Abstract
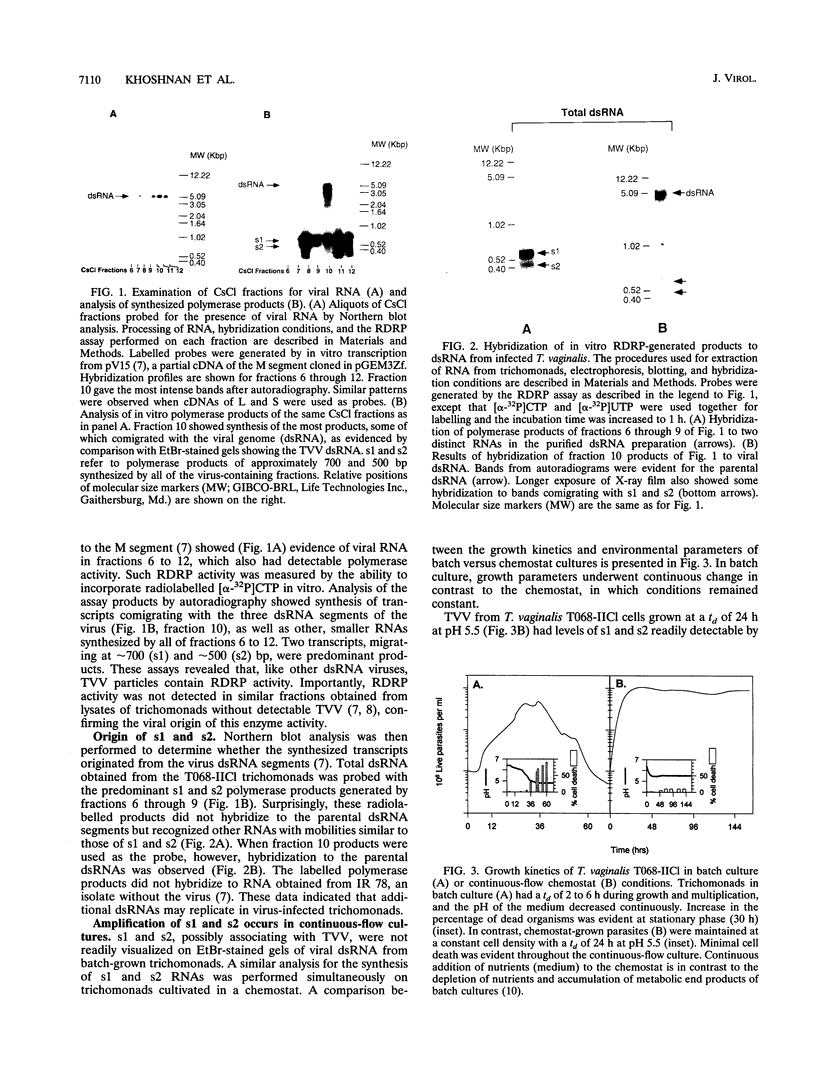
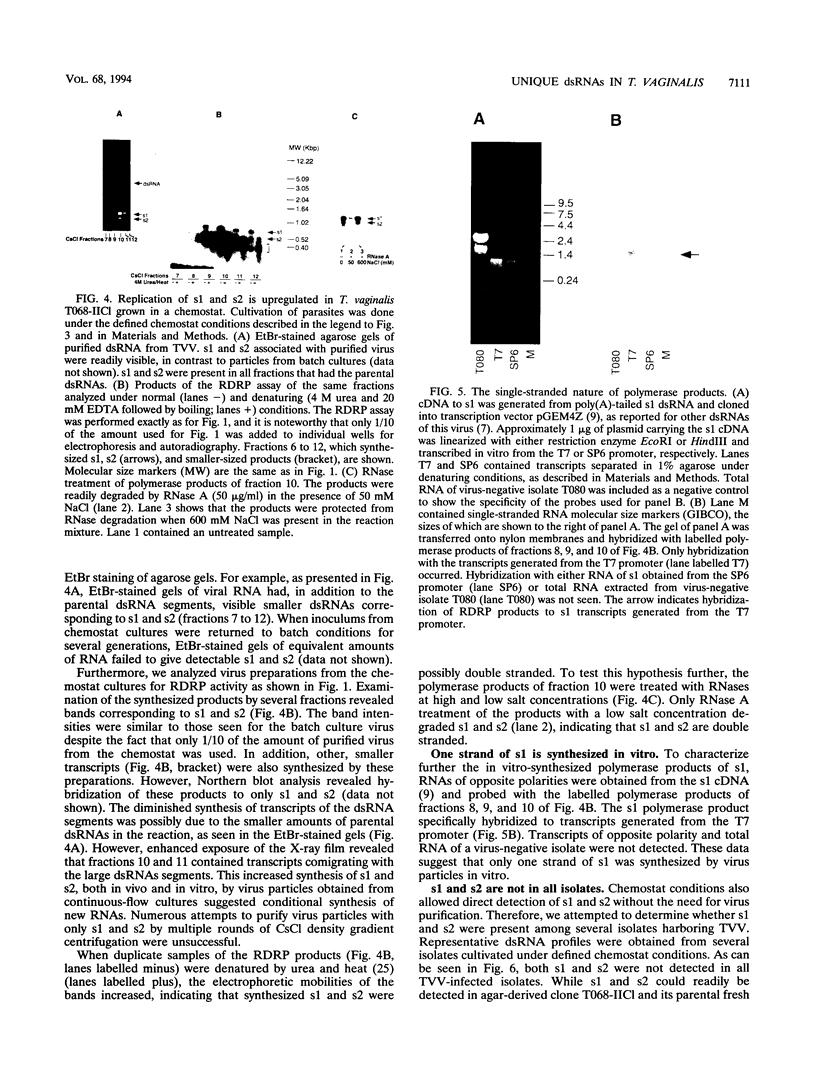
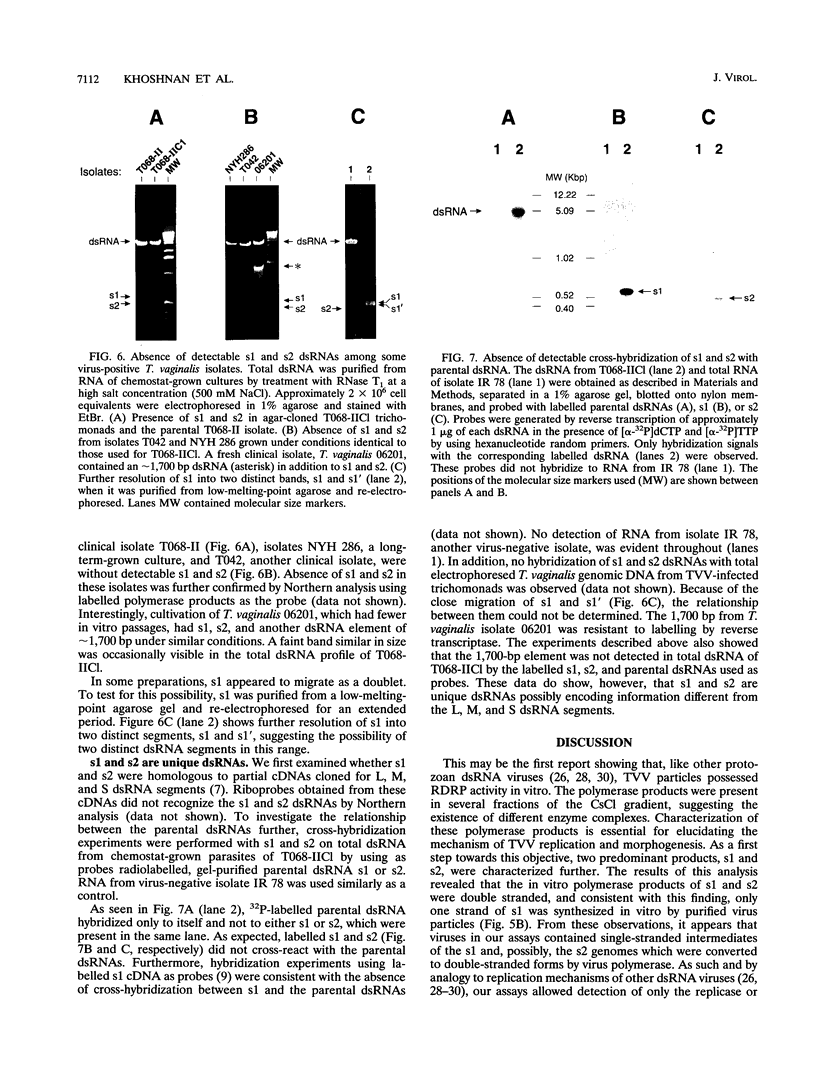
Most Trichomonas vaginalis isolates are carriers of the multisegmented double-stranded RNA (dsRNA) virus. In vitro polymerase assays were performed to demonstrate the RNA-dependent RNA polymerase (RDRP) activity of purified particles. Transcripts which comigrated with the dsRNAs of the virus were readily detected as synthesized products, indicating viral RDRP activity. In addition, smaller-sized dsRNA species, possibly two of approximately 700 bp (s1) and one of 500 bp (s2), were synthesized by purified virus particles of the CsCl gradient surrounding the virus peak. No cross-hybridization with either s1 or s2 and the dsRNA segments occurred, suggesting that s1 and s2 were synthesized from different templates. An RNase A protection assay revealed that the synthesized s1 and s2 polymerase products were double stranded. Furthermore, hybridization of products with strand-specific RNA of s1 generated from cDNA indicated that only one strand was synthesized in vitro. s1 and s2 were not visualized in ethidium bromide-stained agarose gels of dsRNA of infected trichomonads grown in batch cultures. However, dsRNA profiles of the same infected organisms cultivated under defined continuous-flow conditions contained readily detectable levels of s1 and s2, indicating that amplification of s1 and s2 occurred under specific environmental conditions. These newly discovered dsRNAs were not detected in all of the virus-carrying isolates. Finally, it is noteworthy that the s1 and s2 dsRNAs and the RDRP activity were not detected in trichomonal isolates without virus or in virus-negative progeny derived from virus-positive parental isolates. These data indicate the possibility of variations in the number of dsRNAs and/or of the presence of satellites in trichomonads infected with the multisegmented virus.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderete J. F., Demes P., Gombosová A., Valent M., Yánoska A., Fabusová H., Kasmala L., Garza G. E., Metcalfe E. C. Phenotypes and protein-epitope phenotypic variation among fresh isolates of Trichomonas vaginalis. Infect Immun. 1987 May;55(5):1037–1041. doi: 10.1128/iai.55.5.1037-1041.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Kasmala L., Metcalfe E., Garza G. E. Phenotypic variation and diversity among Trichomonas vaginalis isolates and correlation of phenotype with trichomonal virulence determinants. Infect Immun. 1986 Aug;53(2):285–293. doi: 10.1128/iai.53.2.285-293.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete J. F., Newton E., Dennis C., Neale K. A. The vagina of women infected with Trichomonas vaginalis has numerous proteinases and antibody to trichomonad proteinases. Genitourin Med. 1991 Dec;67(6):469–474. doi: 10.1136/sti.67.6.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Diamond L. S. Techniques of axenic cultivation of Entamoeba histolytica Schaudinn, 1903 and E. histolytica-like amebae. J Parasitol. 1968 Oct;54(5):1047–1056. [PubMed] [Google Scholar]
- Khoshnan A., Alderete J. F. Multiple double-stranded RNA segments are associated with virus particles infecting Trichomonas vaginalis. J Virol. 1993 Dec;67(12):6950–6955. doi: 10.1128/jvi.67.12.6950-6955.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoshnan A., Alderete J. F. Trichomonas vaginalis with a double-stranded RNA virus has upregulated levels of phenotypically variable immunogen mRNA. J Virol. 1994 Jun;68(6):4035–4038. doi: 10.1128/jvi.68.6.4035-4038.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehker M. W., Alderete J. F. Properties of Trichomonas vaginalis grown under chemostat controlled growth conditions. Genitourin Med. 1990 Jun;66(3):193–199. doi: 10.1136/sti.66.3.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y., Fishel R., Wickner R. B. Circular single-stranded RNA replicon in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7628–7632. doi: 10.1073/pnas.87.19.7628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson J. L. Viruses of protozoan parasites. Exp Parasitol. 1990 Jan;70(1):111–113. doi: 10.1016/0014-4894(90)90091-p. [DOI] [PubMed] [Google Scholar]
- Roditi I., Wyler T., Smith N., Braun R. Virus-like particles in Eimeria nieschulzi are associated with multiple RNA segments. Mol Biochem Parasitol. 1994 Feb;63(2):275–282. doi: 10.1016/0166-6851(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Cousiño N., Esteban L. M., Esteban R. Molecular cloning and characterization of W double-stranded RNA, a linear molecule present in Saccharomyces cerevisiae. Identification of its single-stranded RNA form as 20 S RNA. J Biol Chem. 1991 Jul 5;266(19):12772–12778. [PubMed] [Google Scholar]
- Stuart K. D., Weeks R., Guilbride L., Myler P. J. Molecular organization of Leishmania RNA virus 1. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8596–8600. doi: 10.1073/pnas.89.18.8596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai J. H., Su H. M., Tsai J., Shaio M. F., Wang C. C. The divergence of Trichomonas vaginalis virus RNAs among various isolates of Trichomonas vaginalis. Exp Parasitol. 1993 May;76(3):278–286. doi: 10.1006/expr.1993.1033. [DOI] [PubMed] [Google Scholar]
- Tipper D. J., Schmitt M. J. Yeast dsRNA viruses: replication and killer phenotypes. Mol Microbiol. 1991 Oct;5(10):2331–2338. doi: 10.1111/j.1365-2958.1991.tb02078.x. [DOI] [PubMed] [Google Scholar]
- Wang A. L., Wang C. C. A linear double-stranded RNA in Trichomonas vaginalis. J Biol Chem. 1985 Mar 25;260(6):3697–3702. [PubMed] [Google Scholar]
- Wang A. L., Wang C. C. The double-stranded RNA in Trichomonas vaginalis may originate from virus-like particles. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7956–7960. doi: 10.1073/pnas.83.20.7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. L., Wang C. C. Viruses of the protozoa. Annu Rev Microbiol. 1991;45:251–263. doi: 10.1146/annurev.mi.45.100191.001343. [DOI] [PubMed] [Google Scholar]
- Wang A. L., Yang H. M., Shen K. A., Wang C. C. Giardiavirus double-stranded RNA genome encodes a capsid polypeptide and a gag-pol-like fusion protein by a translation frameshift. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8595–8599. doi: 10.1073/pnas.90.18.8595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A., Wang C. C., Alderete J. F. Trichomonas vaginalis phenotypic variation occurs only among trichomonads infected with the double-stranded RNA virus. J Exp Med. 1987 Jul 1;166(1):142–150. doi: 10.1084/jem.166.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks R. S., Patterson J. L., Stuart K., Widmer G. Transcribing and replicating particles in a double-stranded RNA virus from Leishmania. Mol Biochem Parasitol. 1992 Jun;52(2):207–213. doi: 10.1016/0166-6851(92)90053-m. [DOI] [PubMed] [Google Scholar]
- Weeks R., Aline R. F., Jr, Myler P. J., Stuart K. LRV1 viral particles in Leishmania guyanensis contain double-stranded or single-stranded RNA. J Virol. 1992 Mar;66(3):1389–1393. doi: 10.1128/jvi.66.3.1389-1393.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesolowski M., Wickner R. B. Two new double-stranded RNA molecules showing non-mendelian inheritance and heat inducibility in Saccharomyces cerevisiae. Mol Cell Biol. 1984 Jan;4(1):181–187. doi: 10.1128/mcb.4.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T. C., Wang C. C. RNA dependent RNA polymerase activity associated with the double-stranded RNA virus of Giardia lamblia. Nucleic Acids Res. 1990 Feb 11;18(3):553–559. doi: 10.1093/nar/18.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner R. B. Double-stranded and single-stranded RNA viruses of Saccharomyces cerevisiae. Annu Rev Microbiol. 1992;46:347–375. doi: 10.1146/annurev.mi.46.100192.002023. [DOI] [PubMed] [Google Scholar]
- Widmer G., Keenan M. C., Patterson J. L. RNA polymerase activity is associated with viral particles isolated from Leishmania braziliensis subsp. guyanensis. J Virol. 1990 Aug;64(8):3712–3715. doi: 10.1128/jvi.64.8.3712-3715.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]