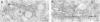Abstract
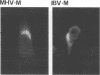
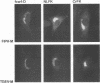
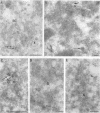
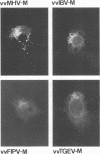
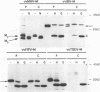
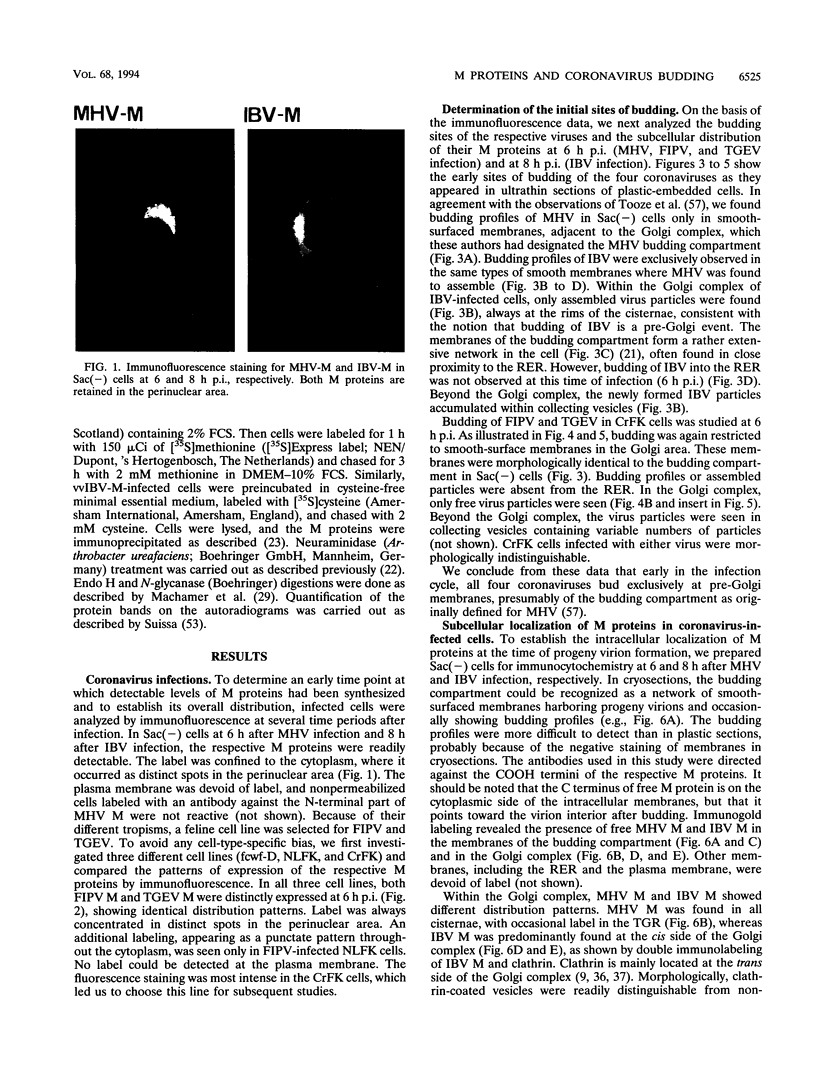
The prevailing hypothesis is that the intracellular site of budding of coronaviruses is determined by the localization of its membrane protein M (previously called E1). We tested this by analyzing the site of budding of four different coronaviruses in relation to the intracellular localization of their M proteins. Mouse hepatitis virus (MHV) and infectious bronchitis virus (IBV) grown in Sac(-) cells, and feline infectious peritonitis virus (FIPV) and transmissible gastroenteritis virus (TGEV) grown in CrFK cells, all budded exclusively into smooth-walled, tubulovesicular membranes located intermediately between the rough endoplasmic reticulum and Golgi complex, identical to the so-called budding compartment previously identified for MHV. Indirect immunofluorescence staining of the infected cells showed that all four M proteins accumulated in a perinuclear region. Immunogold microscopy localized MHV M and IBV M in the budding compartment; in addition, a dense labeling in the Golgi complex occurred, MHV M predominantly in trans-Golgi cisternae and trans-Golgi reticulum and IBV M mainly in the cis and medial Golgi cisternae. The corresponding M proteins of the four viruses, when independently expressed in a recombinant vaccinia virus system, also accumulated in the perinuclear area. Quantitative pulse-chase analysis of metabolically labeled cells showed that in each case the majority of the M glycoproteins carried oligosaccharide side chains with Golgi-specific modifications within 4 h after synthesis. Immunoelectron microscopy localized recombinant MHV M and IBV M to the same membranes as the respective proteins in coronavirus-infected cells, with the same cis-trans distribution over the Golgi complex. Our results demonstrate that some of the M proteins of the four viruses are transported beyond the budding compartment and are differentially retained by intrinsic retention signals; in addition to M, other viral and/or cellular factors are probably required to determine the site of budding.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J., Patel S., Riddle P. Lysosomal sorting mutants of coronavirus E1 protein, a Golgi membrane protein. J Cell Sci. 1990 Feb;95(Pt 2):191–197. doi: 10.1242/jcs.95.2.191. [DOI] [PubMed] [Google Scholar]
- Becker W. B., McIntosh K., Dees J. H., Chanock R. M. Morphogenesis of avian infectious bronchitis virus and a related human virus (strain 229E). J Virol. 1967 Oct;1(5):1019–1027. doi: 10.1128/jvi.1.5.1019-1027.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S., Brechling K., Moss B. Vaccinia virus expression vector: coexpression of beta-galactosidase provides visual screening of recombinant virus plaques. Mol Cell Biol. 1985 Dec;5(12):3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. S., Ariel N., Huang A. S. Membrane anchors of vesicular stomatitis virus: characterization and incorporation into virions. J Virol. 1988 Aug;62(8):2552–2556. doi: 10.1128/jvi.62.8.2552-2556.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVID-FERREIRA J. F., MANAKER R. A. AN ELECTRON MICROSCOPE STUDY OF THE DEVELOPMENT OF A MOUSE HEPATITIS VIRUS IN TISSUE CULTURE CELLS. J Cell Biol. 1965 Jan;24:57–78. doi: 10.1083/jcb.24.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Den Boon J. A., Snijder E. J., Locker J. K., Horzinek M. C., Rottier P. J. Another triple-spanning envelope protein among intracellularly budding RNA viruses: the torovirus E protein. Virology. 1991 Jun;182(2):655–663. doi: 10.1016/0042-6822(91)90606-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois-Dalcq M. E., Doller E. W., Haspel M. V., Holmes K. V. Cell tropism and expression of mouse hepatitis viruses (MHV) in mouse spinal cord cultures. Virology. 1982 Jun;119(2):317–331. doi: 10.1016/0042-6822(82)90092-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuze H. J., Slot J. W., Strous G. J., Hasilik A., von Figura K. Possible pathways for lysosomal enzyme delivery. J Cell Biol. 1985 Dec;101(6):2253–2262. doi: 10.1083/jcb.101.6.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G., Pfeiffer S., Simons K., Matlin K. Exit of newly synthesized membrane proteins from the trans cisterna of the Golgi complex to the plasma membrane. J Cell Biol. 1985 Sep;101(3):949–964. doi: 10.1083/jcb.101.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G., Rottier P. Cell biology of viruses that assemble along the biosynthetic pathway. Semin Cell Biol. 1992 Oct;3(5):367–381. doi: 10.1016/1043-4682(92)90022-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauri H. P., Schweizer A. The endoplasmic reticulum-Golgi intermediate compartment. Curr Opin Cell Biol. 1992 Aug;4(4):600–608. doi: 10.1016/0955-0674(92)90078-Q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille A., Klumperman J., Geuze H. J., Peters C., Brodsky F. M., von Figura K. Lysosomal acid phosphatase is internalized via clathrin-coated pits. Eur J Cell Biol. 1992 Oct;59(1):106–115. [PubMed] [Google Scholar]
- Holmes K. V., Doller E. W., Sturman L. S. Tunicamycin resistant glycosylation of coronavirus glycoprotein: demonstration of a novel type of viral glycoprotein. Virology. 1981 Dec;115(2):334–344. doi: 10.1016/0042-6822(81)90115-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu V. W., Yuan L. C., Nuchtern J. G., Lippincott-Schwartz J., Hammerling G. J., Klausner R. D. A recycling pathway between the endoplasmic reticulum and the Golgi apparatus for retention of unassembled MHC class I molecules. Nature. 1991 Aug 1;352(6334):441–444. doi: 10.1038/352441a0. [DOI] [PubMed] [Google Scholar]
- Jacobs L., de Groot R., van der Zeijst B. A., Horzinek M. C., Spaan W. The nucleotide sequence of the peplomer gene of porcine transmissible gastroenteritis virus (TGEV): comparison with the sequence of the peplomer protein of feline infectious peritonitis virus (FIPV). Virus Res. 1987 Nov;8(4):363–371. doi: 10.1016/0168-1702(87)90008-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobse-Geels H. E., Horzinek M. C. Expression of feline infectious peritonitis coronavirus antigens on the surface of feline macrophage-like cells. J Gen Virol. 1983 Sep;64(Pt 9):1859–1866. doi: 10.1099/0022-1317-64-9-1859. [DOI] [PubMed] [Google Scholar]
- Jamieson J. D., Palade G. E. Intracellular transport of secretory proteins in the pancreatic exocrine cell. I. Role of the peripheral elements of the Golgi complex. J Cell Biol. 1967 Aug;34(2):577–596. doi: 10.1083/jcb.34.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumperman J., Boekestijn J. C., Mulder A. M., Fransen J. A., Ginsel L. A. Intracellular localization and endocytosis of brush border enzymes in the enterocyte-like cell line Caco-2. Eur J Cell Biol. 1991 Feb;54(1):76–84. [PubMed] [Google Scholar]
- Klumperman J., Hille A., Veenendaal T., Oorschot V., Stoorvogel W., von Figura K., Geuze H. J. Differences in the endosomal distributions of the two mannose 6-phosphate receptors. J Cell Biol. 1993 Jun;121(5):997–1010. doi: 10.1083/jcb.121.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Krijnse-Locker J., Ericsson M., Rottier P. J., Griffiths G. Characterization of the budding compartment of mouse hepatitis virus: evidence that transport from the RER to the Golgi complex requires only one vesicular transport step. J Cell Biol. 1994 Jan;124(1-2):55–70. doi: 10.1083/jcb.124.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laude H., Rasschaert D., Huet J. C. Sequence and N-terminal processing of the transmembrane protein E1 of the coronavirus transmissible gastroenteritis virus. J Gen Virol. 1987 Jun;68(Pt 6):1687–1693. doi: 10.1099/0022-1317-68-6-1687. [DOI] [PubMed] [Google Scholar]
- Laviada M. D., Videgain S. P., Moreno L., Alonso F., Enjuanes L., Escribano J. M. Expression of swine transmissible gastroenteritis virus envelope antigens on the surface of infected cells: epitopes externally exposed. Virus Res. 1990 Jul;16(3):247–254. doi: 10.1016/0168-1702(90)90051-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J. Bidirectional membrane traffic between the endoplasmic reticulum and Golgi apparatus. Trends Cell Biol. 1993 Mar;3(3):81–88. doi: 10.1016/0962-8924(93)90078-f. [DOI] [PubMed] [Google Scholar]
- Locker J. K., Griffiths G., Horzinek M. C., Rottier P. J. O-glycosylation of the coronavirus M protein. Differential localization of sialyltransferases in N- and O-linked glycosylation. J Biol Chem. 1992 Jul 15;267(20):14094–14101. doi: 10.1016/S0021-9258(19)49683-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locker J. K., Rose J. K., Horzinek M. C., Rottier P. J. Membrane assembly of the triple-spanning coronavirus M protein. Individual transmembrane domains show preferred orientation. J Biol Chem. 1992 Oct 25;267(30):21911–21918. doi: 10.1016/S0021-9258(19)36699-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotti L. V., Torrisi M. R., Pascale M. C., Bonatti S. Immunocytochemical analysis of the transfer of vesicular stomatitis virus G glycoprotein from the intermediate compartment to the Golgi complex. J Cell Biol. 1992 Jul;118(1):43–50. doi: 10.1083/jcb.118.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machamer C. E., Grim M. G., Esquela A., Chung S. W., Rolls M., Ryan K., Swift A. M. Retention of a cis Golgi protein requires polar residues on one face of a predicted alpha-helix in the transmembrane domain. Mol Biol Cell. 1993 Jul;4(7):695–704. doi: 10.1091/mbc.4.7.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machamer C. E., Rose J. K. A specific transmembrane domain of a coronavirus E1 glycoprotein is required for its retention in the Golgi region. J Cell Biol. 1987 Sep;105(3):1205–1214. doi: 10.1083/jcb.105.3.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metsikkö K., Simons K. The budding mechanism of spikeless vesicular stomatitis virus particles. EMBO J. 1986 Aug;5(8):1913–1920. doi: 10.1002/j.1460-2075.1986.tb04444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemann H., Geyer R., Klenk H. D., Linder D., Stirm S., Wirth M. The carbohydrates of mouse hepatitis virus (MHV) A59: structures of the O-glycosidically linked oligosaccharides of glycoprotein E1. EMBO J. 1984 Mar;3(3):665–670. doi: 10.1002/j.1460-2075.1984.tb01864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemann H., Mayer T., Tamura T. Signals for membrane-associated transport in eukaryotic cells. Subcell Biochem. 1989;15:307–365. doi: 10.1007/978-1-4899-1675-4_10. [DOI] [PubMed] [Google Scholar]
- Niemann H., Mayer T., Wirth M., Tamura T. Expression of the E1 gene of mouse hepatitis virus (MHV A59) in vivo and in vitro. Adv Exp Med Biol. 1987;218:83–97. doi: 10.1007/978-1-4684-1280-2_10. [DOI] [PubMed] [Google Scholar]
- Oprins A., Duden R., Kreis T. E., Geuze H. J., Slot J. W. Beta-COP localizes mainly to the cis-Golgi side in exocrine pancreas. J Cell Biol. 1993 Apr;121(1):49–59. doi: 10.1083/jcb.121.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L., Ravazzola M., Amherdt M., Louvard D., Perrelet A. Clathrin-immunoreactive sites in the Golgi apparatus are concentrated at the trans pole in polypeptide hormone-secreting cells. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5385–5389. doi: 10.1073/pnas.82.16.5385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. Recycling of proteins between the endoplasmic reticulum and Golgi complex. Curr Opin Cell Biol. 1991 Aug;3(4):585–591. doi: 10.1016/0955-0674(91)90027-v. [DOI] [PubMed] [Google Scholar]
- Pulford D. J., Britton P. Expression and cellular localisation of porcine transmissible gastroenteritis virus N and M proteins by recombinant vaccinia viruses. Virus Res. 1991 Mar;18(2-3):203–217. doi: 10.1016/0168-1702(91)90019-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottier P. J., Horzinek M. C., van der Zeijst B. A. Viral protein synthesis in mouse hepatitis virus strain A59-infected cells: effect of tunicamycin. J Virol. 1981 Nov;40(2):350–357. doi: 10.1128/jvi.40.2.350-357.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottier P. J., Rose J. K. Coronavirus E1 glycoprotein expressed from cloned cDNA localizes in the Golgi region. J Virol. 1987 Jun;61(6):2042–2045. doi: 10.1128/jvi.61.6.2042-2045.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraste J., Palade G. E., Farquhar M. G. Antibodies to rat pancreas Golgi subfractions: identification of a 58-kD cis-Golgi protein. J Cell Biol. 1987 Nov;105(5):2021–2029. doi: 10.1083/jcb.105.5.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer A., Fransen J. A., Bächi T., Ginsel L., Hauri H. P. Identification, by a monoclonal antibody, of a 53-kD protein associated with a tubulo-vesicular compartment at the cis-side of the Golgi apparatus. J Cell Biol. 1988 Nov;107(5):1643–1653. doi: 10.1083/jcb.107.5.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer A., Fransen J. A., Matter K., Kreis T. E., Ginsel L., Hauri H. P. Identification of an intermediate compartment involved in protein transport from endoplasmic reticulum to Golgi apparatus. Eur J Cell Biol. 1990 Dec;53(2):185–196. [PubMed] [Google Scholar]
- Slot J. W., Geuze H. J., Gigengack S., Lienhard G. E., James D. E. Immuno-localization of the insulin regulatable glucose transporter in brown adipose tissue of the rat. J Cell Biol. 1991 Apr;113(1):123–135. doi: 10.1083/jcb.113.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaan W. J., Rottier P. J., Horzinek M. C., van der Zeijst B. A. Isolation and identification of virus-specific mRNAs in cells infected with mouse hepatitis virus (MHV-A59). Virology. 1981 Jan 30;108(2):424–434. doi: 10.1016/0042-6822(81)90449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaan W., Cavanagh D., Horzinek M. C. Coronaviruses: structure and genome expression. J Gen Virol. 1988 Dec;69(Pt 12):2939–2952. doi: 10.1099/0022-1317-69-12-2939. [DOI] [PubMed] [Google Scholar]
- Stern D. F., Sefton B. M. Coronavirus proteins: biogenesis of avian infectious bronchitis virus virion proteins. J Virol. 1982 Dec;44(3):794–803. doi: 10.1128/jvi.44.3.794-803.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suissa M. Spectrophotometric quantitation of silver grains eluted from autoradiograms. Anal Biochem. 1983 Sep;133(2):511–514. doi: 10.1016/0003-2697(83)90117-3. [DOI] [PubMed] [Google Scholar]
- Swift A. M., Machamer C. E. A Golgi retention signal in a membrane-spanning domain of coronavirus E1 protein. J Cell Biol. 1991 Oct;115(1):19–30. doi: 10.1083/jcb.115.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To L. T., Bernard S., Lantier I. Fixed-cell immunoperoxidase technique for the study of surface antigens induced by the coronavirus of transmissible gastroenteritis (TGEV). Vet Microbiol. 1991 Nov;29(3-4):361–368. doi: 10.1016/0378-1135(91)90143-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze J., Tooze S. A. Infection of AtT20 murine pituitary tumour cells by mouse hepatitis virus strain A59: virus budding is restricted to the Golgi region. Eur J Cell Biol. 1985 May;37:203–212. [PubMed] [Google Scholar]
- Tooze J., Tooze S., Warren G. Replication of coronavirus MHV-A59 in sac- cells: determination of the first site of budding of progeny virions. Eur J Cell Biol. 1984 Mar;33(2):281–293. [PubMed] [Google Scholar]
- Tooze S. A., Tooze J., Warren G. Site of addition of N-acetyl-galactosamine to the E1 glycoprotein of mouse hepatitis virus-A59. J Cell Biol. 1988 May;106(5):1475–1487. doi: 10.1083/jcb.106.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennema H., Rijnbrand R., Heijnen L., Horzinek M. C., Spaan W. J. Enhancement of the vaccinia virus/phage T7 RNA polymerase expression system using encephalomyocarditis virus 5'-untranslated region sequences. Gene. 1991 Dec 15;108(2):201–209. doi: 10.1016/0378-1119(91)90435-E. [DOI] [PMC free article] [PubMed] [Google Scholar]