Abstract
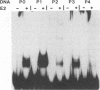
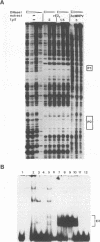
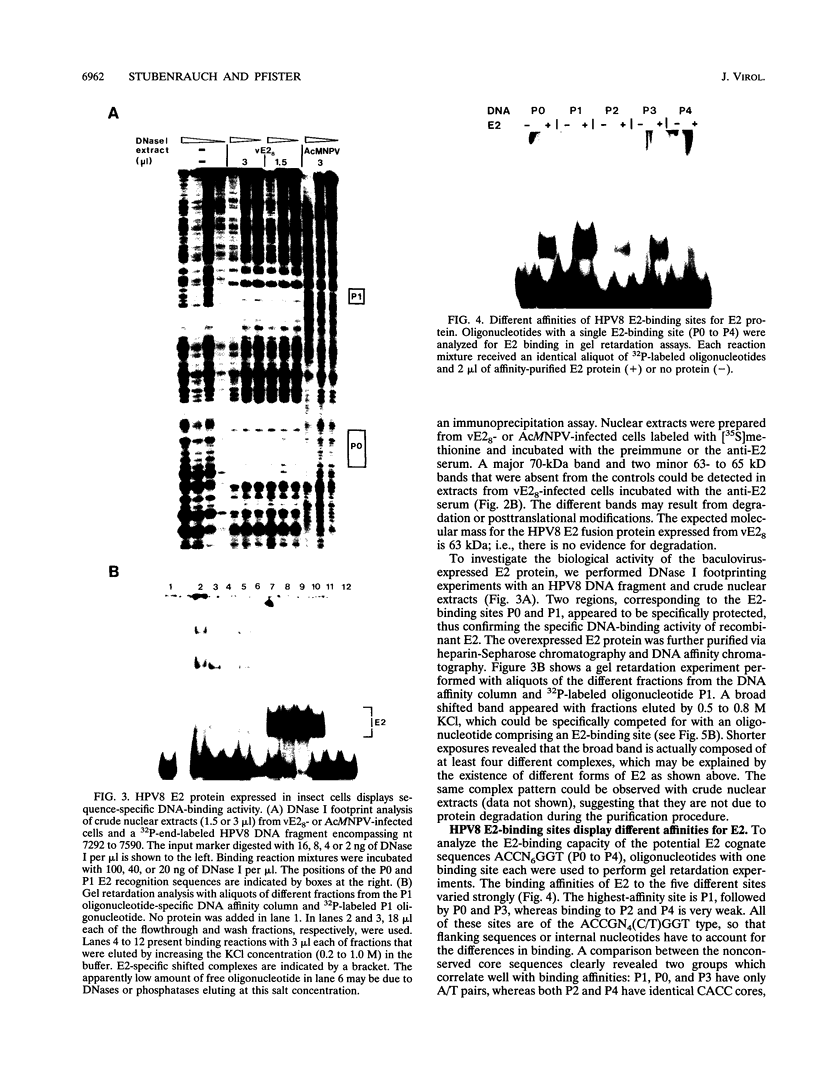
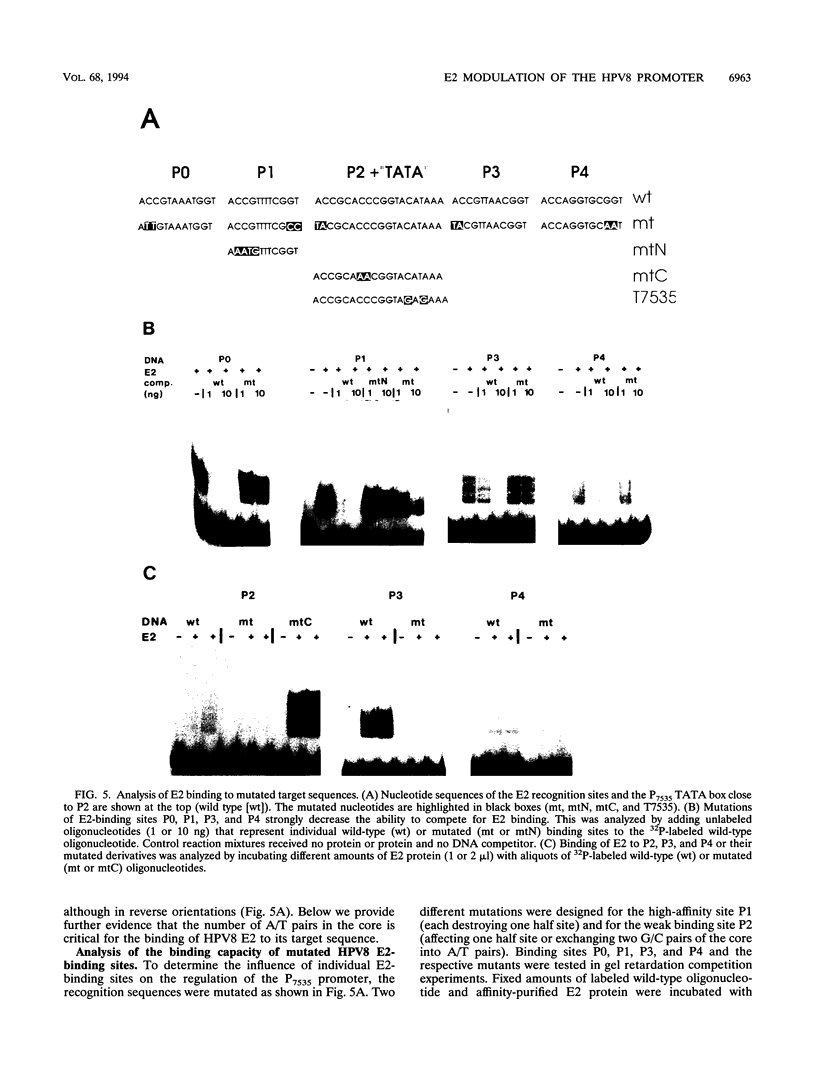
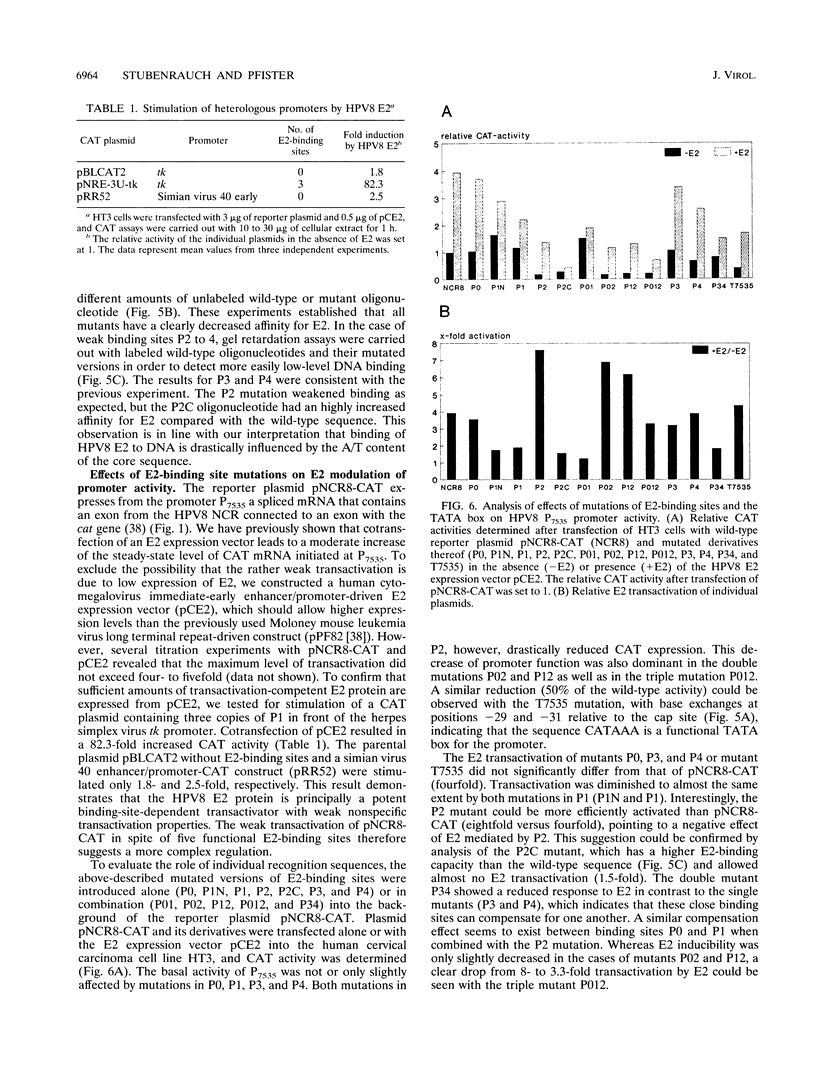
The constitutively active promoter P7535 of the epidermodysplasia verruciformis-associated human papillomavirus type 8 (HPV8) is transactivated by the viral E2 protein. The distribution of potential E2-binding sites (ACCN6GGT) in the viral transcription control region is highly conserved among epidermodysplasia verruciformis-associated human papillomaviruses and differs completely from that of other papillomaviruses. To investigate the role of E2-binding sites P0 to P4 in P7535 regulation, we analyzed their binding affinities in gel retardation experiments using a full-length HPV8 E2 protein expressed from a recombinant baculovirus. Binding site P1 within a transcriptional silencer showed the highest affinity, followed by P0 within the L1 gene and P3 downstream of P7535. P2, 33 nucleotides upstream of the mRNA cap site, and P4 were very weak binders. There is some indication that the number of A/T pairs in the nonconserved core of the recognition sequence is critical for the binding of HPV8 E2. Transient transfection experiments were carried out with an HPV8 E2 expression vector and reporter plasmids containing mutated E2-binding sites in the context of the HPV8 regulatory region. The knockout of the strongest binding site P1 sufficed to clearly diminish transactivation. P0, P3, and P4 mutations had little effect on their own, whereas double mutations P01 and P34 strongly reduced E2 inducibility. Both mutations in P2 severely affected constitutive promoter activity but had opposite effects on transactivation. They revealed an inverse correlation between E2-binding strength and the extent of E2 transactivation. This finding suggests that P2 mediates a negative control of P7535 by E2, counteracting E2 transactivation exerted via the four distal E2 target sequences.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bedrosian C. L., Bastia D. The DNA-binding domain of HPV-16 E2 protein interaction with the viral enhancer: protein-induced DNA bending and role of the nonconserved core sequence in binding site affinity. Virology. 1990 Feb;174(2):557–575. doi: 10.1016/0042-6822(90)90109-5. [DOI] [PubMed] [Google Scholar]
- Bernard B. A., Bailly C., Lenoir M. C., Darmon M., Thierry F., Yaniv M. The human papillomavirus type 18 (HPV18) E2 gene product is a repressor of the HPV18 regulatory region in human keratinocytes. J Virol. 1989 Oct;63(10):4317–4324. doi: 10.1128/jvi.63.10.4317-4324.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dollard S. C., Broker T. R., Chow L. T. Regulation of the human papillomavirus type 11 E6 promoter by viral and host transcription factors in primary human keratinocytes. J Virol. 1993 Mar;67(3):1721–1726. doi: 10.1128/jvi.67.3.1721-1726.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong G., Broker T. R., Chow L. T. Human papillomavirus type 11 E2 proteins repress the homologous E6 promoter by interfering with the binding of host transcription factors to adjacent elements. J Virol. 1994 Feb;68(2):1115–1127. doi: 10.1128/jvi.68.2.1115-1127.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dostatni N., Lambert P. F., Sousa R., Ham J., Howley P. M., Yaniv M. The functional BPV-1 E2 trans-activating protein can act as a repressor by preventing formation of the initiation complex. Genes Dev. 1991 Sep;5(9):1657–1671. doi: 10.1101/gad.5.9.1657. [DOI] [PubMed] [Google Scholar]
- Ensser A., Pfister H. Epidermodysplasia verruciformis associated human papillomaviruses present a subgenus-specific organization of the regulatory genome region. Nucleic Acids Res. 1990 Jul 11;18(13):3919–3922. doi: 10.1093/nar/18.13.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frech B., Zimber-Strobl U., Suentzenich K. O., Pavlish O., Lenoir G. M., Bornkamm G. W., Mueller-Lantzsch N. Identification of Epstein-Barr virus terminal protein 1 (TP1) in extracts of four lymphoid cell lines, expression in insect cells, and detection of antibodies in human sera. J Virol. 1990 Jun;64(6):2759–2767. doi: 10.1128/jvi.64.6.2759-2767.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs P. G., Iftner T., Weninger J., Pfister H. Epidermodysplasia verruciformis-associated human papillomavirus 8: genomic sequence and comparative analysis. J Virol. 1986 May;58(2):626–634. doi: 10.1128/jvi.58.2.626-634.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartenberg M. R., Crothers D. M. DNA sequence determinants of CAP-induced bending and protein binding affinity. Nature. 1988 Jun 30;333(6176):824–829. doi: 10.1038/333824a0. [DOI] [PubMed] [Google Scholar]
- Gauthier J. M., Dostatni N., Lusky M., Yaniv M. Two DNA-bound E2 dimers are required for strong transcriptional activation and for cooperation with cellular factors in most cells. New Biol. 1991 May;3(5):498–509. [PubMed] [Google Scholar]
- Haugen T. H., Turek L. P., Mercurio F. M., Cripe T. P., Olson B. J., Anderson R. D., Seidl D., Karin M., Schiller J. Sequence-specific and general transcriptional activation by the bovine papillomavirus-1 E2 trans-activator require an N-terminal amphipathic helix-containing E2 domain. EMBO J. 1988 Dec 20;7(13):4245–4253. doi: 10.1002/j.1460-2075.1988.tb03322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde R. S., Grossman S. R., Laimins L. A., Sigler P. B. Crystal structure at 1.7 A of the bovine papillomavirus-1 E2 DNA-binding domain bound to its DNA target. Nature. 1992 Oct 8;359(6395):505–512. doi: 10.1038/359505a0. [DOI] [PubMed] [Google Scholar]
- Higuchi R., Krummel B., Saiki R. K. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res. 1988 Aug 11;16(15):7351–7367. doi: 10.1093/nar/16.15.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn S., Pfister H., Fuchs P. G. Constitutive transcriptional activator of Epidermodysplasia verruciformis-associated human papillomavirus 8. Virology. 1993 Oct;196(2):674–681. doi: 10.1006/viro.1993.1524. [DOI] [PubMed] [Google Scholar]
- Knight J. D., Li R., Botchan M. The activation domain of the bovine papillomavirus E2 protein mediates association of DNA-bound dimers to form DNA loops. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3204–3208. doi: 10.1073/pnas.88.8.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krubke J., Kraus J., Delius H., Chow L., Broker T., Iftner T., Pfister H. Genetic relationship among human papillomaviruses associated with benign and malignant tumours of patients with epidermodysplasia verruciformis. J Gen Virol. 1987 Dec;68(Pt 12):3091–3103. doi: 10.1099/0022-1317-68-12-3091. [DOI] [PubMed] [Google Scholar]
- Lambert P. F. Papillomavirus DNA replication. J Virol. 1991 Jul;65(7):3417–3420. doi: 10.1128/jvi.65.7.3417-3420.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Knight J., Bream G., Stenlund A., Botchan M. Specific recognition nucleotides and their DNA context determine the affinity of E2 protein for 17 binding sites in the BPV-1 genome. Genes Dev. 1989 Apr;3(4):510–526. doi: 10.1101/gad.3.4.510. [DOI] [PubMed] [Google Scholar]
- May M., Grassmann K., Pfister H., Fuchs P. G. Transcriptional silencer of the human papillomavirus type 8 late promoter interacts alternatively with the viral trans activator E2 or with a cellular factor. J Virol. 1994 Jun;68(6):3612–3619. doi: 10.1128/jvi.68.6.3612-3619.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May M., Helbl V., Pfister H., Fuchs P. G. Unique topography of DNA-protein interactions in the non-coding region of epidermodysplasia verruciformis-associated human papillomaviruses. J Gen Virol. 1991 Dec;72(Pt 12):2989–2997. doi: 10.1099/0022-1317-72-12-2989. [DOI] [PubMed] [Google Scholar]
- McBride A. A., Romanczuk H., Howley P. M. The papillomavirus E2 regulatory proteins. J Biol Chem. 1991 Oct 5;266(28):18411–18414. [PubMed] [Google Scholar]
- Monini P., Grossman S. R., Pepinsky B., Androphy E. J., Laimins L. A. Cooperative binding of the E2 protein of bovine papillomavirus to adjacent E2-responsive sequences. J Virol. 1991 Apr;65(4):2124–2130. doi: 10.1128/jvi.65.4.2124-2130.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasseri M., Gage J. R., Lorincz A., Wettstein F. O. Human papillomavirus type 16 immortalized cervical keratinocytes contain transcripts encoding E6, E7, and E2 initiated at the P97 promoter and express high levels of E7. Virology. 1991 Sep;184(1):131–140. doi: 10.1016/0042-6822(91)90829-z. [DOI] [PubMed] [Google Scholar]
- Pfister H. Biology and biochemistry of papillomaviruses. Rev Physiol Biochem Pharmacol. 1984;99:111–181. doi: 10.1007/BFb0027716. [DOI] [PubMed] [Google Scholar]
- Reh H., Pfister H. Human papillomavirus type 8 contains cis-active positive and negative transcriptional control sequences. J Gen Virol. 1990 Oct;71(Pt 10):2457–2462. doi: 10.1099/0022-1317-71-10-2457. [DOI] [PubMed] [Google Scholar]
- Romanczuk H., Howley P. M. Disruption of either the E1 or the E2 regulatory gene of human papillomavirus type 16 increases viral immortalization capacity. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):3159–3163. doi: 10.1073/pnas.89.7.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanczuk H., Thierry F., Howley P. M. Mutational analysis of cis elements involved in E2 modulation of human papillomavirus type 16 P97 and type 18 P105 promoters. J Virol. 1990 Jun;64(6):2849–2859. doi: 10.1128/jvi.64.6.2849-2859.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotenberg M. O., Chiang C. M., Ho M. L., Broker T. R., Chow L. T. Characterization of cDNAs of spliced HPV-11 E2 mRNA and other HPV mRNAs recovered via retrovirus-mediated gene transfer. Virology. 1989 Oct;172(2):468–477. doi: 10.1016/0042-6822(89)90189-x. [DOI] [PubMed] [Google Scholar]
- Sowden M., Harrison S., Ashfield R., Kingsman A. J., Kingsman S. M. Multiple cooperative interactions constrain BPV-1 E2 dependent activation of transcription. Nucleic Acids Res. 1989 Apr 25;17(8):2959–2972. doi: 10.1093/nar/17.8.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalholz B. A., Byrne J. C., Howley P. M. Evidence for cooperativity between E2 binding sites in E2 trans-regulation of bovine papillomavirus type 1. J Virol. 1988 Sep;62(9):3143–3150. doi: 10.1128/jvi.62.9.3143-3150.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steger G., Olszewsky M., Stockfleth E., Pfister H. Prevalence of antibodies to human papillomavirus type 8 in human sera. J Virol. 1990 Sep;64(9):4399–4406. doi: 10.1128/jvi.64.9.4399-4406.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenlund A., Botchan M. R. The E2 trans-activator can act as a repressor by interfering with a cellular transcription factor. Genes Dev. 1990 Jan;4(1):123–136. doi: 10.1101/gad.4.1.123. [DOI] [PubMed] [Google Scholar]
- Stubenrauch F., Malejczyk J., Fuchs P. G., Pfister H. Late promoter of human papillomavirus type 8 and its regulation. J Virol. 1992 Jun;66(6):3485–3493. doi: 10.1128/jvi.66.6.3485-3493.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymanski P., Stenlund A. Regulation of early gene expression from the bovine papillomavirus genome in transiently transfected C127 cells. J Virol. 1991 Nov;65(11):5710–5720. doi: 10.1128/jvi.65.11.5710-5720.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan S. H., Gloss B., Bernard H. U. During negative regulation of the human papillomavirus-16 E6 promoter, the viral E2 protein can displace Sp1 from a proximal promoter element. Nucleic Acids Res. 1992 Jan 25;20(2):251–256. doi: 10.1093/nar/20.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierry F., Howley P. M. Functional analysis of E2-mediated repression of the HPV18 P105 promoter. New Biol. 1991 Jan;3(1):90–100. [PubMed] [Google Scholar]
- Tjian R. T antigen binding and the control of SV40 gene expression. Cell. 1981 Oct;26(1 Pt 1):1–2. doi: 10.1016/0092-8674(81)90026-x. [DOI] [PubMed] [Google Scholar]
- Vande Pol S. B., Howley P. M. A bovine papillomavirus constitutive enhancer is negatively regulated by the E2 repressor through competitive binding for a cellular factor. J Virol. 1990 Nov;64(11):5420–5429. doi: 10.1128/jvi.64.11.5420-5429.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]