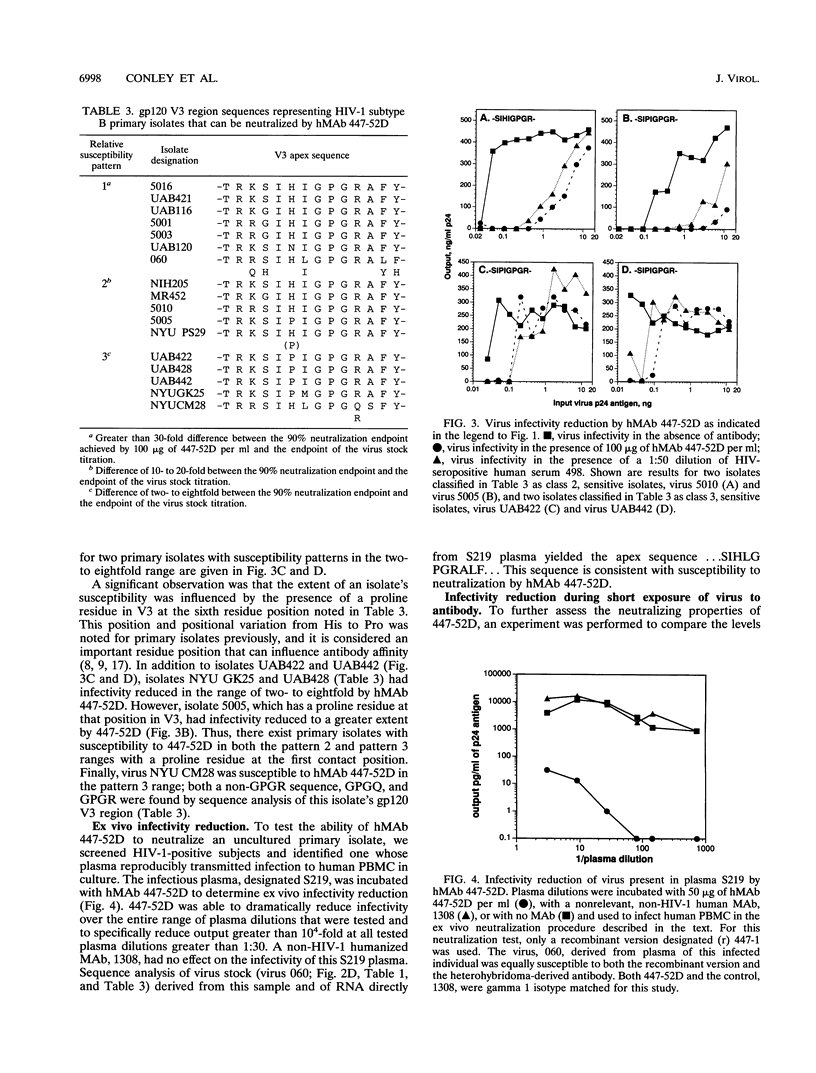
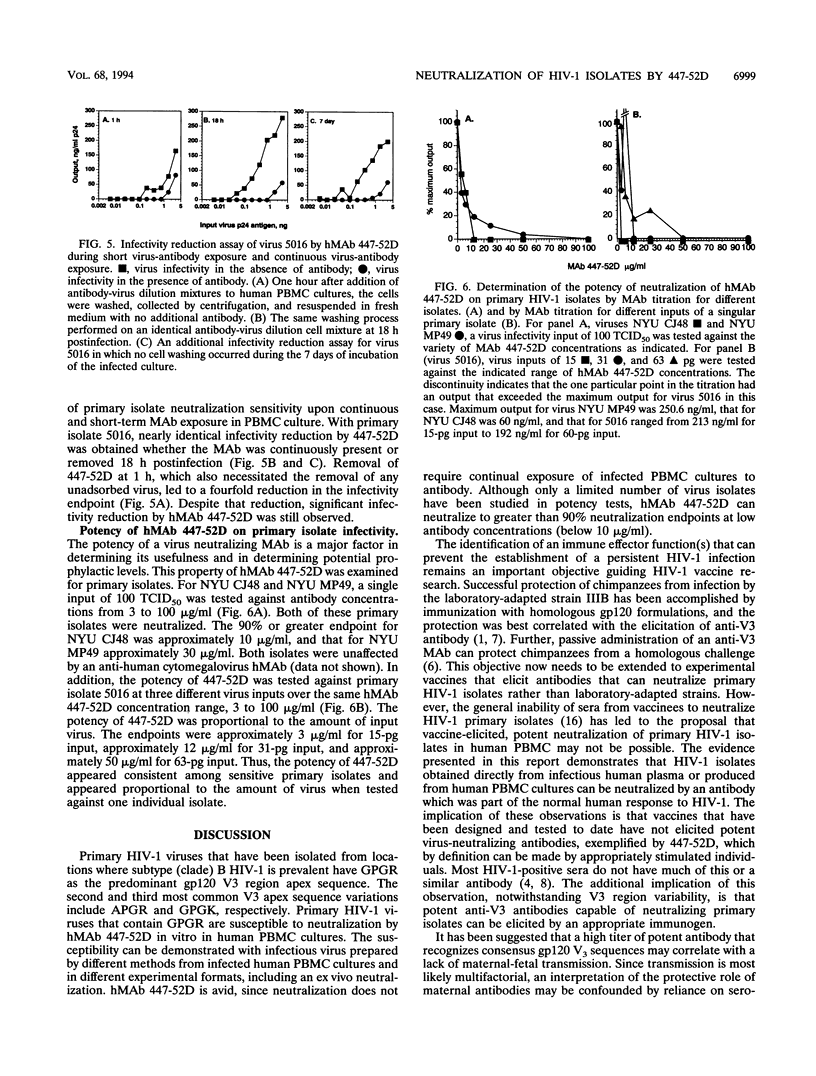
Abstract
Human monoclonal antibody 447-52D binds to the V3 determinant of the human immunodeficiency virus type 1 (HIV-1) gp120 external glycoprotein. Its binding requires the expression of the GPxR sequence at the center of the V3 domain. HIV-1 variants that are adapted to replication in T-lymphoid cell lines and express this sequence motif are efficiently neutralized by the antibody (M. K. Gorny, A. J. Conley, S. Karwowska, A. Buchbinder, J.-Y. Xu, E. A. Emini, S. Koenig, and S. Zolla-Pazner, J. Virol. 66:7538-7542, 1992). In the present study, the antiviral activity of 447-52D was further defined with regard to its ability to mediate neutralization of primary HIV-1 clinical isolates. Again, the antibody was found to potently neutralize those isolates that expressed the binding sequence. We confirmed that this determinant is commonly expressed by virus isolates belonging to the subtype (clade) B sequence classification. As such, 447-52D may be useful for prophylactic and immunotherapeutic intervention. In addition, the study demonstrated that neutralization of primary HIV-1 isolates is possible if mediated by an appropriate antibody.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berman P. W., Gregory T. J., Riddle L., Nakamura G. R., Champe M. A., Porter J. P., Wurm F. M., Hershberg R. D., Cobb E. K., Eichberg J. W. Protection of chimpanzees from infection by HIV-1 after vaccination with recombinant glycoprotein gp120 but not gp160. Nature. 1990 Jun 14;345(6276):622–625. doi: 10.1038/345622a0. [DOI] [PubMed] [Google Scholar]
- Burger H., Weiser B., Flaherty K., Gulla J., Nguyen P. N., Gibbs R. A. Evolution of human immunodeficiency virus type 1 nucleotide sequence diversity among close contacts. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11236–11240. doi: 10.1073/pnas.88.24.11236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley A. J., Kessler J. A., 2nd, Boots L. J., Tung J. S., Arnold B. A., Keller P. M., Shaw A. R., Emini E. A. Neutralization of divergent human immunodeficiency virus type 1 variants and primary isolates by IAM-41-2F5, an anti-gp41 human monoclonal antibody. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3348–3352. doi: 10.1073/pnas.91.8.3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rossi A., Zanotto C., Mammano F., Ometto L., Del Mistro A., Chieco-Bianchi L. Pattern of antibody response against the V3 loop in children with vertically acquired immunodeficiency virus type 1 (HIV-1) infection. AIDS Res Hum Retroviruses. 1993 Mar;9(3):221–228. doi: 10.1089/aid.1993.9.221. [DOI] [PubMed] [Google Scholar]
- Emini E. A., Schleif W. A., Nunberg J. H., Conley A. J., Eda Y., Tokiyoshi S., Putney S. D., Matsushita S., Cobb K. E., Jett C. M. Prevention of HIV-1 infection in chimpanzees by gp120 V3 domain-specific monoclonal antibody. Nature. 1992 Feb 20;355(6362):728–730. doi: 10.1038/355728a0. [DOI] [PubMed] [Google Scholar]
- Girard M., Kieny M. P., Pinter A., Barre-Sinoussi F., Nara P., Kolbe H., Kusumi K., Chaput A., Reinhart T., Muchmore E. Immunization of chimpanzees confers protection against challenge with human immunodeficiency virus. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):542–546. doi: 10.1073/pnas.88.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorny M. K., Conley A. J., Karwowska S., Buchbinder A., Xu J. Y., Emini E. A., Koenig S., Zolla-Pazner S. Neutralization of diverse human immunodeficiency virus type 1 variants by an anti-V3 human monoclonal antibody. J Virol. 1992 Dec;66(12):7538–7542. doi: 10.1128/jvi.66.12.7538-7542.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller P. M., Arnold B. A., Shaw A. R., Tolman R. L., Van Middlesworth F., Bondy S., Rusiecki V. K., Koenig S., Zolla-Pazner S., Conard P. Identification of HIV vaccine candidate peptides by screening random phage epitope libraries. Virology. 1993 Apr;193(2):709–716. doi: 10.1006/viro.1993.1179. [DOI] [PubMed] [Google Scholar]
- LaRosa G. J., Davide J. P., Weinhold K., Waterbury J. A., Profy A. T., Lewis J. A., Langlois A. J., Dreesman G. R., Boswell R. N., Shadduck P. Conserved sequence and structural elements in the HIV-1 principal neutralizing determinant. Science. 1990 Aug 24;249(4971):932–935. doi: 10.1126/science.2392685. [DOI] [PubMed] [Google Scholar]
- Laal S., Burda S., Sharpe S., Zolla-Pazner S. A rapid, automated microtiter assay for measuring neutralization of HIV-1. AIDS Res Hum Retroviruses. 1993 Aug;9(8):781–785. doi: 10.1089/aid.1993.9.781. [DOI] [PubMed] [Google Scholar]
- Scarlatti G., Leitner T., Halapi E., Wahlberg J., Marchisio P., Clerici-Schoeller M. A., Wigzell H., Fenyö E. M., Albert J., Uhlén M. Comparison of variable region 3 sequences of human immunodeficiency virus type 1 from infected children with the RNA and DNA sequences of the virus populations of their mothers. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1721–1725. doi: 10.1073/pnas.90.5.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfs T. F., Zwart G., Bakker M., Valk M., Kuiken C. L., Goudsmit J. Naturally occurring mutations within HIV-1 V3 genomic RNA lead to antigenic variation dependent on a single amino acid substitution. Virology. 1991 Nov;185(1):195–205. doi: 10.1016/0042-6822(91)90767-6. [DOI] [PubMed] [Google Scholar]


