Abstract
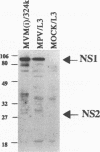
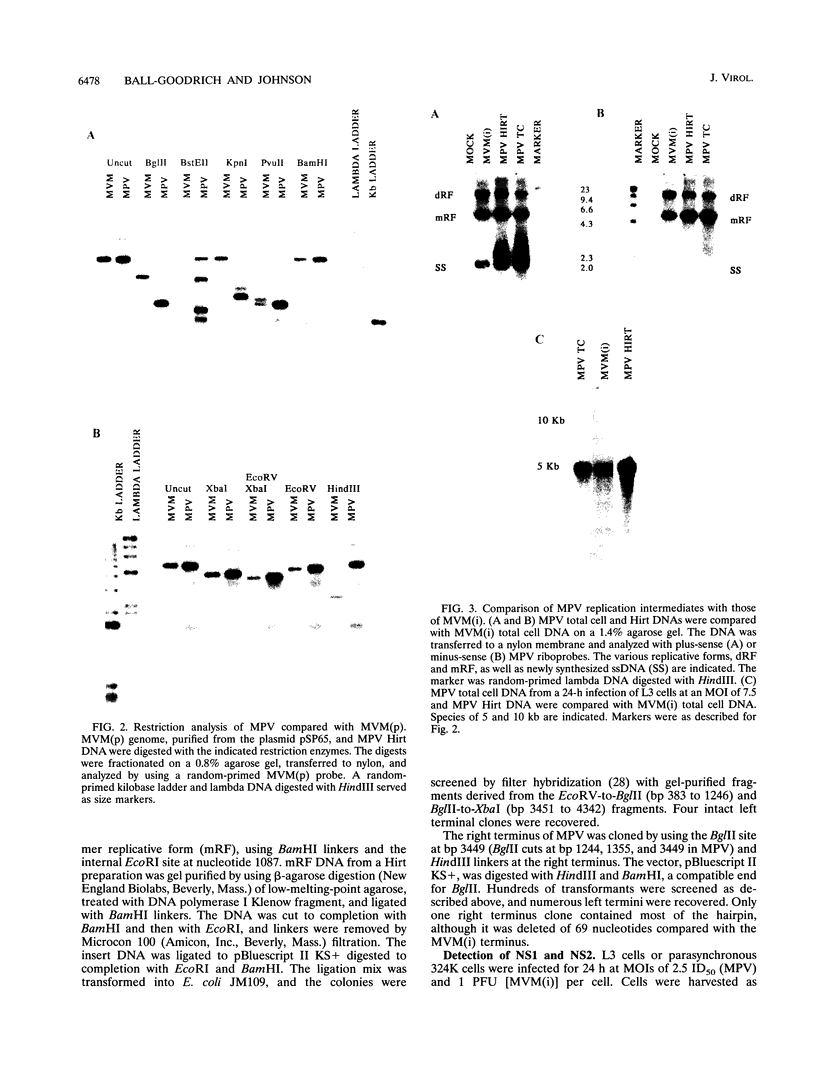
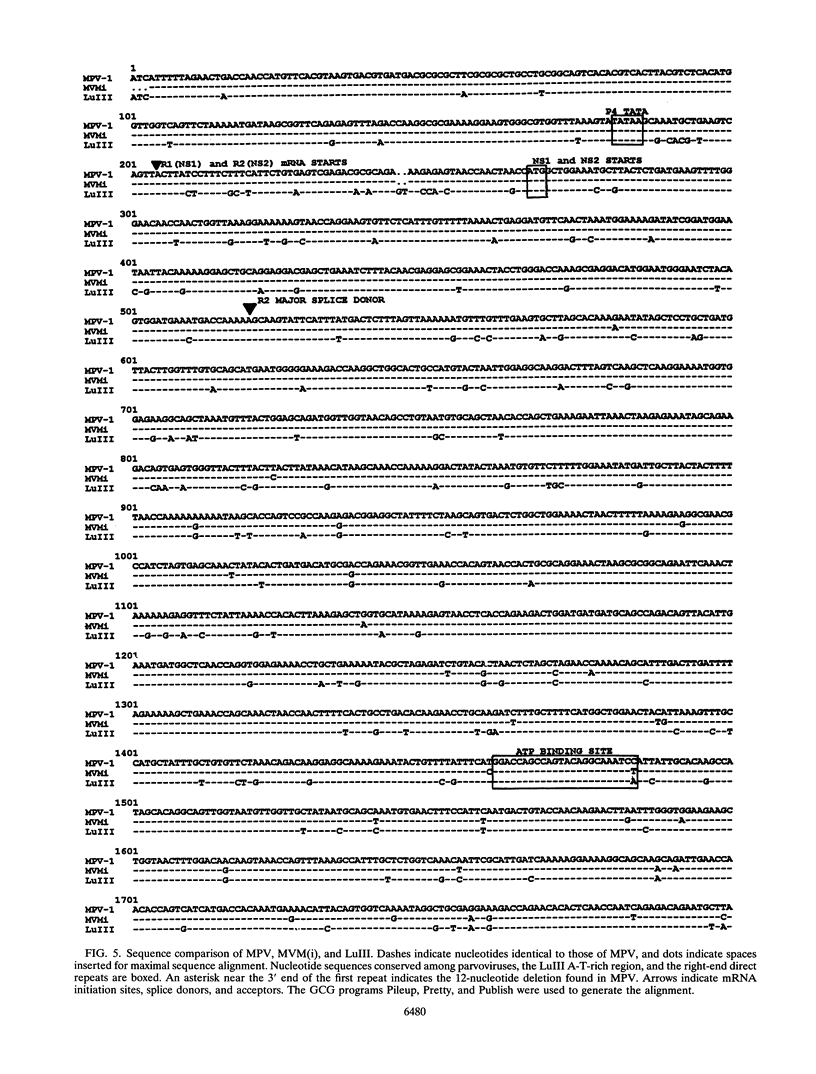
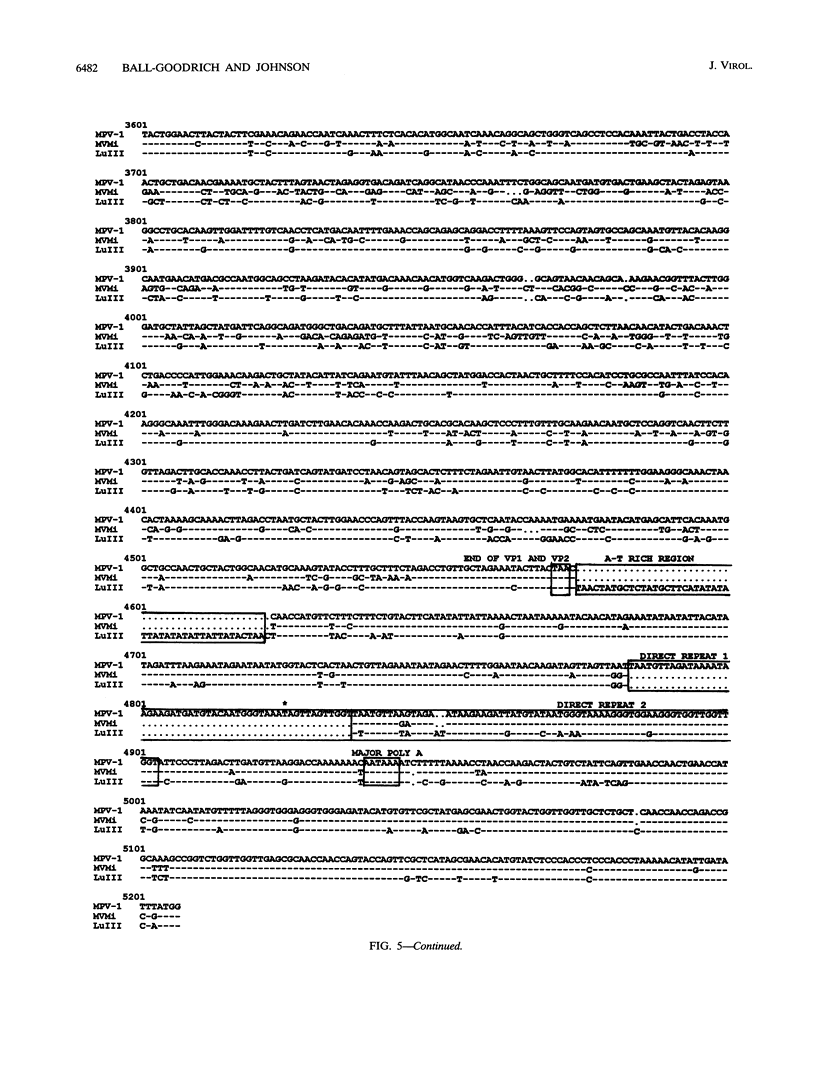
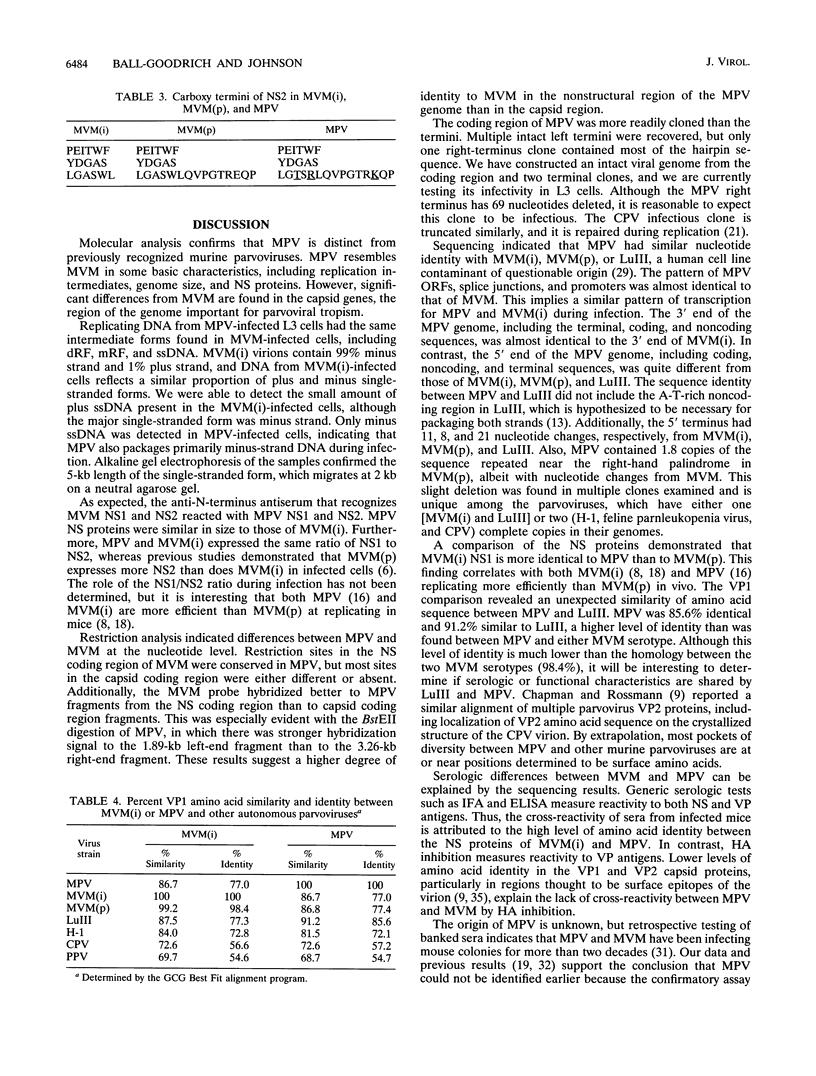
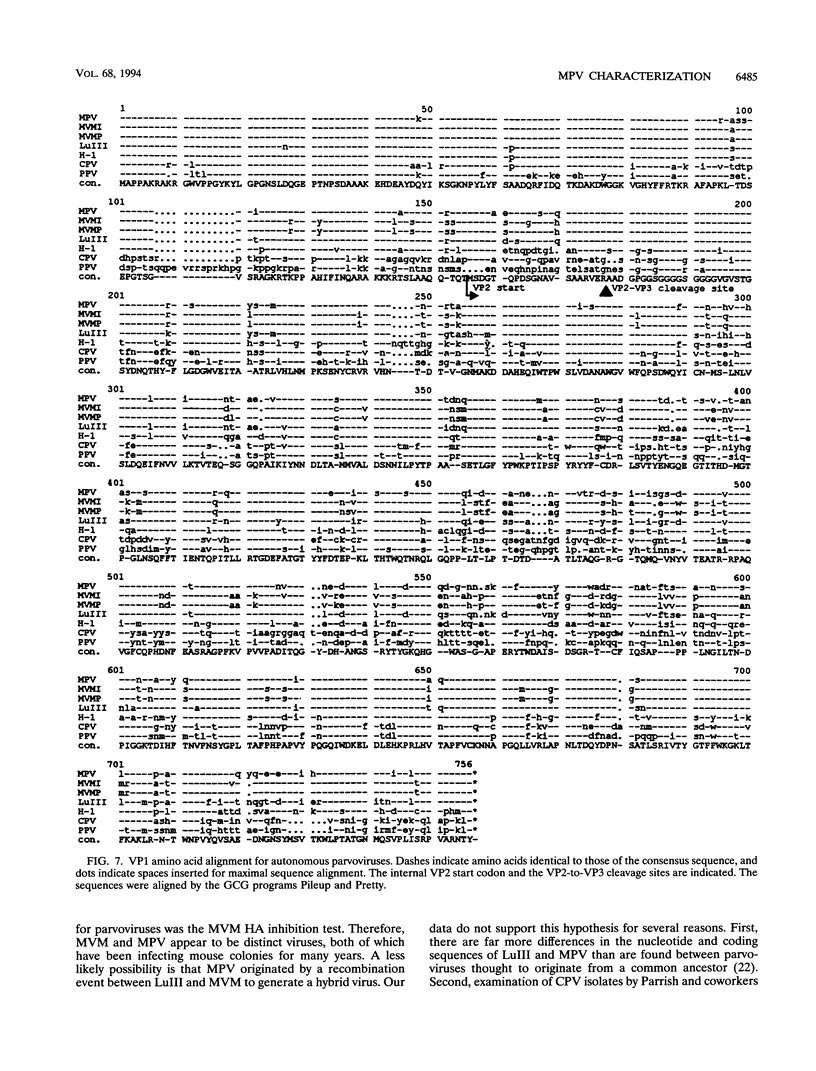
Mouse parvovirus (MPV), formerly known as orphan parvovirus, is a newly recognized rodent parvovirus distinct from both serotypes of minute virus of mice (MVM). Restriction analysis of the MPV genome indicated that many restriction sites in the capsid region were different from those of MVM, but most sites in the nonstructural (NS) region of the genome were conserved. MPV resembled MVM in genome size, replication intermediates, and NS proteins. Replication intermediates in infected cells were the same for MPV and MVM, including packaging of the 5-kb minus (V) strand. Furthermore, the MPV NS proteins were the same size as and present at the same ratio as the MVM(i) proteins in infected cells. Cloning and sequencing of the MPV genome revealed a genome organization closely resembling that of MVM, with conservation of open reading frames, promoter sequences, and splice sites. The left terminal hairpin was identical to that of MVM(i), but the right terminus was not conserved. Also, the MPV genome was unique in that it contained 1.8 copies of the terminal repeat sequence rather than the 1 or 2 copies found in other parvoviruses. The predicted amino acid sequence of the NS proteins of MPV and MVM(i) were nearly identical. In contrast, the predicted amino acid sequence of the capsid proteins of MPV was different from sequences of other parvoviruses. These results confirm that MPV is a distinct murine parvovirus and account for the antigenic differences between MPV and MVM.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonietti J. P., Sahli R., Beard P., Hirt B. Characterization of the cell type-specific determinant in the genome of minute virus of mice. J Virol. 1988 Feb;62(2):552–557. doi: 10.1128/jvi.62.2.552-557.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astell C. R., Gardiner E. M., Tattersall P. DNA sequence of the lymphotropic variant of minute virus of mice, MVM(i), and comparison with the DNA sequence of the fibrotropic prototype strain. J Virol. 1986 Feb;57(2):656–669. doi: 10.1128/jvi.57.2.656-669.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astell C. R., Thomson M., Merchlinsky M., Ward D. C. The complete DNA sequence of minute virus of mice, an autonomous parvovirus. Nucleic Acids Res. 1983 Feb 25;11(4):999–1018. doi: 10.1093/nar/11.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball-Goodrich L. J., Moir R. D., Tattersall P. Parvoviral target cell specificity: acquisition of fibrotropism by a mutant of the lymphotropic strain of minute virus of mice involves multiple amino acid substitutions within the capsid. Virology. 1991 Sep;184(1):175–186. doi: 10.1016/0042-6822(91)90834-x. [DOI] [PubMed] [Google Scholar]
- Ball-Goodrich L. J., Tattersall P. Two amino acid substitutions within the capsid are coordinately required for acquisition of fibrotropism by the lymphotropic strain of minute virus of mice. J Virol. 1992 Jun;66(6):3415–3423. doi: 10.1128/jvi.66.6.3415-3423.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates R. C., Snyder C. E., Banerjee P. T., Mitra S. Autonomous parvovirus LuIII encapsidates equal amounts of plus and minus DNA strands. J Virol. 1984 Feb;49(2):319–324. doi: 10.1128/jvi.49.2.319-324.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownstein D. G., Smith A. L., Jacoby R. O., Johnson E. A., Hansen G., Tattersall P. Pathogenesis of infection with a virulent allotropic variant of minute virus of mice and regulation by host genotype. Lab Invest. 1991 Sep;65(3):357–364. [PubMed] [Google Scholar]
- Chapman M. S., Rossmann M. G. Structure, sequence, and function correlations among parvoviruses. Virology. 1993 Jun;194(2):491–508. doi: 10.1006/viro.1993.1288. [DOI] [PubMed] [Google Scholar]
- Clemens K. E., Pintel D. Minute virus of mice (MVM) mRNAs predominantly polyadenylate at a single site. Virology. 1987 Oct;160(2):511–514. doi: 10.1016/0042-6822(87)90028-6. [DOI] [PubMed] [Google Scholar]
- Cotmore S. F., Tattersall P. Organization of nonstructural genes of the autonomous parvovirus minute virus of mice. J Virol. 1986 Jun;58(3):724–732. doi: 10.1128/jvi.58.3.724-732.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotmore S. F., Tattersall P. The autonomously replicating parvoviruses of vertebrates. Adv Virus Res. 1987;33:91–174. doi: 10.1016/s0065-3527(08)60317-6. [DOI] [PubMed] [Google Scholar]
- Diffoot N., Chen K. C., Bates R. C., Lederman M. The complete nucleotide sequence of parvovirus LuIII and localization of a unique sequence possibly responsible for its encapsidation pattern. Virology. 1993 Jan;192(1):339–345. doi: 10.1006/viro.1993.1040. [DOI] [PubMed] [Google Scholar]
- Gardiner E. M., Tattersall P. Mapping of the fibrotropic and lymphotropic host range determinants of the parvovirus minute virus of mice. J Virol. 1988 Aug;62(8):2605–2613. doi: 10.1128/jvi.62.8.2605-2613.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Jongeneel C. V., Sahli R., McMaster G. K., Hirt B. A precise map of splice junctions in the mRNAs of minute virus of mice, an autonomous parvovirus. J Virol. 1986 Sep;59(3):564–573. doi: 10.1128/jvi.59.3.564-573.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimsey P. B., Engers H. D., Hirt B., Jongeneel C. V. Pathogenicity of fibroblast- and lymphocyte-specific variants of minute virus of mice. J Virol. 1986 Jul;59(1):8–13. doi: 10.1128/jvi.59.1.8-13.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKisic M. D., Lancki D. W., Otto G., Padrid P., Snook S., Cronin D. C., 2nd, Lohmar P. D., Wong T., Fitch F. W. Identification and propagation of a putative immunosuppressive orphan parvovirus in cloned T cells. J Immunol. 1993 Jan 15;150(2):419–428. [PubMed] [Google Scholar]
- Morgan W. R., Ward D. C. Three splicing patterns are used to excise the small intron common to all minute virus of mice RNAs. J Virol. 1986 Dec;60(3):1170–1174. doi: 10.1128/jvi.60.3.1170-1174.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish C. R., Aquadro C. F., Strassheim M. L., Evermann J. F., Sgro J. Y., Mohammed H. O. Rapid antigenic-type replacement and DNA sequence evolution of canine parvovirus. J Virol. 1991 Dec;65(12):6544–6552. doi: 10.1128/jvi.65.12.6544-6552.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish C. R. Emergence, natural history, and variation of canine, mink, and feline parvoviruses. Adv Virus Res. 1990;38:403–450. doi: 10.1016/S0065-3527(08)60867-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish C. R. Mapping specific functions in the capsid structure of canine parvovirus and feline panleukopenia virus using infectious plasmid clones. Virology. 1991 Jul;183(1):195–205. doi: 10.1016/0042-6822(91)90132-u. [DOI] [PubMed] [Google Scholar]
- Pintel D., Dadachanji D., Astell C. R., Ward D. C. The genome of minute virus of mice, an autonomous parvovirus, encodes two overlapping transcription units. Nucleic Acids Res. 1983 Feb 25;11(4):1019–1038. doi: 10.1093/nar/11.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed A. P., Jones E. V., Miller T. J. Nucleotide sequence and genome organization of canine parvovirus. J Virol. 1988 Jan;62(1):266–276. doi: 10.1128/jvi.62.1.266-276.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd, Paradiso P. R. Parvovirus genome: nucleotide sequence of H-1 and mapping of its genes by hybrid-arrested translation. J Virol. 1983 Jan;45(1):173–184. doi: 10.1128/jvi.45.1.173-184.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahli R., McMaster G. K., Hirt B. DNA sequence comparison between two tissue-specific variants of the autonomous parvovirus, minute virus of mice. Nucleic Acids Res. 1985 May 24;13(10):3617–3633. doi: 10.1093/nar/13.10.3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. L., Jacoby R. O., Johnson E. A., Paturzo F., Bhatt P. N. In vivo studies with an "orphan" parvovirus of mice. Lab Anim Sci. 1993 Apr;43(2):175–182. [PubMed] [Google Scholar]
- Smith A. L. Response of weanling random-bred mice to inoculation with minute virus of mice. Lab Anim Sci. 1983 Feb;33(1):37–39. [PubMed] [Google Scholar]
- Tattersall P., Bratton J. Reciprocal productive and restrictive virus-cell interactions of immunosuppressive and prototype strains of minute virus of mice. J Virol. 1983 Jun;46(3):944–955. doi: 10.1128/jvi.46.3.944-955.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersall P., Shatkin A. J., Ward D. C. Sequence homology between the structural polypeptides of minute virus of mice. J Mol Biol. 1977 Apr 25;111(4):375–394. doi: 10.1016/s0022-2836(77)80060-0. [DOI] [PubMed] [Google Scholar]
- Tsao J., Chapman M. S., Agbandje M., Keller W., Smith K., Wu H., Luo M., Smith T. J., Rossmann M. G., Compans R. W. The three-dimensional structure of canine parvovirus and its functional implications. Science. 1991 Mar 22;251(5000):1456–1464. doi: 10.1126/science.2006420. [DOI] [PubMed] [Google Scholar]
- Vasudevacharya J., Basak S., Srinivas R. V., Compans R. W. Nucleotide sequence analysis of the capsid genes and the right-hand terminal palindrome of porcine parvovirus, strain NADL-2. Virology. 1989 Dec;173(2):368–377. doi: 10.1016/0042-6822(89)90549-7. [DOI] [PubMed] [Google Scholar]