Abstract
Using as substrates a series of chimeric proteins containing various fragments of the hepatitis C virus precursor polyprotein between Escherichia coli maltose binding protein and dihydrofolate reductase, we analyzed the substrate requirements of hepatitis C viral serine proteinase (Cpro-2) for intermolecular polypeptide cleavage in E. coli. Cpro-2-dependent substrate cleavage was observed in E. coli cells simultaneously transformed with expression plasmids for the Cpro-2 molecule and substrate protein. The cleavage sites were estimated by determining the amino (N)-terminal amino acid sequences of dihydrofolate reductase-fused processed products purified partially by affinity chromatography from the lysates, indicating that cleavage occurred at sites identical to those observed in eukaryotic cells. Mutation analysis using the chimeric substrate indicated that the presence of cysteine and small uncharged residues at positions P1 and P1', respectively, of the putative cleavage site is necessary for cleavage and that acidic residues in the region upstream of the cleavage site are required for efficient cleavage.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashorn P., McQuade T. J., Thaisrivongs S., Tomasselli A. G., Tarpley W. G., Moss B. An inhibitor of the protease blocks maturation of human and simian immunodeficiency viruses and spread of infection. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7472–7476. doi: 10.1073/pnas.87.19.7472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartenschlager R., Ahlborn-Laake L., Mous J., Jacobsen H. Nonstructural protein 3 of the hepatitis C virus encodes a serine-type proteinase required for cleavage at the NS3/4 and NS4/5 junctions. J Virol. 1993 Jul;67(7):3835–3844. doi: 10.1128/jvi.67.7.3835-3844.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo Q. L., Kuo G., Weiner A. J., Overby L. R., Bradley D. W., Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989 Apr 21;244(4902):359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- Eckart M. R., Selby M., Masiarz F., Lee C., Berger K., Crawford K., Kuo C., Kuo G., Houghton M., Choo Q. L. The hepatitis C virus encodes a serine protease involved in processing of the putative nonstructural proteins from the viral polyprotein precursor. Biochem Biophys Res Commun. 1993 Apr 30;192(2):399–406. doi: 10.1006/bbrc.1993.1429. [DOI] [PubMed] [Google Scholar]
- Failla C., Tomei L., De Francesco R. Both NS3 and NS4A are required for proteolytic processing of hepatitis C virus nonstructural proteins. J Virol. 1994 Jun;68(6):3753–3760. doi: 10.1128/jvi.68.6.3753-3760.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grakoui A., McCourt D. W., Wychowski C., Feinstone S. M., Rice C. M. Characterization of the hepatitis C virus-encoded serine proteinase: determination of proteinase-dependent polyprotein cleavage sites. J Virol. 1993 May;67(5):2832–2843. doi: 10.1128/jvi.67.5.2832-2843.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grakoui A., Wychowski C., Lin C., Feinstone S. M., Rice C. M. Expression and identification of hepatitis C virus polyprotein cleavage products. J Virol. 1993 Mar;67(3):1385–1395. doi: 10.1128/jvi.67.3.1385-1395.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijikata M., Kato N., Ootsuyama Y., Nakagawa M., Shimotohno K. Gene mapping of the putative structural region of the hepatitis C virus genome by in vitro processing analysis. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5547–5551. doi: 10.1073/pnas.88.13.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
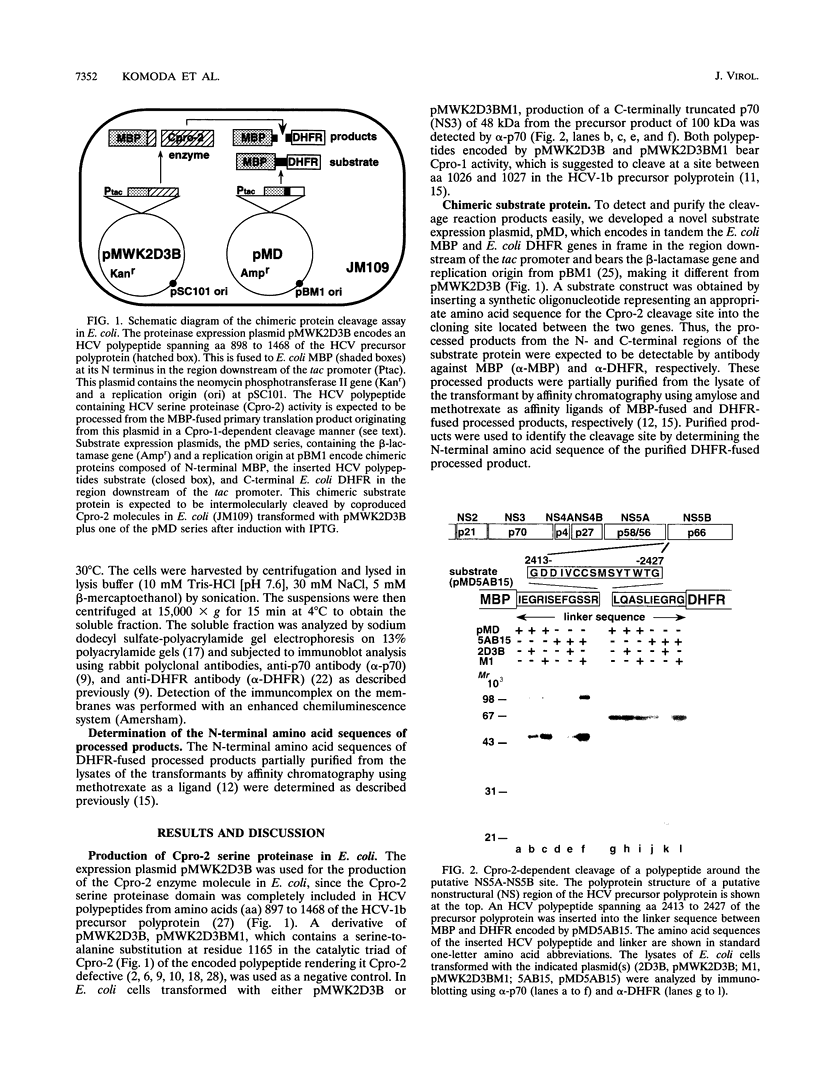
- Hijikata M., Mizushima H., Akagi T., Mori S., Kakiuchi N., Kato N., Tanaka T., Kimura K., Shimotohno K. Two distinct proteinase activities required for the processing of a putative nonstructural precursor protein of hepatitis C virus. J Virol. 1993 Aug;67(8):4665–4675. doi: 10.1128/jvi.67.8.4665-4675.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijikata M., Mizushima H., Tanji Y., Komoda Y., Hirowatari Y., Akagi T., Kato N., Kimura K., Shimotohno K. Proteolytic processing and membrane association of putative nonstructural proteins of hepatitis C virus. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10773–10777. doi: 10.1073/pnas.90.22.10773. [DOI] [PMC free article] [PubMed] [Google Scholar]
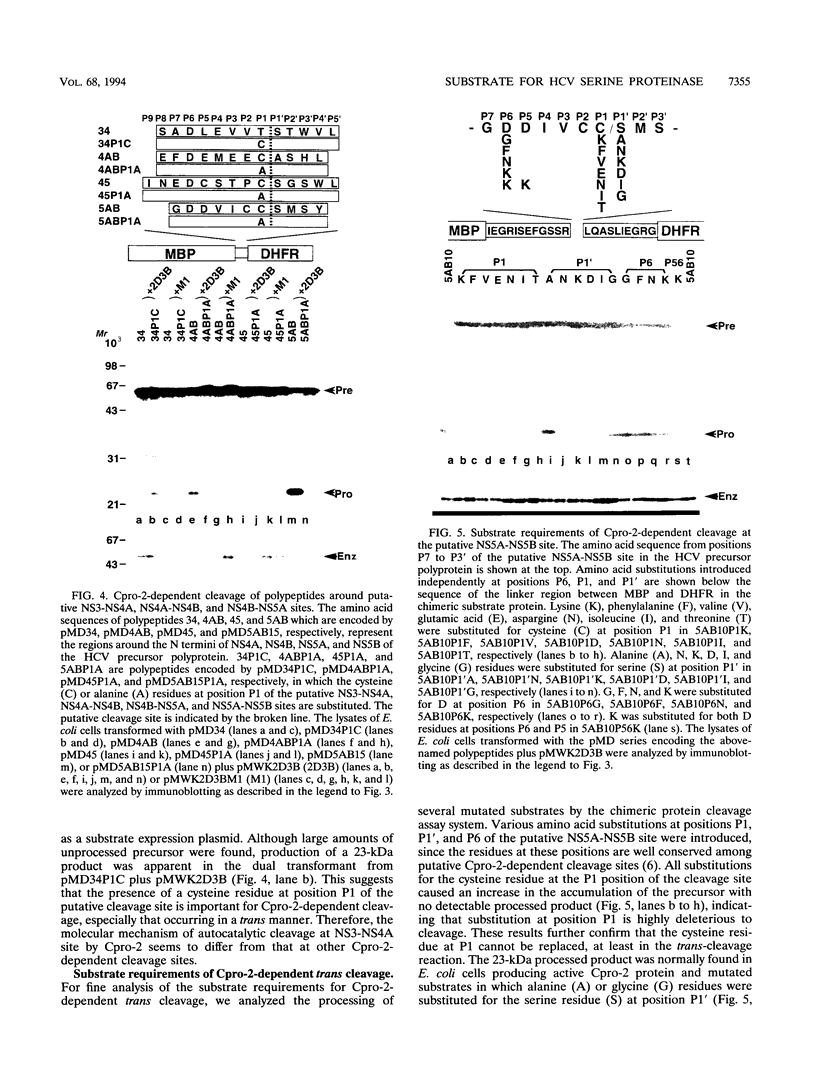
- Hirowatari Y., Hijikata M., Tanji Y., Nyunoya H., Mizushima H., Kimura K., Tanaka T., Kato N., Shimotohno K. Two proteinase activities in HCV polypeptide expressed in insect cells using baculovirus vector. Arch Virol. 1993;133(3-4):349–356. doi: 10.1007/BF01313774. [DOI] [PubMed] [Google Scholar]
- Iwakura M., Furusawa K., Kokubu T., Ohashi S., Tanaka Y., Shimura Y., Tsuda K. Dihydrofolate reductase as a new "affinity handle". J Biochem. 1992 Jan;111(1):37–45. doi: 10.1093/oxfordjournals.jbchem.a123715. [DOI] [PubMed] [Google Scholar]
- Iwakura M., Tanaka T. Dihydrofolate reductase gene as a versatile expression marker. J Biochem. 1992 Jan;111(1):31–36. doi: 10.1093/oxfordjournals.jbchem.a123714. [DOI] [PubMed] [Google Scholar]
- Kato N., Hijikata M., Nakagawa M., Ootsuyama Y., Muraiso K., Ohkoshi S., Shimotohno K. Molecular structure of the Japanese hepatitis C viral genome. FEBS Lett. 1991 Mar 25;280(2):325–328. doi: 10.1016/0014-5793(91)80322-t. [DOI] [PubMed] [Google Scholar]
- Komoda Y., Hijikata M., Tanji Y., Hirowatari Y., Mizushima H., Kimura K., Shimotohno K. Processing of hepatitis C viral polyprotein in Escherichia coli. Gene. 1994 Aug 5;145(2):221–226. doi: 10.1016/0378-1119(94)90009-4. [DOI] [PubMed] [Google Scholar]
- Kuo G., Choo Q. L., Alter H. J., Gitnick G. L., Redeker A. G., Purcell R. H., Miyamura T., Dienstag J. L., Alter M. J., Stevens C. E. An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science. 1989 Apr 21;244(4902):362–364. doi: 10.1126/science.2496467. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Manabe S., Fuke I., Tanishita O., Kaji C., Gomi Y., Yoshida S., Mori C., Takamizawa A., Yosida I., Okayama H. Production of nonstructural proteins of hepatitis C virus requires a putative viral protease encoded by NS3. Virology. 1994 Feb;198(2):636–644. doi: 10.1006/viro.1994.1075. [DOI] [PubMed] [Google Scholar]
- Meek T. D., Lambert D. M., Dreyer G. B., Carr T. J., Tomaszek T. A., Jr, Moore M. L., Strickler J. E., Debouck C., Hyland L. J., Matthews T. J. Inhibition of HIV-1 protease in infected T-lymphocytes by synthetic peptide analogues. Nature. 1990 Jan 4;343(6253):90–92. doi: 10.1038/343090a0. [DOI] [PubMed] [Google Scholar]
- Mizushima H., Hijikata M., Tanji Y., Kimura K., Shimotohno K. Analysis of N-terminal processing of hepatitis C virus nonstructural protein 2. J Virol. 1994 Apr;68(4):2731–2734. doi: 10.1128/jvi.68.4.2731-2734.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkoshi S., Kojima H., Tawaraya H., Miyajima T., Kamimura T., Asakura H., Satoh A., Hirose S., Hijikata M., Kato N. Prevalence of antibody against non-A, non-B hepatitis virus in Japanese patients with hepatocellular carcinoma. Jpn J Cancer Res. 1990 Jun-Jul;81(6-7):550–553. doi: 10.1111/j.1349-7006.1990.tb02605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osumi T., Tsukamoto T., Hata S., Yokota S., Miura S., Fujiki Y., Hijikata M., Miyazawa S., Hashimoto T. Amino-terminal presequence of the precursor of peroxisomal 3-ketoacyl-CoA thiolase is a cleavable signal peptide for peroxisomal targeting. Biochem Biophys Res Commun. 1991 Dec 31;181(3):947–954. doi: 10.1016/0006-291x(91)92028-i. [DOI] [PubMed] [Google Scholar]
- Roberts N. A., Martin J. A., Kinchington D., Broadhurst A. V., Craig J. C., Duncan I. B., Galpin S. A., Handa B. K., Kay J., Kröhn A. Rational design of peptide-based HIV proteinase inhibitors. Science. 1990 Apr 20;248(4953):358–361. doi: 10.1126/science.2183354. [DOI] [PubMed] [Google Scholar]
- Saito I., Miyamura T., Ohbayashi A., Harada H., Katayama T., Kikuchi S., Watanabe Y., Koi S., Onji M., Ohta Y. Hepatitis C virus infection is associated with the development of hepatocellular carcinoma. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6547–6549. doi: 10.1073/pnas.87.17.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selby M. J., Choo Q. L., Berger K., Kuo G., Glazer E., Eckart M., Lee C., Chien D., Kuo C., Houghton M. Expression, identification and subcellular localization of the proteins encoded by the hepatitis C viral genome. J Gen Virol. 1993 Jun;74(Pt 6):1103–1113. doi: 10.1099/0022-1317-74-6-1103. [DOI] [PubMed] [Google Scholar]
- Tanji Y., Hijikata M., Hirowatari Y., Shimotohno K. Identification of the domain required for trans-cleavage activity of hepatitis C viral serine proteinase. Gene. 1994 Aug 5;145(2):215–219. doi: 10.1016/0378-1119(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Tomei L., Failla C., Santolini E., De Francesco R., La Monica N. NS3 is a serine protease required for processing of hepatitis C virus polyprotein. J Virol. 1993 Jul;67(7):4017–4026. doi: 10.1128/jvi.67.7.4017-4026.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]