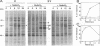Abstract
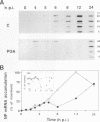
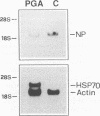
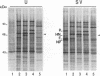
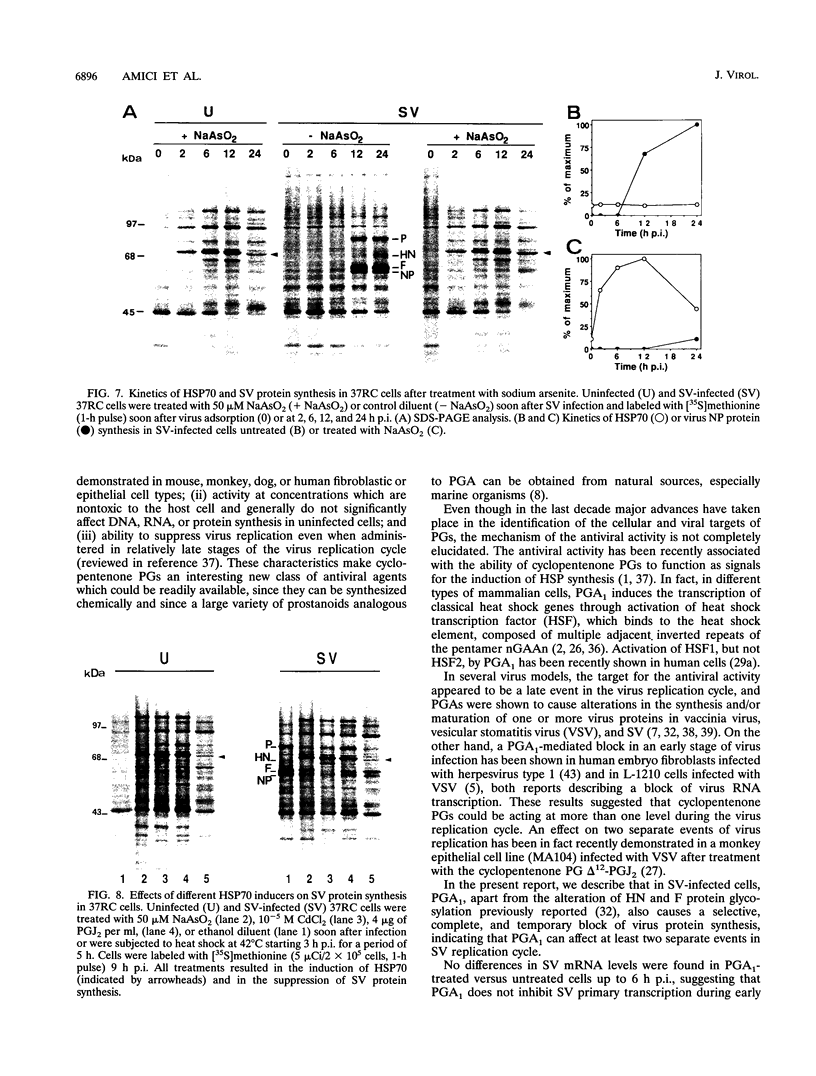
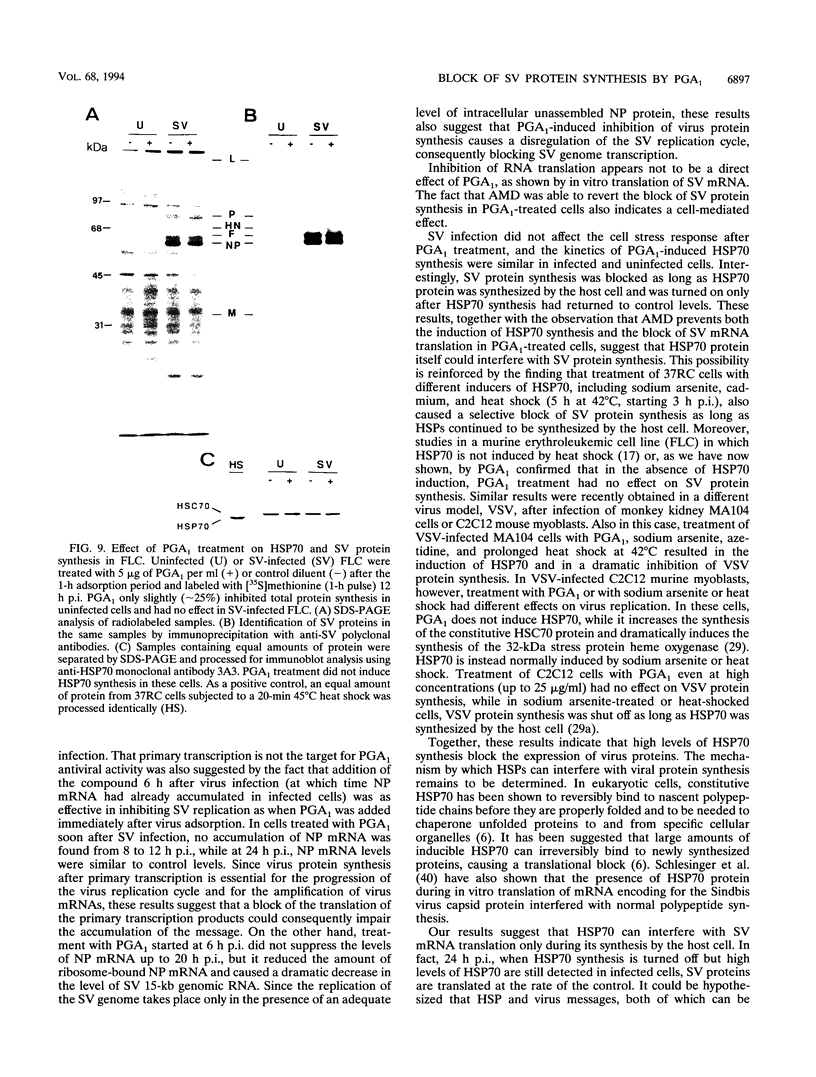
Cyclopentenone prostaglandins are potent inhibitors of virus replication. The antiviral activity has been associated with the induction of 70-kDa heat shock protein (HSP70) synthesis. In this report, we describe that in African green monkey kidney cells infected with Sendai virus (SV) and treated with prostaglandin A1 (PGA1), SV protein synthesis was selectively blocked as long as HSP70 was being synthesized by the host cell. The block appeared to be at the translational level, as indicated by the following (i) PGA1 had no effect on SV primary transcription, and a dramatic decrease in the abundance of SV mRNA occurred only at later stages of infection; and (ii) treatment with PGA1 started at 6 h postinfection, at which time SV mRNA had already accumulated in infected cells, did not suppress the levels of NP mRNA, but it reduced the amount of ribosome-bound NP mRNA and caused a dramatic decrease in the level of genomic RNA. The PGA1-induced block of SV protein synthesis appeared to be cell mediated, since it was prevented by actinomycin D, while PGA1 had no effect on SV mRNA translation in vitro. The possibility that HSP70 could be a mediator of the antiviral effect is suggested by the fact that treatment with other classical inducers of HSP70, including sodium arsenite, cadmium, and heat shock at 42 degrees C for 5 h, also selectively prevented SV protein synthesis as long as heat shock protein synthesis occurred. Moreover, SV protein synthesis was not inhibited by PGA1 in murine Friend erythroleukemic cells, which lack the ability to induce HSP70 expression in response to PGA1.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amici C., Santoro M. G. Suppression of virus replication by prostaglandin A is associated with heat shock protein synthesis. J Gen Virol. 1991 Aug;72(Pt 8):1877–1885. doi: 10.1099/0022-1317-72-8-1877. [DOI] [PubMed] [Google Scholar]
- Amici C., Sistonen L., Santoro M. G., Morimoto R. I. Antiproliferative prostaglandins activate heat shock transcription factor. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6227–6231. doi: 10.1073/pnas.89.14.6227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankel H., Mittnacht S., Jacobsen H. Antiviral activity of prostaglandin A on encephalomyocarditis virus-infected cells: a unique effect unrelated to interferon. J Gen Virol. 1985 Nov;66(Pt 11):2355–2364. doi: 10.1099/0022-1317-66-11-2355. [DOI] [PubMed] [Google Scholar]
- Ankel H., Turriziani O., Antonelli G. Prostaglandin A inhibits replication of human immunodeficiency virus during acute infection. J Gen Virol. 1991 Nov;72(Pt 11):2797–2800. doi: 10.1099/0022-1317-72-11-2797. [DOI] [PubMed] [Google Scholar]
- Bader T., Ankel H. Inhibition of primary transcription of vesicular stomatitis virus by prostaglandin A1. J Gen Virol. 1990 Dec;71(Pt 12):2823–2832. doi: 10.1099/0022-1317-71-12-2823. [DOI] [PubMed] [Google Scholar]
- Beckmann R. P., Mizzen L. E., Welch W. J. Interaction of Hsp 70 with newly synthesized proteins: implications for protein folding and assembly. Science. 1990 May 18;248(4957):850–854. doi: 10.1126/science.2188360. [DOI] [PubMed] [Google Scholar]
- Benavente J., Esteban M., Jaffe B. M., Santoro M. G. Selective inhibition of viral gene expression as the mechanism of the antiviral action of PGA1 in vaccinia virus-infected cells. J Gen Virol. 1984 Mar;65(Pt 3):599–608. doi: 10.1099/0022-1317-65-3-599. [DOI] [PubMed] [Google Scholar]
- Coccia E. M., Profita V., Fiorucci G., Romeo G., Affabris E., Testa U., Hentze M. W., Battistini A. Modulation of ferritin H-chain expression in Friend erythroleukemia cells: transcriptional and translational regulation by hemin. Mol Cell Biol. 1992 Jul;12(7):3015–3022. doi: 10.1128/mcb.12.7.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove J. W., Brown I. R. Heat shock protein in mammalian brain and other organs after a physiologically relevant increase in body temperature induced by D-lysergic acid diethylamide. Proc Natl Acad Sci U S A. 1983 Jan;80(2):569–573. doi: 10.1073/pnas.80.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Onofrio C., Alvino E., Garaci E., Bonmassar E., Santoro M. G. Selection of HTLV-I positive clones is prevented by prostaglandin A in infected cord blood cultures. Br J Cancer. 1990 Feb;61(2):207–214. doi: 10.1038/bjc.1990.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marco A., Santoro M. G. Antiviral effect of short hyperthermic treatment at specific stages of vesicular stomatitis virus replication cycle. J Gen Virol. 1993 Aug;74(Pt 8):1685–1690. doi: 10.1099/0022-1317-74-8-1685. [DOI] [PubMed] [Google Scholar]
- Dinarello C. A., Wolff S. M. Molecular basis of fever in humans. Am J Med. 1982 May;72(5):799–819. doi: 10.1016/0002-9343(82)90548-4. [DOI] [PubMed] [Google Scholar]
- Dowling P. C., Giorgi C., Roux L., Dethlefsen L. A., Galantowicz M. E., Blumberg B. M., Kolakofsky D. Molecular cloning of the 3'-proximal third of Sendai virus genome. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5213–5216. doi: 10.1073/pnas.80.17.5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima M. Prostaglandin J2--anti-tumour and anti-viral activities and the mechanisms involved. Eicosanoids. 1990;3(4):189–199. [PubMed] [Google Scholar]
- Galinski M. S. Paramyxoviridae: transcription and replication. Adv Virus Res. 1991;39:129–162. doi: 10.1016/s0065-3527(08)60794-0. [DOI] [PubMed] [Google Scholar]
- Hensold J. O., Hunt C. R., Calderwood S. K., Housman D. E., Kingston R. E. DNA binding of heat shock factor to the heat shock element is insufficient for transcriptional activation in murine erythroleukemia cells. Mol Cell Biol. 1990 Apr;10(4):1600–1608. doi: 10.1128/mcb.10.4.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes-Fulford M., McGrath M. S., Hanks D., Erickson S., Pulliam L. Effects of dimethyl prostaglandin A1 on herpes simplex virus and human immunodeficiency virus replication. Antimicrob Agents Chemother. 1992 Oct;36(10):2253–2258. doi: 10.1128/aac.36.10.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S. H., Sorof S. Preferential binding of growth inhibitory prostaglandins by the target protein of a carcinogen. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9401–9405. doi: 10.1073/pnas.87.23.9401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastromarino P., Conti C., Petruzziello R., De Marco A., Pica F., Santoro M. G. Inhibition of Sindbis virus replication by cyclopentenone prostaglandins: a cell-mediated event associated with heat-shock protein synthesis. Antiviral Res. 1993 Mar;20(3):209–222. doi: 10.1016/0166-3542(93)90021-a. [DOI] [PubMed] [Google Scholar]
- Model P., Webster R. E., Zinder N. D. Characterization of Op3, a lysis-defective mutant of bacteriophage f2. Cell. 1979 Oct;18(2):235–246. doi: 10.1016/0092-8674(79)90043-6. [DOI] [PubMed] [Google Scholar]
- Muñoz A., Alonso M. A., Carrasco L. Synthesis of heat-shock proteins in HeLa cells: inhibition by virus infection. Virology. 1984 Aug;137(1):150–159. doi: 10.1016/0042-6822(84)90018-7. [DOI] [PubMed] [Google Scholar]
- Nuss D. L., Koch G. Differential inhibition of vesicular stomatitis virus polypeptide synthesis by hypertonic initiation block. J Virol. 1975 Jan;17(1):283–286. doi: 10.1128/jvi.17.1.283-286.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno K., Fukushima M., Fujiwara M., Narumiya S. Induction of 68,000-dalton heat shock proteins by cyclopentenone prostaglandins. Its association with prostaglandin-induced G1 block in cell cycle progression. J Biol Chem. 1988 Dec 25;263(36):19764–19770. [PubMed] [Google Scholar]
- Pica F., De Marco A., De Cesare F., Santoro M. G. Inhibition of vesicular stomatitis virus replication by delta 12-prostaglandin J2 is regulated at two separate levels and is associated with induction of stress protein synthesis. Antiviral Res. 1993 Mar;20(3):193–208. doi: 10.1016/0166-3542(93)90020-j. [DOI] [PubMed] [Google Scholar]
- Santoro M. G., Amici C., Elia G., Benedetto A., Garaci E. Inhibition of virus protein glycosylation as the mechanism of the antiviral action of prostaglandin A in Sendai virus-infected cells. J Gen Virol. 1989 Apr;70(Pt 4):789–800. doi: 10.1099/0022-1317-70-4-789. [DOI] [PubMed] [Google Scholar]
- Santoro M. G., Benedetto A., Carruba G., Garaci E., Jaffe B. M. Prostaglandin A compounds as antiviral agents. Science. 1980 Aug 29;209(4460):1032–1034. doi: 10.1126/science.6157190. [DOI] [PubMed] [Google Scholar]
- Santoro M. G., Benedetto A., Jaffe B. M. Prostaglandin A1 induces differentiation in Friend erythroleukemia cells. Prostaglandins. 1979 May;17(5):719–727. doi: 10.1016/s0090-6980(79)80043-x. [DOI] [PubMed] [Google Scholar]
- Santoro M. G., Favalli C., Mastino A., Jaffe B. M., Esteban M., Garaci E. Antiviral activity of a synthetic analog of prostaglandin A in mice infected with influenza A virus. Arch Virol. 1988;99(1-2):89–100. doi: 10.1007/BF01311026. [DOI] [PubMed] [Google Scholar]
- Santoro M. G., Garaci E., Amici C. Induction of heat shock protein synthesis by prostaglandins with antineoplastic and antiviral activity. Adv Prostaglandin Thromboxane Leukot Res. 1991;21B:867–874. [PubMed] [Google Scholar]
- Santoro M. G., Garaci E., Amici C. Prostaglandins with antiproliferative activity induce the synthesis of a heat shock protein in human cells. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8407–8411. doi: 10.1073/pnas.86.21.8407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro M. G., Jaffe B. M., Esteban M. Prostaglandin A inhibits the replication of vesicular stomatitis virus: effect on virus glycoprotein. J Gen Virol. 1983 Dec;64(Pt 12):2797–2801. doi: 10.1099/0022-1317-64-12-2797. [DOI] [PubMed] [Google Scholar]
- Santoro M. G., Jaffe B. M., Garaci E., Esteban M. Antiviral effect of prostaglandins of the A series: inhibition of vaccinia virus replication in cultured cells. J Gen Virol. 1982 Dec;63(2):435–440. doi: 10.1099/0022-1317-63-2-435. [DOI] [PubMed] [Google Scholar]
- Vidal S., Kolakofsky D. Modified model for the switch from Sendai virus transcription to replication. J Virol. 1989 May;63(5):1951–1958. doi: 10.1128/jvi.63.5.1951-1958.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N., Fukushima M., Tsurumi T., Maeno K., Nishiyama Y. Mechanism of inhibition of herpes simplex virus replication by delta 7-prostaglandin A1 and delta 12-prostaglandin J2. Biochem Biophys Res Commun. 1987 Aug 14;146(3):1425–1431. doi: 10.1016/0006-291x(87)90809-6. [DOI] [PubMed] [Google Scholar]