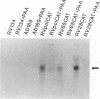
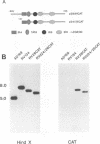
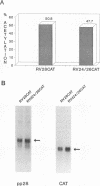
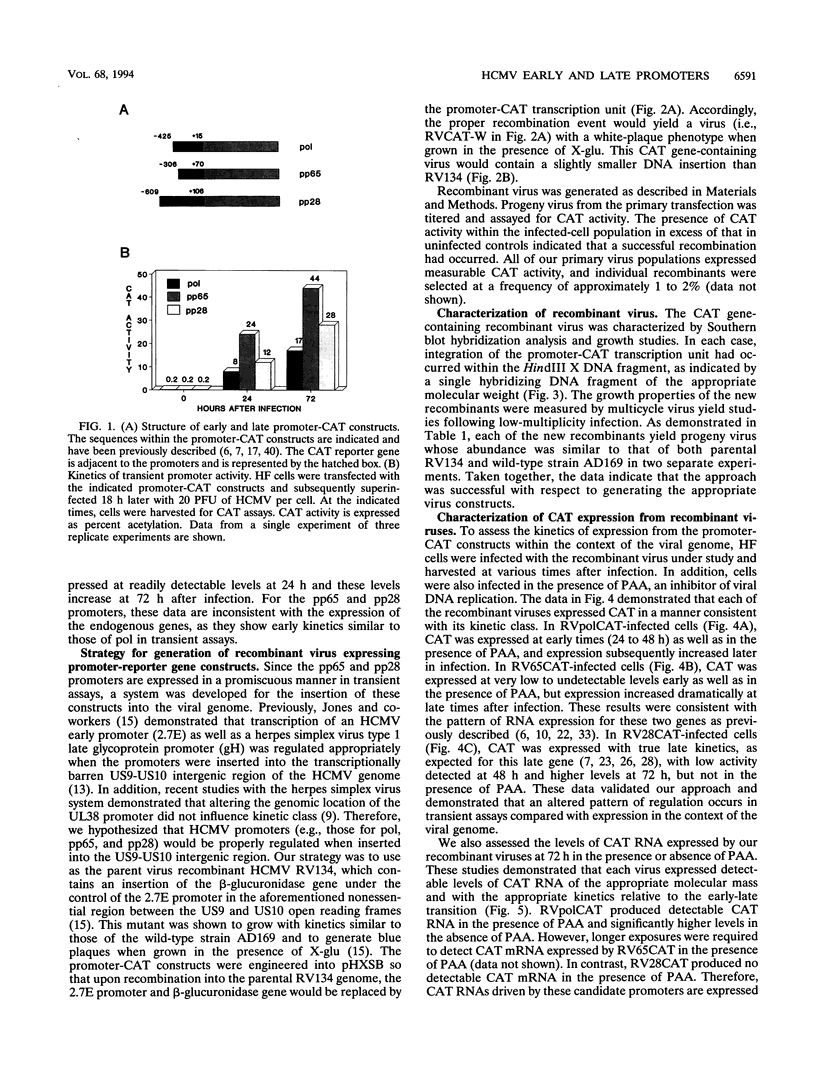
Abstract
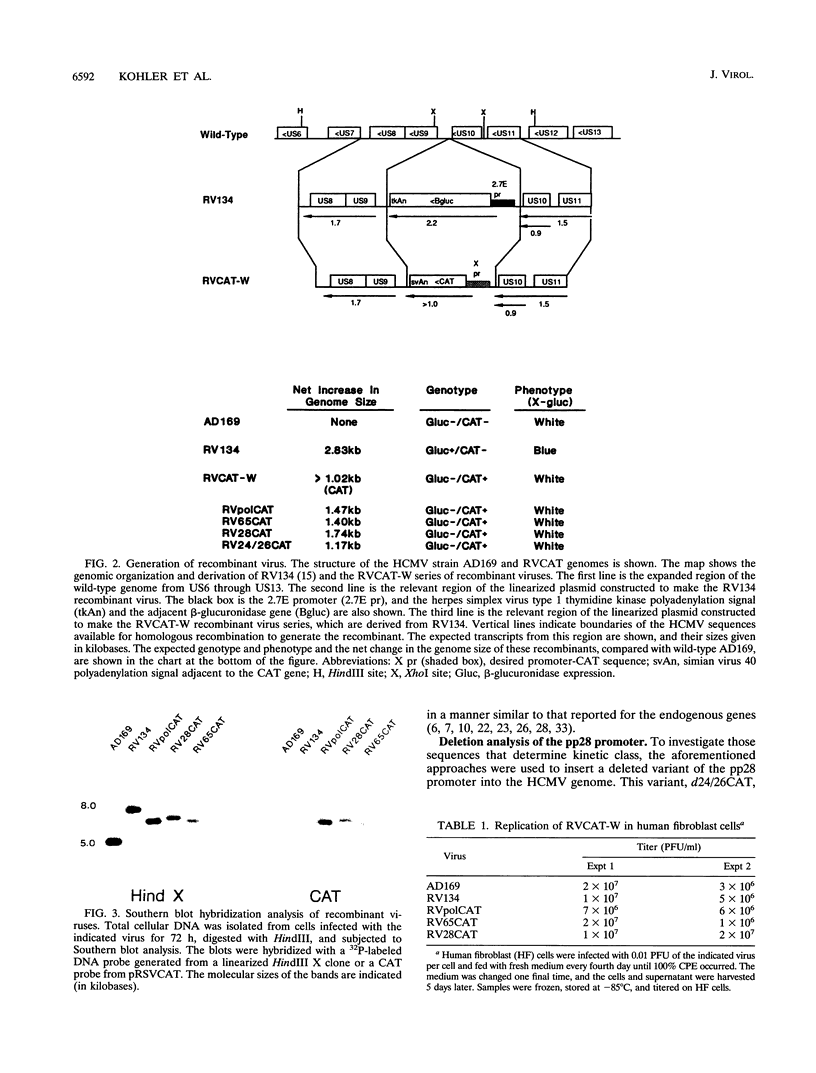
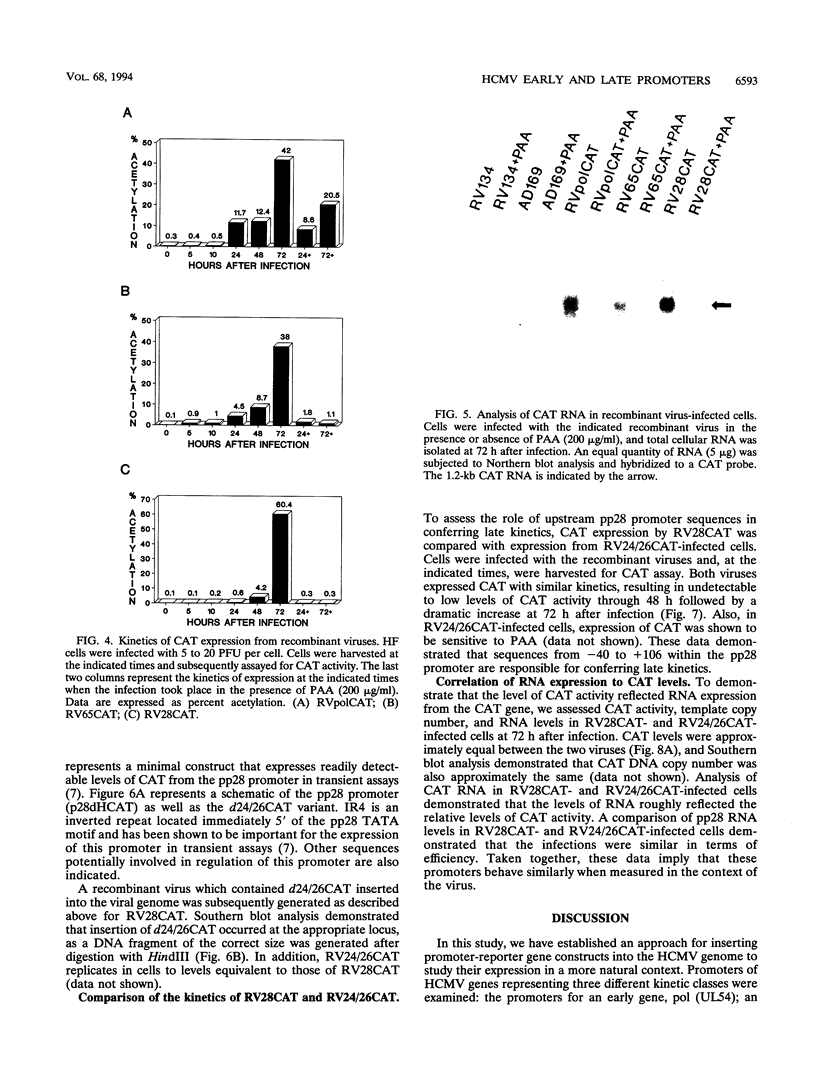
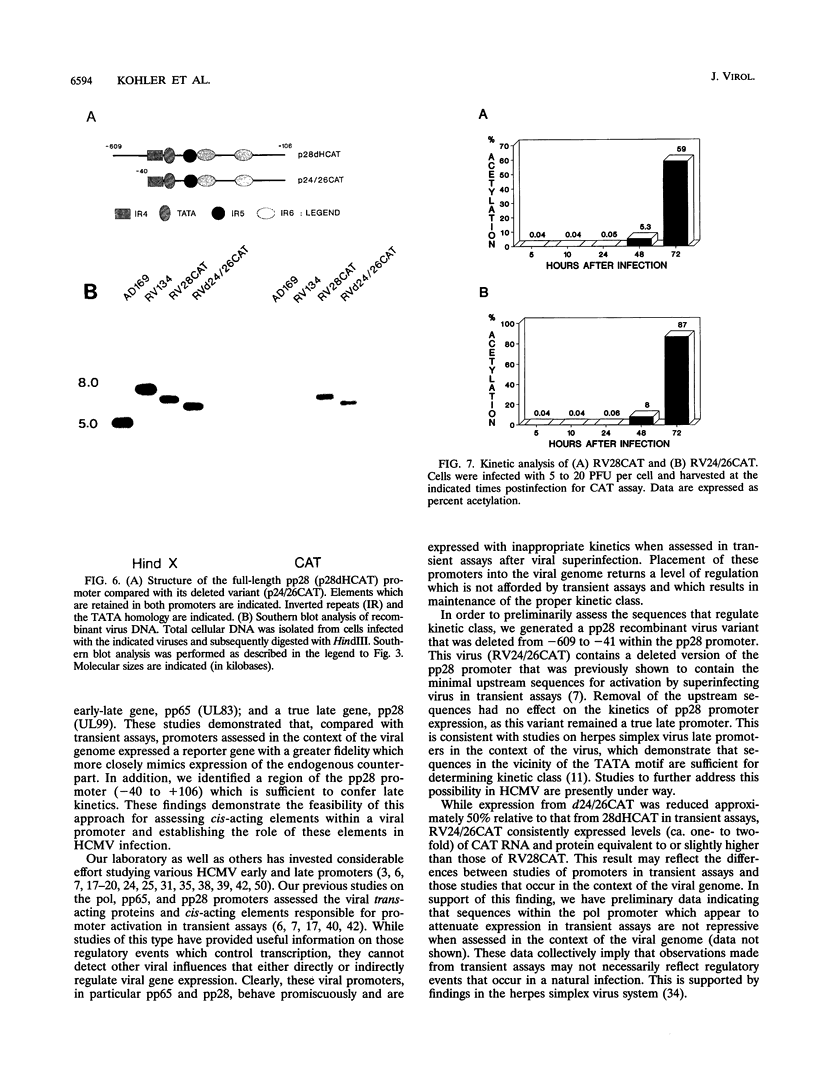
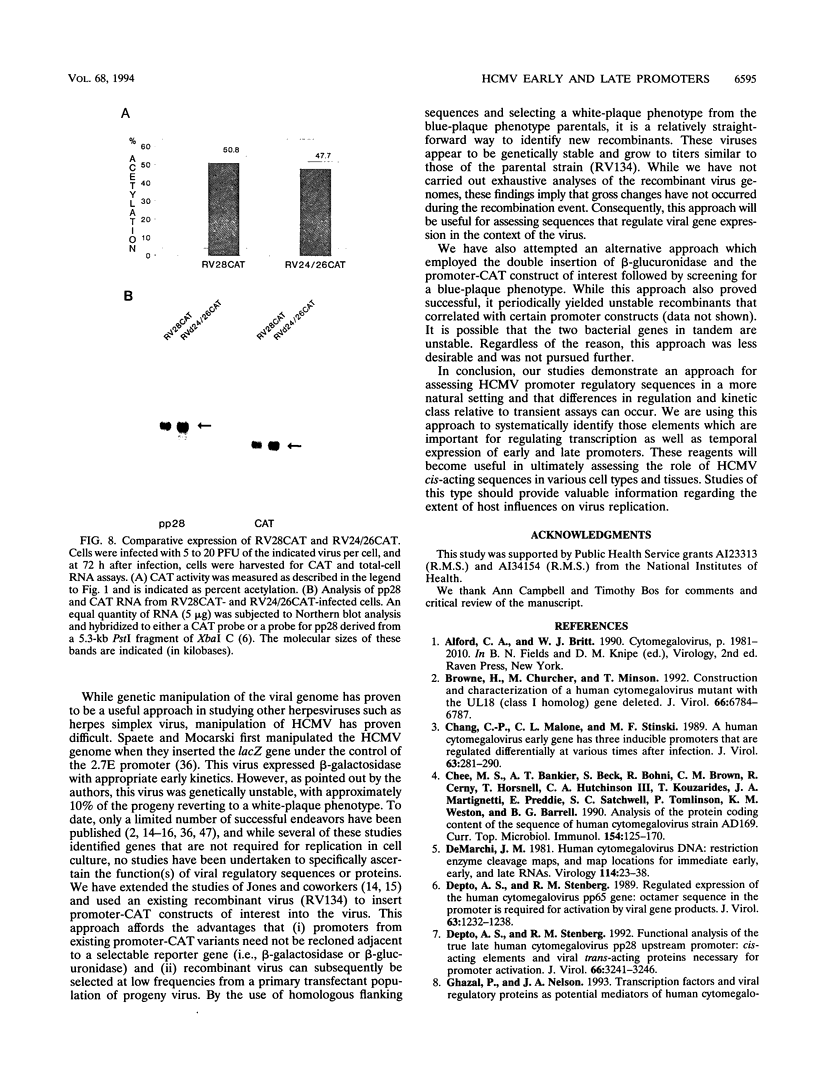
We have developed a system to study human cytomegalovirus (HCMV) cis-acting promoter elements within the context of the viral genome. A recombinant HCMV (RV134) containing a marker gene (beta-glucuronidase) was used to insert HCMV promoter-chloramphenicol acetyltransferase gene constructs into the viral genome between open reading frames US9 and US10. Using this system, we have studied the promoters for the early DNA polymerase gene (UL54), the early-late lower matrix phosphoprotein gene (pp65, UL83), and the true late 28-kDa structural phosphoprotein gene (pp28, UL99). Transient-expression assays demonstrated that the pp65 and pp28 promoters are activated earlier and to higher levels than typically observed with the endogenous gene. In contrast, insertion of these promoters into the viral genome resulted in kinetics which mimicked that of the endogenous genes. In addition, we have also tested a variant of the pp28 promoter (d24/26CAT) which is deleted from -609 to -41. This promoter behaved similarly to the wild-type pp28 promoter, indicating that sequences from -40 to +106 are sufficient for conferring true late kinetics. Taken together, these data demonstrate that the viral genome affords a level of regulation on HCMV gene expression that has been previously unrealized. Therefore, these experiments provide a model system for the analysis of cis-acting promoter regulatory elements in the context of the viral genome.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Browne H., Churcher M., Minson T. Construction and characterization of a human cytomegalovirus mutant with the UL18 (class I homolog) gene deleted. J Virol. 1992 Nov;66(11):6784–6787. doi: 10.1128/jvi.66.11.6784-6787.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. P., Malone C. L., Stinski M. F. A human cytomegalovirus early gene has three inducible promoters that are regulated differentially at various times after infection. J Virol. 1989 Jan;63(1):281–290. doi: 10.1128/jvi.63.1.281-290.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee M. S., Bankier A. T., Beck S., Bohni R., Brown C. M., Cerny R., Horsnell T., Hutchison C. A., 3rd, Kouzarides T., Martignetti J. A. Analysis of the protein-coding content of the sequence of human cytomegalovirus strain AD169. Curr Top Microbiol Immunol. 1990;154:125–169. doi: 10.1007/978-3-642-74980-3_6. [DOI] [PubMed] [Google Scholar]
- Demarchi J. M. Human cytomegalovirus DNA: restriction enzyme cleavage maps and map locations for immediate-early, early, and late RNAs. Virology. 1981 Oct 15;114(1):23–38. doi: 10.1016/0042-6822(81)90249-x. [DOI] [PubMed] [Google Scholar]
- Depto A. S., Stenberg R. M. Functional analysis of the true late human cytomegalovirus pp28 upstream promoter: cis-acting elements and viral trans-acting proteins necessary for promoter activation. J Virol. 1992 May;66(5):3241–3246. doi: 10.1128/jvi.66.5.3241-3246.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depto A. S., Stenberg R. M. Regulated expression of the human cytomegalovirus pp65 gene: octamer sequence in the promoter is required for activation by viral gene products. J Virol. 1989 Mar;63(3):1232–1238. doi: 10.1128/jvi.63.3.1232-1238.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodart S. A., Guzowski J. F., Rice M. K., Wagner E. K. Effect of genomic location on expression of beta-galactosidase mRNA controlled by the herpes simplex virus type 1 UL38 promoter. J Virol. 1992 May;66(5):2973–2981. doi: 10.1128/jvi.66.5.2973-2981.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronn R., Jahn G., Bürkle A., Freese U. K., Fleckenstein B., zur Hausen H. Genomic localization, sequence analysis, and transcription of the putative human cytomegalovirus DNA polymerase gene. J Virol. 1987 Jan;61(1):119–124. doi: 10.1128/jvi.61.1.119-124.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homa F. L., Otal T. M., Glorioso J. C., Levine M. Transcriptional control signals of a herpes simplex virus type 1 late (gamma 2) gene lie within bases -34 to +124 relative to the 5' terminus of the mRNA. Mol Cell Biol. 1986 Nov;6(11):3652–3666. doi: 10.1128/mcb.6.11.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn G., Knust E., Schmolla H., Sarre T., Nelson J. A., McDougall J. K., Fleckenstein B. Predominant immediate-early transcripts of human cytomegalovirus AD 169. J Virol. 1984 Feb;49(2):363–370. doi: 10.1128/jvi.49.2.363-370.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. R., Muzithras V. P. A cluster of dispensable genes within the human cytomegalovirus genome short component: IRS1, US1 through US5, and the US6 family. J Virol. 1992 Apr;66(4):2541–2546. doi: 10.1128/jvi.66.4.2541-2546.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. R., Muzithras V. P. Fine mapping of transcripts expressed from the US6 gene family of human cytomegalovirus strain AD169. J Virol. 1991 Apr;65(4):2024–2036. doi: 10.1128/jvi.65.4.2024-2036.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. R., Muzithras V. P., Gluzman Y. Replacement mutagenesis of the human cytomegalovirus genome: US10 and US11 gene products are nonessential. J Virol. 1991 Nov;65(11):5860–5872. doi: 10.1128/jvi.65.11.5860-5872.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye J., Browne H., Stoffel M., Minson T. The UL16 gene of human cytomegalovirus encodes a glycoprotein that is dispensable for growth in vitro. J Virol. 1992 Nov;66(11):6609–6615. doi: 10.1128/jvi.66.11.6609-6615.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerry J. A., Priddy M. A., Stenberg R. M. Identification of sequence elements in the human cytomegalovirus DNA polymerase gene promoter required for activation by viral gene products. J Virol. 1994 Jul;68(7):4167–4176. doi: 10.1128/jvi.68.7.4167-4176.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klucher K. M., Rabert D. K., Spector D. H. Sequences in the human cytomegalovirus 2.7-kilobase RNA promoter which mediate its regulation as an early gene. J Virol. 1989 Dec;63(12):5334–5343. doi: 10.1128/jvi.63.12.5334-5343.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klucher K. M., Sommer M., Kadonaga J. T., Spector D. H. In vivo and in vitro analysis of transcriptional activation mediated by the human cytomegalovirus major immediate-early proteins. Mol Cell Biol. 1993 Feb;13(2):1238–1250. doi: 10.1128/mcb.13.2.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klucher K. M., Spector D. H. The human cytomegalovirus 2.7-kilobase RNA promoter contains a functional binding site for the adenovirus major late transcription factor. J Virol. 1990 Sep;64(9):4189–4198. doi: 10.1128/jvi.64.9.4189-4198.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouzarides T., Bankier A. T., Satchwell S. C., Preddy E., Barrell B. G. An immediate early gene of human cytomegalovirus encodes a potential membrane glycoprotein. Virology. 1988 Jul;165(1):151–164. doi: 10.1016/0042-6822(88)90668-x. [DOI] [PubMed] [Google Scholar]
- Kouzarides T., Bankier A. T., Satchwell S. C., Weston K., Tomlinson P., Barrell B. G. Sequence and transcription analysis of the human cytomegalovirus DNA polymerase gene. J Virol. 1987 Jan;61(1):125–133. doi: 10.1128/jvi.61.1.125-133.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahijani R. S., Otteson E. W., Adlish J. D., St Jeor S. C. Characterization of a human cytomegalovirus 1.6-kilobase late mRNA and identification of its putative protein product. J Virol. 1991 Jan;65(1):373–381. doi: 10.1128/jvi.65.1.373-381.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leach F. S., Mocarski E. S. Regulation of cytomegalovirus late-gene expression: differential use of three start sites in the transcriptional activation of ICP36 gene expression. J Virol. 1989 Apr;63(4):1783–1791. doi: 10.1128/jvi.63.4.1783-1791.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone C. L., Vesole D. H., Stinski M. F. Transactivation of a human cytomegalovirus early promoter by gene products from the immediate-early gene IE2 and augmentation by IE1: mutational analysis of the viral proteins. J Virol. 1990 Apr;64(4):1498–1506. doi: 10.1128/jvi.64.4.1498-1506.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J., Lahijani R. S., St Jeor S. C. Analysis of a region of the human cytomegalovirus (AD169) genome coding for a 25-kilodalton virion protein. J Virol. 1989 Jan;63(1):233–241. doi: 10.1128/jvi.63.1.233-241.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough S. H., Spector D. H. Transcription in human fibroblasts permissively infected by human cytomegalovirus strain AD169. Virology. 1983 Feb;125(1):31–46. doi: 10.1016/0042-6822(83)90061-2. [DOI] [PubMed] [Google Scholar]
- Meyer H., Bankier A. T., Landini M. P., Brown C. M., Barrell B. G., Rüger B., Mach M. Identification and procaryotic expression of the gene coding for the highly immunogenic 28-kilodalton structural phosphoprotein (pp28) of human cytomegalovirus. J Virol. 1988 Jul;62(7):2243–2250. doi: 10.1128/jvi.62.7.2243-2250.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nankervis G. A., Kumar M. L. Diseases produced by cytomegaloviruses. Med Clin North Am. 1978 Sep;62(5):1021–1035. doi: 10.1016/s0025-7125(16)31752-7. [DOI] [PubMed] [Google Scholar]
- Nelson J. A., Ghazal P., Wiley C. A. Role of opportunistic viral infections in AIDS. AIDS. 1990 Jan;4(1):1–10. doi: 10.1097/00002030-199001000-00001. [DOI] [PubMed] [Google Scholar]
- Puchtler E., Stamminger T. An inducible promoter mediates abundant expression from the immediate-early 2 gene region of human cytomegalovirus at late times after infection. J Virol. 1991 Nov;65(11):6301–6306. doi: 10.1128/jvi.65.11.6301-6306.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüger B., Klages S., Walla B., Albrecht J., Fleckenstein B., Tomlinson P., Barrell B. Primary structure and transcription of the genes coding for the two virion phosphoproteins pp65 and pp71 of human cytomegalovirus. J Virol. 1987 Feb;61(2):446–453. doi: 10.1128/jvi.61.2.446-453.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver S., Roizman B. gamma 2-Thymidine kinase chimeras are identically transcribed but regulated a gamma 2 genes in herpes simplex virus genomes and as beta genes in cell genomes. Mol Cell Biol. 1985 Mar;5(3):518–528. doi: 10.1128/mcb.5.3.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaete R. R., Mocarski E. S. Insertion and deletion mutagenesis of the human cytomegalovirus genome. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7213–7217. doi: 10.1073/pnas.84.20.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaete R. R., Mocarski E. S. Regulation of cytomegalovirus gene expression: alpha and beta promoters are trans activated by viral functions in permissive human fibroblasts. J Virol. 1985 Oct;56(1):135–143. doi: 10.1128/jvi.56.1.135-143.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector D. H., Klucher K. M., Rabert D. K., Wright D. A. Human cytomegalovirus early gene expression. Curr Top Microbiol Immunol. 1990;154:21–45. doi: 10.1007/978-3-642-74980-3_2. [DOI] [PubMed] [Google Scholar]
- Staprans S. I., Rabert D. K., Spector D. H. Identification of sequence requirements and trans-acting functions necessary for regulated expression of a human cytomegalovirus early gene. J Virol. 1988 Sep;62(9):3463–3473. doi: 10.1128/jvi.62.9.3463-3473.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasiak P. C., Mocarski E. S. Transactivation of the cytomegalovirus ICP36 gene promoter requires the alpha gene product TRS1 in addition to IE1 and IE2. J Virol. 1992 Feb;66(2):1050–1058. doi: 10.1128/jvi.66.2.1050-1058.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenberg R. M., Depto A. S., Fortney J., Nelson J. A. Regulated expression of early and late RNAs and proteins from the human cytomegalovirus immediate-early gene region. J Virol. 1989 Jun;63(6):2699–2708. doi: 10.1128/jvi.63.6.2699-2708.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenberg R. M., Fortney J., Barlow S. W., Magrane B. P., Nelson J. A., Ghazal P. Promoter-specific trans activation and repression by human cytomegalovirus immediate-early proteins involves common and unique protein domains. J Virol. 1990 Apr;64(4):1556–1565. doi: 10.1128/jvi.64.4.1556-1565.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenberg R. M., Stinski M. F. Autoregulation of the human cytomegalovirus major immediate-early gene. J Virol. 1985 Dec;56(3):676–682. doi: 10.1128/jvi.56.3.676-682.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenberg R. M., Thomsen D. R., Stinski M. F. Structural analysis of the major immediate early gene of human cytomegalovirus. J Virol. 1984 Jan;49(1):190–199. doi: 10.1128/jvi.49.1.190-199.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenberg R. M., Witte P. R., Stinski M. F. Multiple spliced and unspliced transcripts from human cytomegalovirus immediate-early region 2 and evidence for a common initiation site within immediate-early region 1. J Virol. 1985 Dec;56(3):665–675. doi: 10.1128/jvi.56.3.665-675.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinski M. F., Thomsen D. R., Stenberg R. M., Goldstein L. C. Organization and expression of the immediate early genes of human cytomegalovirus. J Virol. 1983 Apr;46(1):1–14. doi: 10.1128/jvi.46.1.1-14.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takekoshi M., Maeda-Takekoshi F., Ihara S., Sakuma S., Watanabe Y. Site-specific stable insertion into the human cytomegalovirus genome of a foreign gene under control of the SV40 promoter. Gene. 1991 May 30;101(2):209–213. doi: 10.1016/0378-1119(91)90413-6. [DOI] [PubMed] [Google Scholar]
- Tenney D. J., Colberg-Poley A. M. Expression of the human cytomegalovirus UL36-38 immediate early region during permissive infection. Virology. 1991 May;182(1):199–210. doi: 10.1016/0042-6822(91)90663-v. [DOI] [PubMed] [Google Scholar]
- Tenney D. J., Colberg-Poley A. M. Human cytomegalovirus UL36-38 and US3 immediate-early genes: temporally regulated expression of nuclear, cytoplasmic, and polysome-associated transcripts during infection. J Virol. 1991 Dec;65(12):6724–6734. doi: 10.1128/jvi.65.12.6724-6734.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade E. J., Klucher K. M., Spector D. H. An AP-1 binding site is the predominant cis-acting regulatory element in the 1.2-kilobase early RNA promoter of human cytomegalovirus. J Virol. 1992 Apr;66(4):2407–2417. doi: 10.1128/jvi.66.4.2407-2417.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wathen M. W., Stinski M. F. Temporal patterns of human cytomegalovirus transcription: mapping the viral RNAs synthesized at immediate early, early, and late times after infection. J Virol. 1982 Feb;41(2):462–477. doi: 10.1128/jvi.41.2.462-477.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston K. An enhancer element in the short unique region of human cytomegalovirus regulates the production of a group of abundant immediate early transcripts. Virology. 1988 Feb;162(2):406–416. doi: 10.1016/0042-6822(88)90481-3. [DOI] [PubMed] [Google Scholar]
- Wiley C. A., Nelson J. A. Role of human immunodeficiency virus and cytomegalovirus in AIDS encephalitis. Am J Pathol. 1988 Oct;133(1):73–81. [PMC free article] [PubMed] [Google Scholar]
- Wilkinson G. W., Akrigg A., Greenaway P. J. Transcription of the immediate early genes of human cytomegalovirus strain AD169. Virus Res. 1984;1(2):101–106. doi: 10.1016/0168-1702(84)90067-4. [DOI] [PubMed] [Google Scholar]