proportions. FASEB J. 1991 Jul;5(10):2437–2455. doi: 10.1096/fasebj.5.10.1712328. [DOI] [PubMed] [Google Scholar]
- Nara P. L., Hwang K. M., Rausch D. M., Lifson J. D., Eiden L. E. CD4 antigen-based antireceptor peptides inhibit infectivity of human immunodeficiency virus in vitro at multiple stages of the viral life cycle. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7139–7143. doi: 10.1073/pnas.86.18.7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardelli B., Lu Y. A., Shiu D. R., Delpierre-Defoort C., Profy A. T., Tam J. P. A chemically defined synthetic vaccine model for HIV-1. J Immunol. 1992 Feb 1;148(3):914–920. [PubMed] [Google Scholar]
- Nehete P. N., Arlinghaus R. B., Sastry K. J. Inhibition of human immunodeficiency virus type 1 infection and syncytium formation in human cells by V3 loop synthetic peptides from gp120. J Virol. 1993 Nov;67(11):6841–6846. doi: 10.1128/jvi.67.11.6841-6846.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomizu M., Yamamura K., Kleinman H. K., Yamada Y. Multimeric forms of Tyr-Ile-Gly-Ser-Arg (YIGSR) peptide enhance the inhibition of tumor growth and metastasis. Cancer Res. 1993 Aug 1;53(15):3459–3461. [PubMed] [Google Scholar]
- Palker T. J., Clark M. E., Langlois A. J., Matthews T. J., Weinhold K. J., Randall R. R., Bolognesi D. P., Haynes B. F. Type-specific neutralization of the human immunodeficiency virus with antibodies to env-encoded synthetic peptides. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1932–1936. doi: 10.1073/pnas.85.6.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatier J. M., Zerrouk H., Darbon H., Mabrouk K., Benslimane A., Rochat H., Martin-Eauclaire M. F., Van Rietschoten J. P05, a new leiurotoxin I-like scorpion toxin: synthesis and structure-activity relationships of the alpha-amidated analog, a ligand of Ca(2+)-activated K+ channels with increased affinity. Biochemistry. 1993 Mar 23;32(11):2763–2770. doi: 10.1021/bi00062a005. [DOI] [PubMed] [Google Scholar]
- Spire B., Sire J., Zachar V., Rey F., Barré-Sinoussi F., Galibert F., Hampe A., Chermann J. C. Nucleotide sequence of HIV1-NDK: a highly cytopathic strain of the human immunodeficiency virus. Gene. 1989 Sep 30;81(2):275–284. doi: 10.1016/0378-1119(89)90188-1. [DOI] [PubMed] [Google Scholar]
- Stefano K. A., Collman R., Kolson D., Hoxie J., Nathanson N., Gonzalez-Scarano F. Replication of a macrophage-tropic strain of human immunodeficiency virus type 1 (HIV-1) in a hybrid cell line, CEMx174, suggests that cellular accessory molecules are required for HIV-1 entry. J Virol. 1993 Nov;67(11):6707–6715. doi: 10.1128/jvi.67.11.6707-6715.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam J. P. Synthetic peptide vaccine design: synthesis and properties of a high-density multiple antigenic peptide system. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5409–5413. doi: 10.1073/pnas.85.15.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truneh A., Buck D., Cassatt D. R., Juszczak R., Kassis S., Ryu S. E., Healey D., Sweet R., Sattentau Q. A region in domain 1 of CD4 distinct from the primary gp120 binding site is involved in HIV infection and virus-mediated fusion. J Biol Chem. 1991 Mar 25;266(9):5942–5948. [PubMed] [Google Scholar]
- Yahi N., Baghdiguian S., Moreau H., Fantini J. Galactosyl ceramide (or a closely related molecule) is the receptor for human immunodeficiency virus type 1 on human colon epithelial HT29 cells. J Virol. 1992 Aug;66(8):4848–4854. doi: 10.1128/jvi.66.8.4848-4854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahi N., Fantini J., Hirsch I., Chermann J. C. Structural variability of env and gag gene products from a highly cytopathic strain of HIV-1. Arch Virol. 1992;125(1-4):287–298. doi: 10.1007/BF01309645. [DOI] [PubMed] [Google Scholar]




