Abstract
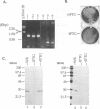
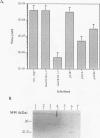
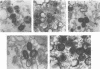
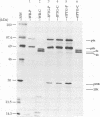
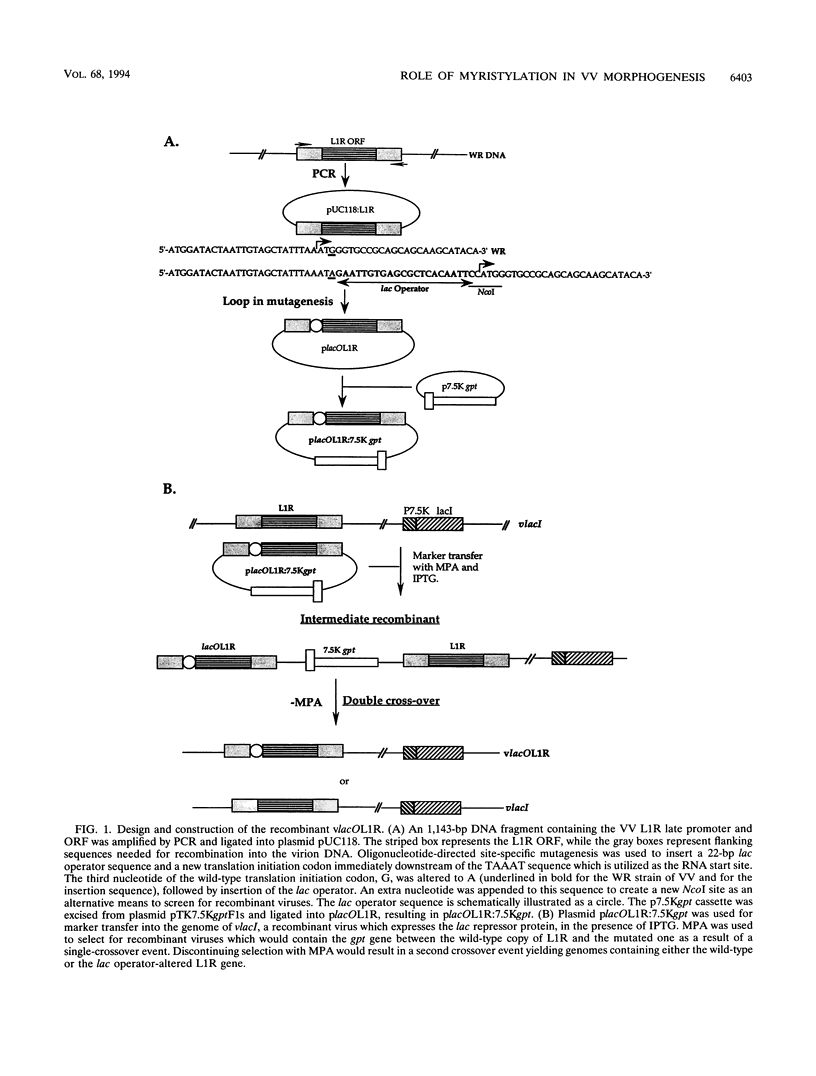
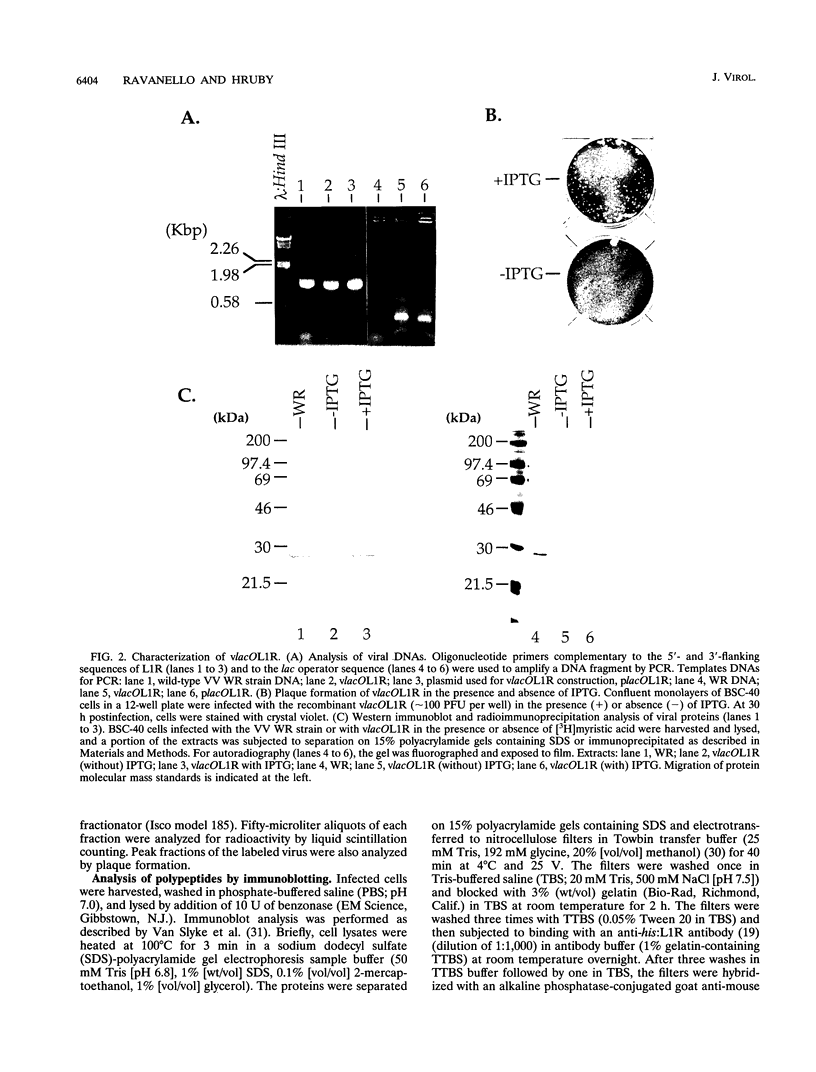
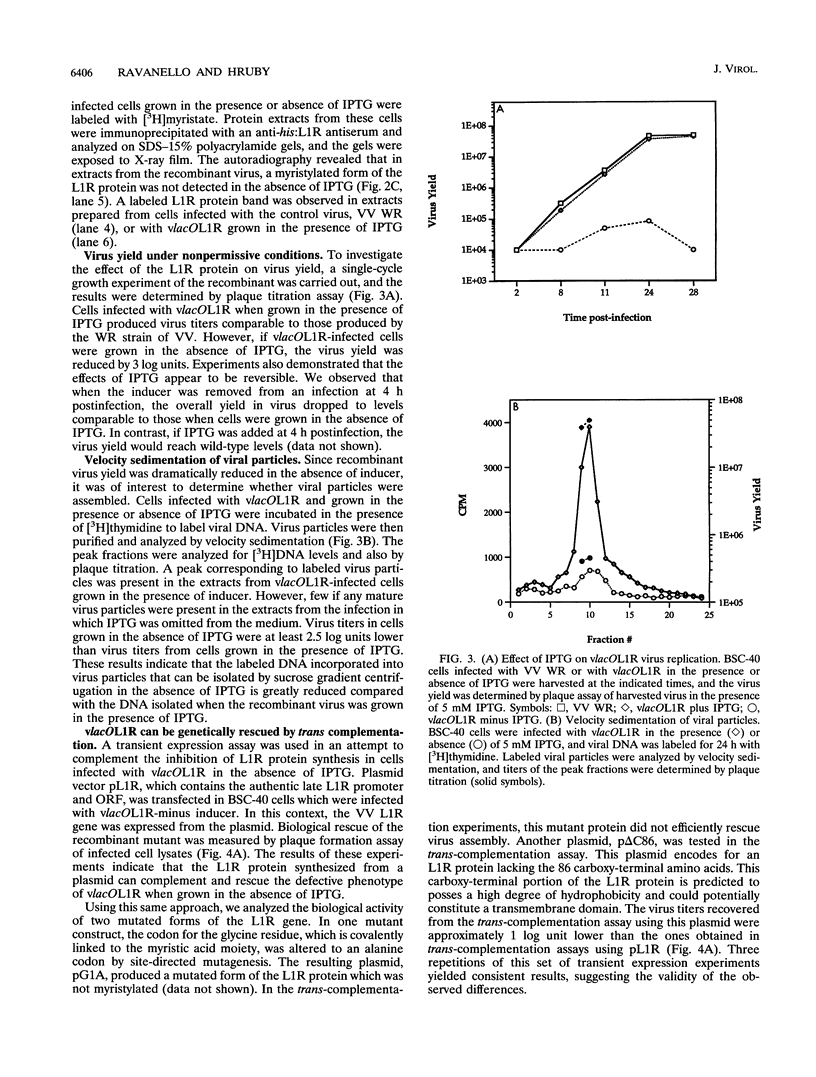
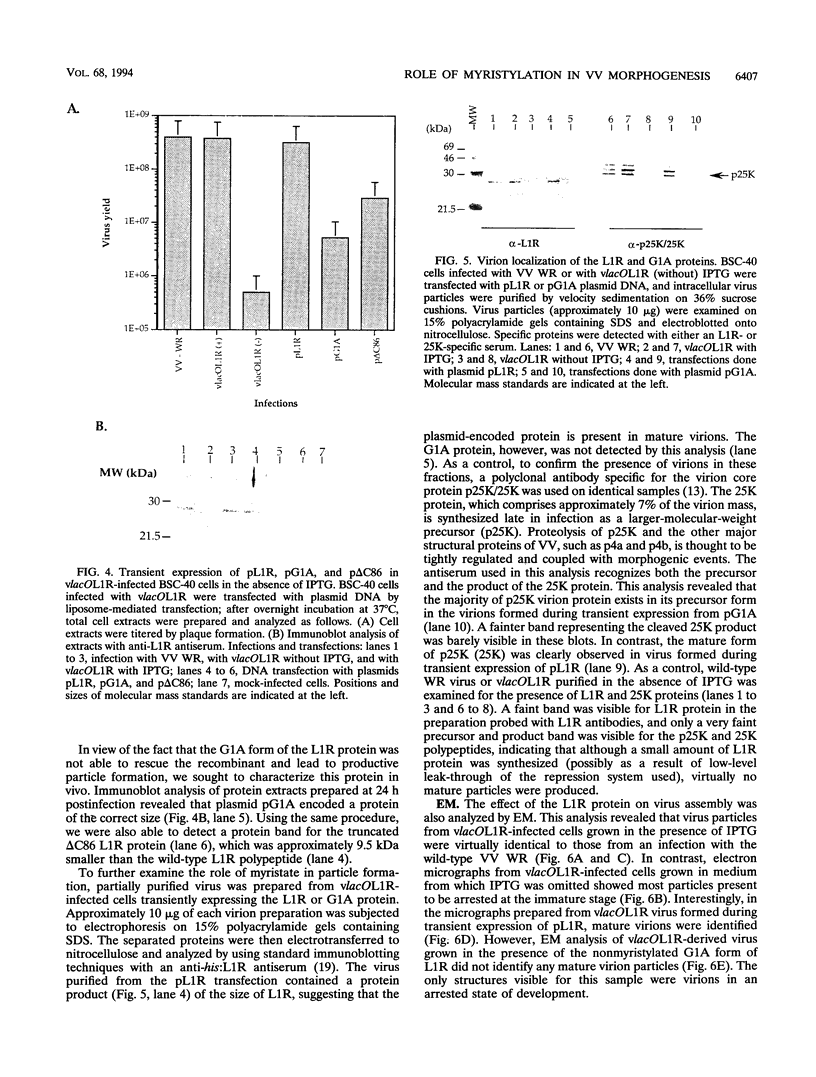
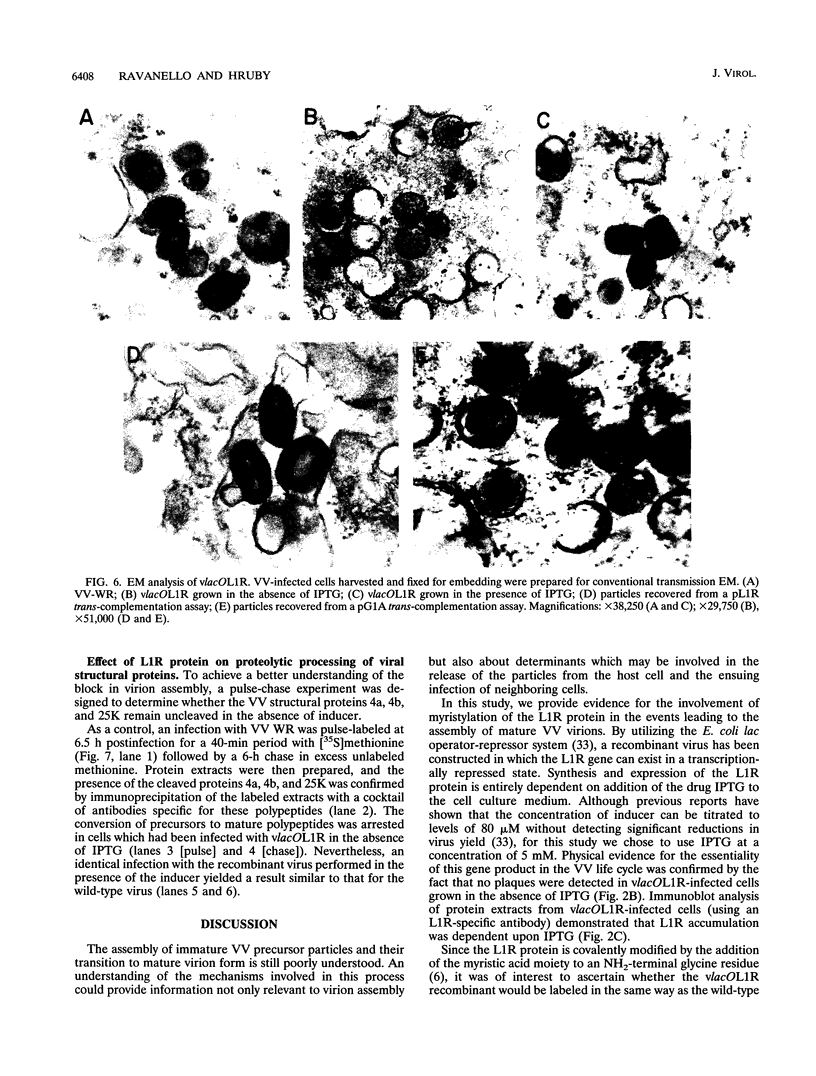
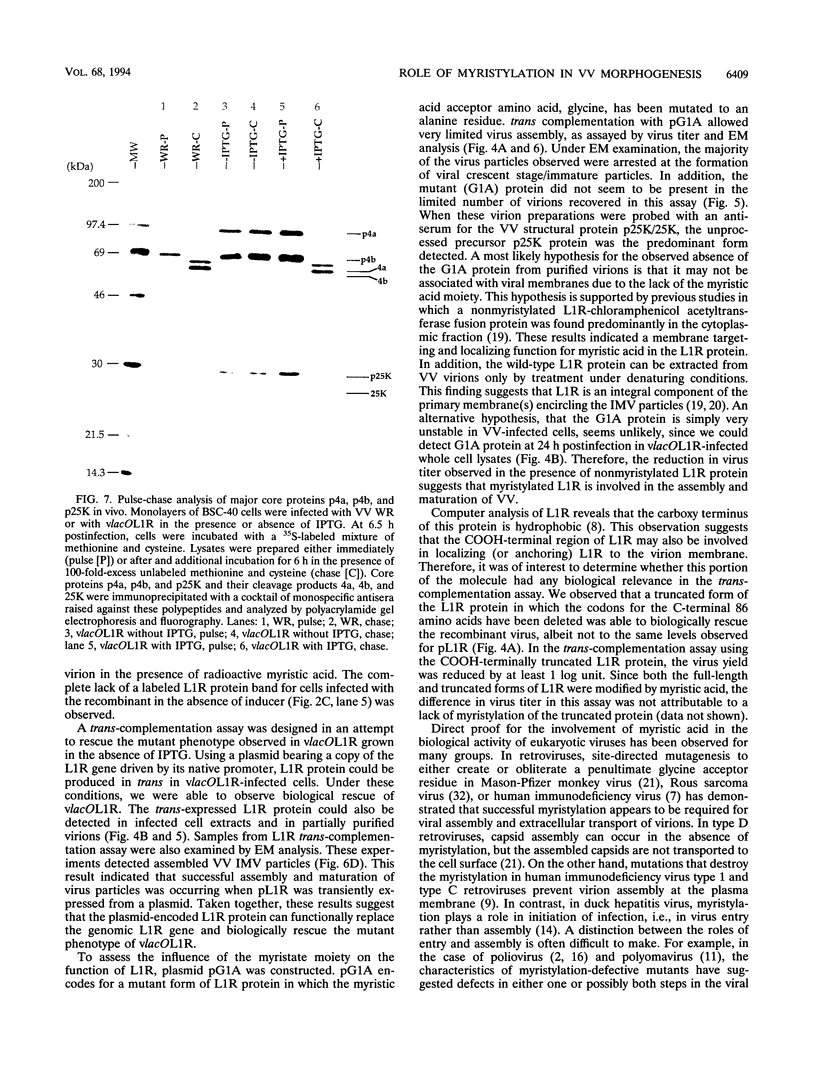
Within vaccinia virus-infected cells, the product of the L1R open reading frame is covalently modified by myristic acid at the penultimate NH2-terminal glycine residue. Previously we have shown that while the L1R protein is a constituent of both intracellular mature virus particles and extracellular enveloped virions which are released from the infected cell, it is associated exclusively with the primary membranes surrounding the virion core. Given this rather specific localization, it was of interest to study the potential role of this essential gene in virus replication and morphogenesis. To this end, we have constructed a recombinant vaccinia virus in which expression of the L1R gene can be transcriptionally repressed. Without the inducer isopropylthiogalactopyranoside (IPTG), synthesis of the L1R protein was blocked, resulting in a total inhibition of plaque formation. Velocity sedimentation of viral particles labeled in the presence of [3H]thymidine, grown in the absence of IPTG, revealed a substantial reduction in viral DNA incorporation into virions. Likewise, proteolysis of the major core proteins p4a, p4b, and p25K, believed to occur during the final stages of virion maturation, was severely impaired. In the absence of L1R expression, only immature virions could be detected by electron microscopy. Transient expression of a plasmid containing the full-length L1R gene driven by its own promoter was able to complement and rescue the defective phenotype. However, a plasmid bearing a mutation in the myristyl acceptor glycine residue was unable to biologically rescue the recombinant, and the protein was not detected in purified virions.trans complementation using a truncated, myristylated form of the L1R protein partially rescued the defective mutant. Collectively, these data suggest that myristic acid mediates essential interactions of the L1R protein with viral membranes and/or other virion components that lead to the productive assembly, maturation, and release of particles.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Chow M., Newman J. F., Filman D., Hogle J. M., Rowlands D. J., Brown F. Myristylation of picornavirus capsid protein VP4 and its structural significance. Nature. 1987 Jun 11;327(6122):482–486. doi: 10.1038/327482a0. [DOI] [PubMed] [Google Scholar]
- Condit R. C., Niles E. G. Orthopoxvirus genetics. Curr Top Microbiol Immunol. 1990;163:1–39. doi: 10.1007/978-3-642-75605-4_1. [DOI] [PubMed] [Google Scholar]
- Franke C. A., Reynolds P. L., Hruby D. E. Fatty acid acylation of vaccinia virus proteins. J Virol. 1989 Oct;63(10):4285–4291. doi: 10.1128/jvi.63.10.4285-4291.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheysen D., Jacobs E., de Foresta F., Thiriart C., Francotte M., Thines D., De Wilde M. Assembly and release of HIV-1 precursor Pr55gag virus-like particles from recombinant baculovirus-infected insect cells. Cell. 1989 Oct 6;59(1):103–112. doi: 10.1016/0092-8674(89)90873-8. [DOI] [PubMed] [Google Scholar]
- Goebel S. J., Johnson G. P., Perkus M. E., Davis S. W., Winslow J. P., Paoletti E. The complete DNA sequence of vaccinia virus. Virology. 1990 Nov;179(1):247-66, 517-63. doi: 10.1016/0042-6822(90)90294-2. [DOI] [PubMed] [Google Scholar]
- Göttlinger H. G., Sodroski J. G., Haseltine W. A. Role of capsid precursor processing and myristoylation in morphogenesis and infectivity of human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5781–5785. doi: 10.1073/pnas.86.15.5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruby D. E., Guarino L. A., Kates J. R. Vaccinia virus replication. I. Requirement for the host-cell nucleus. J Virol. 1979 Feb;29(2):705–715. doi: 10.1128/jvi.29.2.705-715.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauzewicz N., Streuli C. H., Stuart-Smith N., Jones M. D., Wallace S., Griffin B. E. Myristylated polyomavirus VP2: role in the life cycle of the virus. J Virol. 1990 Sep;64(9):4414–4420. doi: 10.1128/jvi.64.9.4414-4420.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P., Hruby D. E. trans processing of vaccinia virus core proteins. J Virol. 1993 Jul;67(7):4252–4263. doi: 10.1128/jvi.67.7.4252-4263.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrae D. R., Bruss V., Ganem D. Myristylation of a duck hepatitis B virus envelope protein is essential for infectivity but not for virus assembly. Virology. 1991 Mar;181(1):359–363. doi: 10.1016/0042-6822(91)90503-4. [DOI] [PubMed] [Google Scholar]
- Miner J. N., Hruby D. E. Rifampicin prevents virosome localization of L65, an essential vaccinia virus polypeptide. Virology. 1989 May;170(1):227–237. doi: 10.1016/0042-6822(89)90370-x. [DOI] [PubMed] [Google Scholar]
- Moscufo N., Simons J., Chow M. Myristoylation is important at multiple stages in poliovirus assembly. J Virol. 1991 May;65(5):2372–2380. doi: 10.1128/jvi.65.5.2372-2380.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Selection for animal cells that express the Escherichia coli gene coding for xanthine-guanine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2072–2076. doi: 10.1073/pnas.78.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne L. Polypeptide composition of extracellular enveloped vaccinia virus. J Virol. 1978 Jul;27(1):28–37. doi: 10.1128/jvi.27.1.28-37.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravanello M. P., Franke C. A., Hruby D. E. An NH2-terminal peptide from the vaccinia virus L1R protein directs the myristylation and virion envelope localization of a heterologous fusion protein. J Biol Chem. 1993 Apr 5;268(10):7585–7593. [PubMed] [Google Scholar]
- Ravanello M. P., Hruby D. E. Characterization of the vaccinia virus L1R myristylprotein as a component of the intracellular virion envelope. J Gen Virol. 1994 Jun;75(Pt 6):1479–1483. doi: 10.1099/0022-1317-75-6-1479. [DOI] [PubMed] [Google Scholar]
- Rhee S. S., Hunter E. Myristylation is required for intracellular transport but not for assembly of D-type retrovirus capsids. J Virol. 1987 Apr;61(4):1045–1053. doi: 10.1128/jvi.61.4.1045-1053.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Buonocore L., Whitt M. A. A new cationic liposome reagent mediating nearly quantitative transfection of animal cells. Biotechniques. 1991 Apr;10(4):520–525. [PubMed] [Google Scholar]
- Sahli R., Freund R., Dubensky T., Garcea R., Bronson R., Benjamin T. Defect in entry and altered pathogenicity of a polyoma virus mutant blocked in VP2 myristylation. Virology. 1993 Jan;192(1):142–153. doi: 10.1006/viro.1993.1016. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz M., Sodeik B., Ericsson M., Wolffe E. J., Shida H., Hiller G., Griffiths G. Assembly of vaccinia virus: the second wrapping cisterna is derived from the trans Golgi network. J Virol. 1994 Jan;68(1):130–147. doi: 10.1128/jvi.68.1.130-147.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodeik B., Doms R. W., Ericsson M., Hiller G., Machamer C. E., van 't Hof W., van Meer G., Moss B., Griffiths G. Assembly of vaccinia virus: role of the intermediate compartment between the endoplasmic reticulum and the Golgi stacks. J Cell Biol. 1993 May;121(3):521–541. doi: 10.1083/jcb.121.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodeik B., Griffiths G., Ericsson M., Moss B., Doms R. W. Assembly of vaccinia virus: effects of rifampin on the intracellular distribution of viral protein p65. J Virol. 1994 Feb;68(2):1103–1114. doi: 10.1128/jvi.68.2.1103-1114.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern W., Dales S. Biogenesis of vaccinia: concerning the origin of the envelope phospholipids. Virology. 1974 Dec;62(2):293–306. doi: 10.1016/0042-6822(74)90393-6. [DOI] [PubMed] [Google Scholar]
- Stern W., Dales S. Biogenesis of vaccinia: isolation and characterization of a surface component that elicits antibody suppressing infectivity and cell-cell fusion. Virology. 1976 Nov;75(1):232–241. doi: 10.1016/0042-6822(76)90022-2. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanslyke J. K., Whitehead S. S., Wilson E. M., Hruby D. E. The multistep proteolytic maturation pathway utilized by vaccinia virus P4a protein: a degenerate conserved cleavage motif within core proteins. Virology. 1991 Aug;183(2):467–478. doi: 10.1016/0042-6822(91)90976-i. [DOI] [PubMed] [Google Scholar]
- Wills J. W., Craven R. C., Achacoso J. A. Creation and expression of myristylated forms of Rous sarcoma virus gag protein in mammalian cells. J Virol. 1989 Oct;63(10):4331–4343. doi: 10.1128/jvi.63.10.4331-4343.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. F., Moss B. Inducer-dependent conditional-lethal mutant animal viruses. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1511–1515. doi: 10.1073/pnas.88.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. F., Moss B. Vaccinia virus morphogenesis is interrupted when expression of the gene encoding an 11-kilodalton phosphorylated protein is prevented by the Escherichia coli lac repressor. J Virol. 1991 Nov;65(11):6101–6110. doi: 10.1128/jvi.65.11.6101-6110.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Moss B. Immature viral envelope formation is interrupted at the same stage by lac operator-mediated repression of the vaccinia virus D13L gene and by the drug rifampicin. Virology. 1992 Apr;187(2):643–653. doi: 10.1016/0042-6822(92)90467-4. [DOI] [PubMed] [Google Scholar]