Abstract
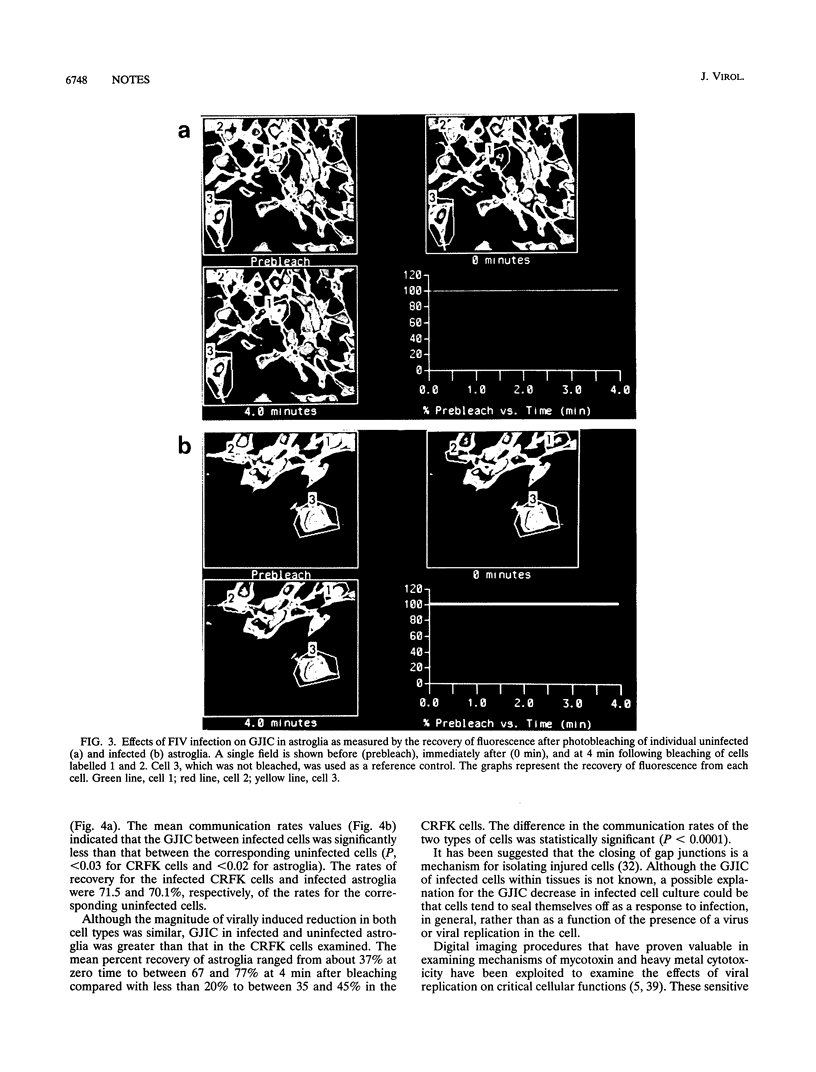
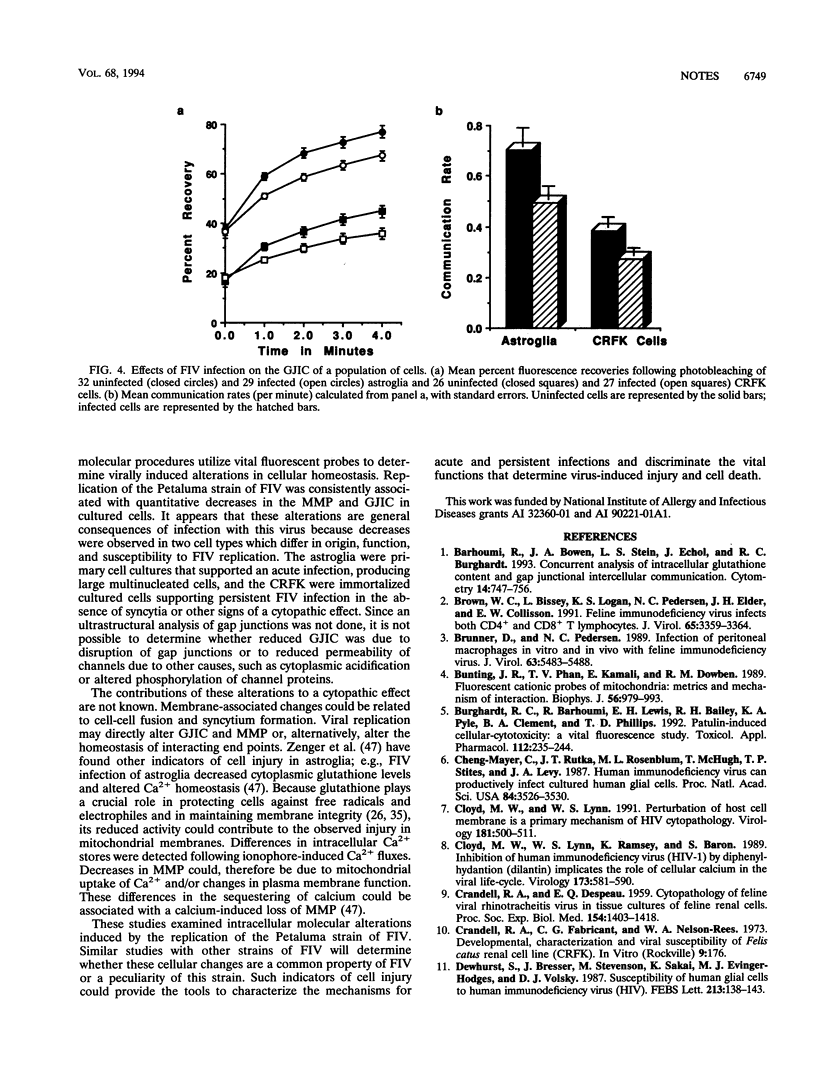
The in vitro effects of viral replication on mitochondrial membrane potential (MMP) and gap junctional intercellular communication (GJIC) were evaluated as two parameters of potential cellular injury. Two distinct cell types were infected with the Petaluma strain of feline immunodeficiency virus (FIV). Primary astroglia supported acute FIV infection, resulting in syncytia within 3 days of infection, whereas immortalized Crandell feline kidney (CRFK) cells of epithelial origin supported persistent FIV infection in the absence of an obvious cytopathic effect. An examination of cells under conditions that included an infection rate of more than 90% for either population revealed that the astroglia produced about four times more virus than the CRFK cells. The mitochondrial uptake of the cationic fluorescent dye rhodamine 123 in infected astroglia was less than 45% of that of normal control cells, whereas the MMP of the CRFK cells, which produced about one-fourth as much virus, was 80.8% of that of the normal cells. Cell-cell communication between adjacent cells was determined by the recovery of fluorescence following photobleaching of a single cell. In spite of the lower level of innate cell-cell communication among cultured CRFK cells than among astroglia, viral replication resulted in a 30% decrease in the GJIC of both astroglia and CRFK cells. These studies indicate that cell injury, as defined by an inhibition of MMP and GJIC, can occur as a result of persistent and acute infection with the Petaluma strain of FIV.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barhoumi R., Bowen J. A., Stein L. S., Echols J., Burghardt R. C. Concurrent analysis of intracellular glutathione content and gap junctional intercellular communication. Cytometry. 1993 Oct;14(7):747–756. doi: 10.1002/cyto.990140707. [DOI] [PubMed] [Google Scholar]
- Brown W. C., Bissey L., Logan K. S., Pedersen N. C., Elder J. H., Collisson E. W. Feline immunodeficiency virus infects both CD4+ and CD8+ T lymphocytes. J Virol. 1991 Jun;65(6):3359–3364. doi: 10.1128/jvi.65.6.3359-3364.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner D., Pedersen N. C. Infection of peritoneal macrophages in vitro and in vivo with feline immunodeficiency virus. J Virol. 1989 Dec;63(12):5483–5488. doi: 10.1128/jvi.63.12.5483-5488.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunting J. R., Phan T. V., Kamali E., Dowben R. M. Fluorescent cationic probes of mitochondria. Metrics and mechanism of interaction. Biophys J. 1989 Nov;56(5):979–993. doi: 10.1016/S0006-3495(89)82743-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghardt R. C., Barhoumi R., Lewis E. H., Bailey R. H., Pyle K. A., Clement B. A., Phillips T. D. Patulin-induced cellular toxicity: a vital fluorescence study. Toxicol Appl Pharmacol. 1992 Feb;112(2):235–244. doi: 10.1016/0041-008x(92)90193-v. [DOI] [PubMed] [Google Scholar]
- Cheng-Mayer C., Rutka J. T., Rosenblum M. L., McHugh T., Stites D. P., Levy J. A. Human immunodeficiency virus can productively infect cultured human glial cells. Proc Natl Acad Sci U S A. 1987 May;84(10):3526–3530. doi: 10.1073/pnas.84.10.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloyd M. W., Lynn W. S. Perturbation of host-cell membrane is a primary mechanism of HIV cytopathology. Virology. 1991 Apr;181(2):500–511. doi: 10.1016/0042-6822(91)90882-c. [DOI] [PubMed] [Google Scholar]
- Cloyd M. W., Lynn W. S., Ramsey K., Baron S. Inhibition of human immunodeficiency virus (HIV-1) infection by diphenylhydantoin (dilantin) implicates role of cellular calcium in virus life cycle. Virology. 1989 Dec;173(2):581–590. doi: 10.1016/0042-6822(89)90569-2. [DOI] [PubMed] [Google Scholar]
- Crandell R. A., Fabricant C. G., Nelson-Rees W. A. Development, characterization, and viral susceptibility of a feline (Felis catus) renal cell line (CRFK). In Vitro. 1973 Nov-Dec;9(3):176–185. doi: 10.1007/BF02618435. [DOI] [PubMed] [Google Scholar]
- Dewhurst S., Bresser J., Stevenson M., Sakai K., Evinger-Hodges M. J., Volsky D. J. Susceptibility of human glial cells to infection with human immunodeficiency virus (HIV). FEBS Lett. 1987 Mar 9;213(1):138–143. doi: 10.1016/0014-5793(87)81479-5. [DOI] [PubMed] [Google Scholar]
- Dickson D. W., Mattiace L. A., Kure K., Hutchins K., Lyman W. D., Brosnan C. F. Microglia in human disease, with an emphasis on acquired immune deficiency syndrome. Lab Invest. 1991 Feb;64(2):135–156. [PubMed] [Google Scholar]
- Dow S. W., Dreitz M. J., Hoover E. A. Feline immunodeficiency virus neurotropism: evidence that astrocytes and microglia are the primary target cells. Vet Immunol Immunopathol. 1992 Dec;35(1-2):23–35. doi: 10.1016/0165-2427(92)90118-a. [DOI] [PubMed] [Google Scholar]
- Dow S. W., Poss M. L., Hoover E. A. Feline immunodeficiency virus: a neurotropic lentivirus. J Acquir Immune Defic Syndr. 1990;3(7):658–668. [PubMed] [Google Scholar]
- Ehrenberg B., Montana V., Wei M. D., Wuskell J. P., Loew L. M. Membrane potential can be determined in individual cells from the nernstian distribution of cationic dyes. Biophys J. 1988 May;53(5):785–794. doi: 10.1016/S0006-3495(88)83158-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fermin C. D., Garry R. F. Membrane alterations linked to early interactions of HIV with the cell surface. Virology. 1992 Dec;191(2):941–946. doi: 10.1016/0042-6822(92)90269-u. [DOI] [PubMed] [Google Scholar]
- Fletcher W. H., Byus C. V., Walsh D. A. Receptor-mediated action without receptor occupancy: a function for cell-cell communication in ovarian follicles. Adv Exp Med Biol. 1987;219:299–323. doi: 10.1007/978-1-4684-5395-9_15. [DOI] [PubMed] [Google Scholar]
- Fontana A., Kristensen F., Dubs R., Gemsa D., Weber E. Production of prostaglandin E and an interleukin-1 like factor by cultured astrocytes and C6 glioma cells. J Immunol. 1982 Dec;129(6):2413–2419. [PubMed] [Google Scholar]
- Frei K., Bodmer S., Schwerdel C., Fontana A. Astrocyte-derived interleukin 3 as a growth factor for microglia cells and peritoneal macrophages. J Immunol. 1986 Dec 1;137(11):3521–3527. [PubMed] [Google Scholar]
- Frohman E. M., van den Noort S., Gupta S. Astrocytes and intracerebral immune responses. J Clin Immunol. 1989 Jan;9(1):1–9. doi: 10.1007/BF00917121. [DOI] [PubMed] [Google Scholar]
- Giulian D., Woodward J., Young D. G., Krebs J. F., Lachman L. B. Interleukin-1 injected into mammalian brain stimulates astrogliosis and neovascularization. J Neurosci. 1988 Jul;8(7):2485–2490. doi: 10.1523/JNEUROSCI.08-07-02485.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewe C., Beck A., Gelderblom H. R. HIV: early virus-cell interactions. J Acquir Immune Defic Syndr. 1990;3(10):965–974. [PubMed] [Google Scholar]
- Harbour D. A., Williams P. D., Gruffydd-Jones T. J., Burbridge J., Pearson G. R. Isolation of a T-lymphotropic lentivirus from a persistently leucopenic domestic cat. Vet Rec. 1988 Jan 23;122(4):84–86. doi: 10.1136/vr.122.4.84. [DOI] [PubMed] [Google Scholar]
- Ishida T., Washizu T., Toriyabe K., Motoyoshi S., Tomoda I., Pedersen N. C. Feline immunodeficiency virus infection in cats of Japan. J Am Vet Med Assoc. 1989 Jan 15;194(2):221–225. [PubMed] [Google Scholar]
- Jain A., Mårtensson J., Stole E., Auld P. A., Meister A. Glutathione deficiency leads to mitochondrial damage in brain. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1913–1917. doi: 10.1073/pnas.88.5.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janzer R. C., Raff M. C. Astrocytes induce blood-brain barrier properties in endothelial cells. Nature. 1987 Jan 15;325(6101):253–257. doi: 10.1038/325253a0. [DOI] [PubMed] [Google Scholar]
- Kimelberg H. K. Occurrence and functional significance of serotonin and catecholamine uptake by astrocytes. Biochem Pharmacol. 1986 Jul 15;35(14):2273–2281. doi: 10.1016/0006-2952(86)90451-x. [DOI] [PubMed] [Google Scholar]
- Kinchington D., Barker W., Galpin S., Apostolov K. Temperature enhancement of syncytium formation by HIV and Sendai virus. J Med Virol. 1992 Jan;36(1):44–48. doi: 10.1002/jmv.1890360109. [DOI] [PubMed] [Google Scholar]
- Loewenstein W. R. Permeability of membrane junctions. Ann N Y Acad Sci. 1966 Jul 14;137(2):441–472. doi: 10.1111/j.1749-6632.1966.tb50175.x. [DOI] [PubMed] [Google Scholar]
- Lynn W. S., Tweedale A., Cloyd M. W. Human immunodeficiency virus (HIV-1) cytotoxicity: perturbation of the cell membrane and depression of phospholipid synthesis. Virology. 1988 Mar;163(1):43–51. doi: 10.1016/0042-6822(88)90232-2. [DOI] [PubMed] [Google Scholar]
- Malipiero U. V., Frei K., Fontana A. Production of hemopoietic colony-stimulating factors by astrocytes. J Immunol. 1990 May 15;144(10):3816–3821. [PubMed] [Google Scholar]
- Meister A., Anderson M. E. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Miller M. A., Garry R. F., Jaynes J. M., Montelaro R. C. A structural correlation between lentivirus transmembrane proteins and natural cytolytic peptides. AIDS Res Hum Retroviruses. 1991 Jun;7(6):511–519. doi: 10.1089/aid.1991.7.511. [DOI] [PubMed] [Google Scholar]
- Pedersen N. C., Ho E. W., Brown M. L., Yamamoto J. K. Isolation of a T-lymphotropic virus from domestic cats with an immunodeficiency-like syndrome. Science. 1987 Feb 13;235(4790):790–793. doi: 10.1126/science.3643650. [DOI] [PubMed] [Google Scholar]
- Pumarola-Sune T., Navia B. A., Cordon-Cardo C., Cho E. S., Price R. W. HIV antigen in the brains of patients with the AIDS dementia complex. Ann Neurol. 1987 May;21(5):490–496. doi: 10.1002/ana.410210513. [DOI] [PubMed] [Google Scholar]
- Rahn C. A., Bombick D. W., Doolittle D. J. Assessment of mitochondrial membrane potential as an indicator of cytotoxicity. Fundam Appl Toxicol. 1991 Apr;16(3):435–448. doi: 10.1016/0272-0590(91)90084-h. [DOI] [PubMed] [Google Scholar]
- Rey M. A., Spire B., Dormont D., Barre-Sinoussi F., Montagnier L., Chermann J. C. Characterization of the RNA dependent DNA polymerase of a new human T-lymphotropic retrovirus (lymphadenopathy associated virus). Biochem Biophys Res Commun. 1984 May 31;121(1):126–133. doi: 10.1016/0006-291x(84)90696-x. [DOI] [PubMed] [Google Scholar]
- Rodriguez M., Pierce M. L., Howie E. A. Immune response gene products (Ia antigens) on glial and endothelial cells in virus-induced demyelination. J Immunol. 1987 May 15;138(10):3438–3442. [PubMed] [Google Scholar]
- Stavrou D., Mehraein P., Mellert W., Bise K., Schmidtke K., Rothemunds E., Funke I., Stocker U., Babaryka I., Zietz C. Evaluation of intracerebral lesions in patients with acquired immunodeficiency syndrome. Neuropathological findings and experimental data. Neuropathol Appl Neurobiol. 1989 May-Jun;15(3):207–222. doi: 10.1111/j.1365-2990.1989.tb01223.x. [DOI] [PubMed] [Google Scholar]
- Wade M. H., Trosko J. E., Schindler M. A fluorescence photobleaching assay of gap junction-mediated communication between human cells. Science. 1986 Apr 25;232(4749):525–528. doi: 10.1126/science.3961495. [DOI] [PubMed] [Google Scholar]
- Yamamoto J. K., Hansen H., Ho E. W., Morishita T. Y., Okuda T., Sawa T. R., Nakamura R. M., Pedersen N. C. Epidemiologic and clinical aspects of feline immunodeficiency virus infection in cats from the continental United States and Canada and possible mode of transmission. J Am Vet Med Assoc. 1989 Jan 15;194(2):213–220. [PubMed] [Google Scholar]
- Yamamoto J. K., Sparger E., Ho E. W., Andersen P. R., O'Connor T. P., Mandell C. P., Lowenstine L., Munn R., Pedersen N. C. Pathogenesis of experimentally induced feline immunodeficiency virus infection in cats. Am J Vet Res. 1988 Aug;49(8):1246–1258. [PubMed] [Google Scholar]