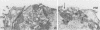Abstract
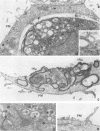
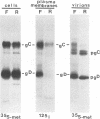
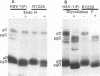
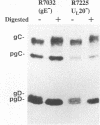
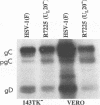
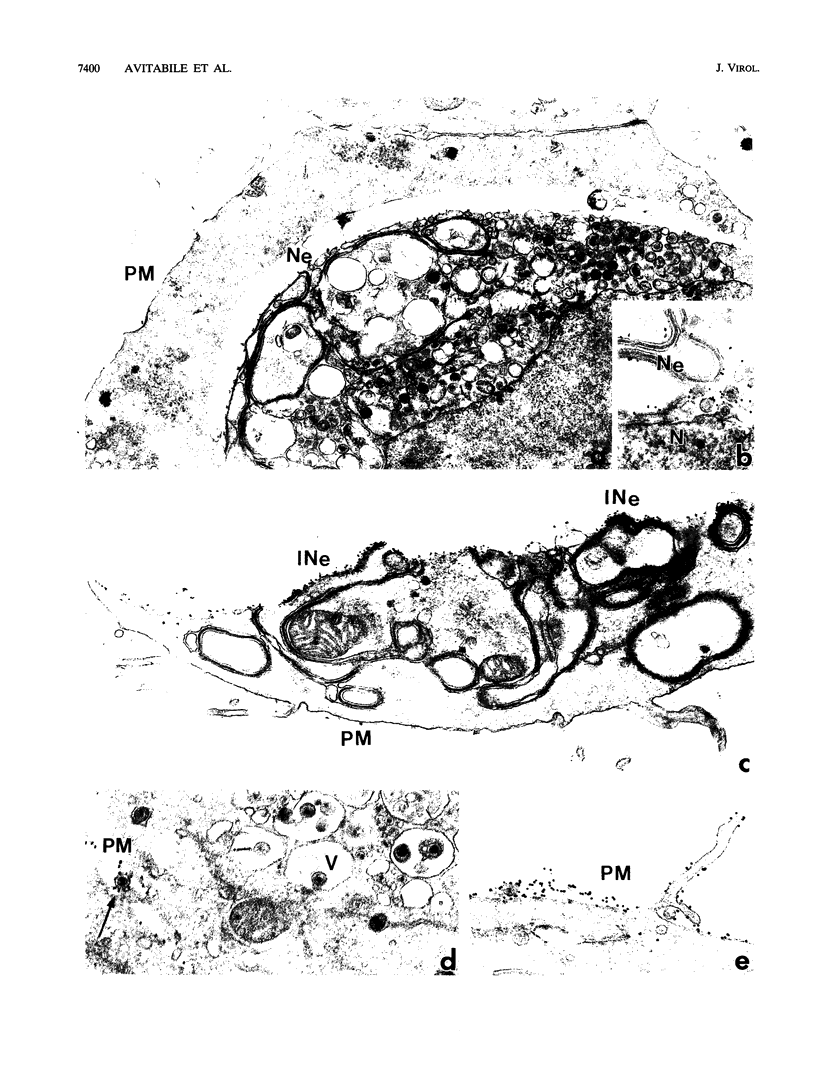
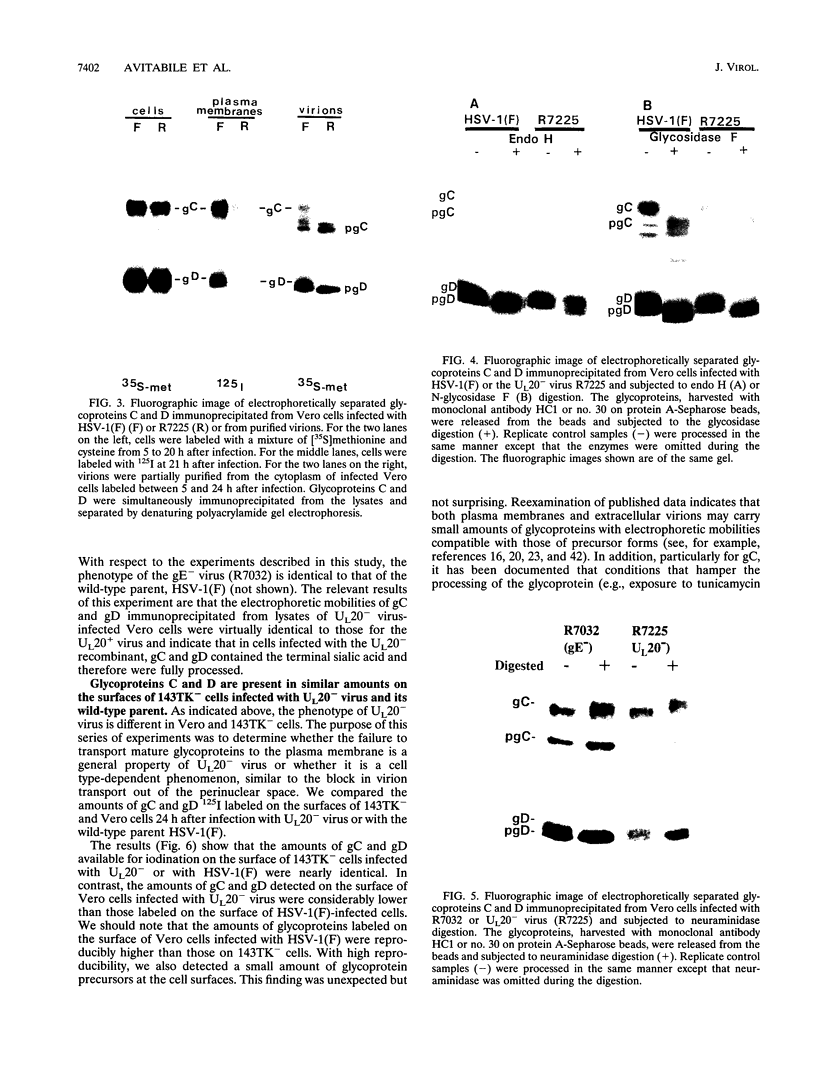
The Golgi apparatus is fragmented and dispersed in Vero cells but not in human 143TK- cells infected with wild-type herpes simplex virus 1. Moreover, a recombinant virus lacking the gene encoding the membrane protein UL20 (UL20- virus) accumulates in the space between the inner and outer nuclear membranes of Vero cells but is exported and spreads from cell to cell in 143TK- cell cultures. Here we report that in Vero cells infected with UL20- virus, the virion envelope glycoproteins were of the immature type, whereas the viral glycoproteins associated with cell membranes were fully processed up to the addition of sialic acid, a trans-Golgi function. Moreover, the amounts of viral glycoproteins accumulating in the plasma membranes were considerably smaller than those detected on the surface of Vero cells infected with wild-type virus. In contrast, the amounts of viral glycoproteins present on the plasma membranes of 143TK- cells infected with wild-type or UL20- virus were nearly identical. We conclude that (i) in Vero cells infected with UL20- virus the block in the export of virions is at the entry into the exocytic pathway, and a second block in the exocytosis of viral glycoproteins associated with cytoplasmic membranes is due to an impairment of transport beyond Golgi fragments containing trans-Golgi enzymes and not to a failure of the Golgi oligosaccharide-processing functions; (ii) these defects are manifested in cells in which the Golgi apparatus is fragmented; and (iii) the UL20 protein compensates for these defects by enabling transport to and from the fragmented Golgi apparatus.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baines J. D., Ward P. L., Campadelli-Fiume G., Roizman B. The UL20 gene of herpes simplex virus 1 encodes a function necessary for viral egress. J Virol. 1991 Dec;65(12):6414–6424. doi: 10.1128/jvi.65.12.6414-6424.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campadelli-Fiume G., Lombardo M. T., Foà-Tomasi L., Avitabile E., Serafini-Cessi F. Individual herpes simplex virus 1 glycoproteins display characteristic rates of maturation from precursor to mature form both in infected cells and in cells that constitutively express the glycoproteins. Virus Res. 1988 Apr;10(1):29–40. doi: 10.1016/0168-1702(88)90055-x. [DOI] [PubMed] [Google Scholar]
- Campadelli G., Brandimarti R., Di Lazzaro C., Ward P. L., Roizman B., Torrisi M. R. Fragmentation and dispersal of Golgi proteins and redistribution of glycoproteins and glycolipids processed through the Golgi apparatus after infection with herpes simplex virus 1. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2798–2802. doi: 10.1073/pnas.90.7.2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S., Sarkar S. Studies on endoplasmic reticulum--Golgi complex cycling pathway in herpes simplex virus-infected and brefeldin A-treated human fibroblast cells. Virology. 1992 Nov;191(1):327–337. doi: 10.1016/0042-6822(92)90195-u. [DOI] [PubMed] [Google Scholar]
- Cohen G. H., Dietzschold B., Ponce de Leon M., Long D., Golub E., Varrichio A., Pereira L., Eisenberg R. J. Localization and synthesis of an antigenic determinant of herpes simplex virus glycoprotein D that stimulates the production of neutralizing antibody. J Virol. 1984 Jan;49(1):102–108. doi: 10.1128/jvi.49.1.102-108.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall'Olio F., Malagolini N., Speziali V., Campadelli-Fiume G., Serafini-Cessi F. Sialylated oligosaccharides O-glycosidically linked to glycoprotein C from herpes simplex virus type 1. J Virol. 1985 Oct;56(1):127–134. doi: 10.1128/jvi.56.1.127-134.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai P. J., Schaffer P. A., Minson A. C. Excretion of non-infectious virus particles lacking glycoprotein H by a temperature-sensitive mutant of herpes simplex virus type 1: evidence that gH is essential for virion infectivity. J Gen Virol. 1988 Jun;69(Pt 6):1147–1156. doi: 10.1099/0022-1317-69-6-1147. [DOI] [PubMed] [Google Scholar]
- Eisenberg R. J., Hydrean-Stern C., Cohen G. H. Structural analysis of precursor and product forms of type-common envelope glycoprotein D (CP-1 antigen) of herpes simplex virus type 1. J Virol. 1979 Sep;31(3):608–620. doi: 10.1128/jvi.31.3.608-620.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejercito P. M., Kieff E. D., Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J Gen Virol. 1968 May;2(3):357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- Foà-Tomasi L., Avitabile E., Boscaro A., Brandimarti R., Gualandri R., Manservigi R., Dall'Olio F., Serafini-Cessi F., Fiume G. C. Herpes simplex virus (HSV) glycoprotein H is partially processed in a cell line that expresses the glycoprotein and fully processed in cells infected with deletion or ts mutants in the known HSV glycoproteins. Virology. 1991 Feb;180(2):474–482. doi: 10.1016/0042-6822(91)90061-f. [DOI] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G., Andersson L. C. Role of sialic acid in the mobility of membrane proteins containing O-linked oligosaccharides on polyacrylamide gel electrophoresis in sodium dodecyl sulfate. Eur J Biochem. 1982 Mar 1;122(3):581–586. doi: 10.1111/j.1432-1033.1982.tb06478.x. [DOI] [PubMed] [Google Scholar]
- Gahmberg N., Pettersson R. F., Käriäinen L. Efficient transport of Semliki Forest virus glycoproteins through a Golgi complex morphologically altered by Uukuniemi virus glycoproteins. EMBO J. 1986 Dec 1;5(12):3111–3118. doi: 10.1002/j.1460-2075.1986.tb04617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glorioso J., Szczesiul M. S., Marlin S. D., Levine M. Inhibition of glycosylation of herpes simplex virus glycoproteins: identification of antigenic and immunogenic partially glycosylated glycopeptides on the cell surface membrane. Virology. 1983 Apr 15;126(1):1–18. doi: 10.1016/0042-6822(83)90458-0. [DOI] [PubMed] [Google Scholar]
- Gompels U. A., Minson A. C. Antigenic properties and cellular localization of herpes simplex virus glycoprotein H synthesized in a mammalian cell expression system. J Virol. 1989 Nov;63(11):4744–4755. doi: 10.1128/jvi.63.11.4744-4755.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Proteins specified by herpes simplex virus. XIII. Glycosylation of viral polypeptides. J Virol. 1975 Nov;16(5):1308–1326. doi: 10.1128/jvi.16.5.1308-1326.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasty V., Chang P. W. Release of infectious bovine rhinotracheitis virus from productively infected bovine kidney cells: an electron microscopic study. J Ultrastruct Res. 1972 Mar;38(5):433–443. doi: 10.1016/0022-5320(72)90081-0. [DOI] [PubMed] [Google Scholar]
- Johnson D. C., Smiley J. R. Intracellular transport of herpes simplex virus gD occurs more rapidly in uninfected cells than in infected cells. J Virol. 1985 Jun;54(3):682–689. doi: 10.1128/jvi.54.3.682-689.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean C. A., Efstathiou S., Elliott M. L., Jamieson F. E., McGeoch D. J. Investigation of herpes simplex virus type 1 genes encoding multiply inserted membrane proteins. J Gen Virol. 1991 Apr;72(Pt 4):897–906. doi: 10.1099/0022-1317-72-4-897. [DOI] [PubMed] [Google Scholar]
- McKenzie R. C., Epand R. M., Johnson D. C. Cyclosporine A inhibits herpes simplex virus-induced cell fusion but not virus penetration into cells. Virology. 1987 Jul;159(1):1–9. doi: 10.1016/0042-6822(87)90341-2. [DOI] [PubMed] [Google Scholar]
- Meignier B., Longnecker R., Mavromara-Nazos P., Sears A. E., Roizman B. Virulence of and establishment of latency by genetically engineered deletion mutants of herpes simplex virus 1. Virology. 1988 Jan;162(1):251–254. doi: 10.1016/0042-6822(88)90417-5. [DOI] [PubMed] [Google Scholar]
- Morgan A. J., Smith A. R., Barker R. N., Epstein M. A. A structural investigation of the Epstein-Barr (EB) virus membrane antigen glycoprotein, gp340. J Gen Virol. 1984 Feb;65(Pt 2):397–404. doi: 10.1099/0022-1317-65-2-397. [DOI] [PubMed] [Google Scholar]
- Newport J. W., Forbes D. J. The nucleus: structure, function, and dynamics. Annu Rev Biochem. 1987;56:535–565. doi: 10.1146/annurev.bi.56.070187.002535. [DOI] [PubMed] [Google Scholar]
- Norrild B., Pedersen B. Effect of tunicamycin on the synthesis of herpes simplex virus type 1 glycoproteins and their expression on the cell surface. J Virol. 1982 Aug;43(2):395–402. doi: 10.1128/jvi.43.2.395-402.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L., Klassen T., Baringer J. R. Type-common and type-specific monoclonal antibody to herpes simplex virus type 1. Infect Immun. 1980 Aug;29(2):724–732. doi: 10.1128/iai.29.2.724-732.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry L. J., McGeoch D. J. The DNA sequences of the long repeat region and adjoining parts of the long unique region in the genome of herpes simplex virus type 1. J Gen Virol. 1988 Nov;69(Pt 11):2831–2846. doi: 10.1099/0022-1317-69-11-2831. [DOI] [PubMed] [Google Scholar]
- Pinto da Silva P., Torrisi M. R., Kachar B. Freeze-fracture cytochemistry: localization of wheat-germ agglutinin and concanavalin A binding sites on freeze-fractured pancreatic cells. J Cell Biol. 1981 Nov;91(2 Pt 1):361–372. doi: 10.1083/jcb.91.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puddington L., Lively M. O., Lyles D. S. Role of the nuclear envelope in synthesis, processing, and transport of membrane glycoproteins. J Biol Chem. 1985 May 10;260(9):5641–5647. [PubMed] [Google Scholar]
- Roberts S. R., Ponce de Leon M., Cohen G. H., Eisenberg R. J. Analysis of the intracellular maturation of the herpes simplex virus type 1 glycoprotein gH in infected and transfected cells. Virology. 1991 Oct;184(2):609–624. doi: 10.1016/0042-6822(91)90431-a. [DOI] [PubMed] [Google Scholar]
- Rosenthal K. L., Smiley J. R., South S., Johnson D. C. Cells expressing herpes simplex virus glycoprotein gC but not gB, gD, or gE are recognized by murine virus-specific cytotoxic T lymphocytes. J Virol. 1987 Aug;61(8):2438–2447. doi: 10.1128/jvi.61.8.2438-2447.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J., Roizman B. Concerning the egress of herpes simplex virus from infected cells: electron and light microscope observations. Virology. 1969 May;38(1):42–49. doi: 10.1016/0042-6822(69)90126-3. [DOI] [PubMed] [Google Scholar]
- Seidel-Dugan C., Ponce de Leon M., Friedman H. M., Fries L. F., Frank M. M., Cohen G. H., Eisenberg R. J. C3b receptor activity on transfected cells expressing glycoprotein C of herpes simplex virus types 1 and 2. J Virol. 1988 Nov;62(11):4027–4036. doi: 10.1128/jvi.62.11.4027-4036.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini-Cessi F., Dall'Olio F., Malagolini N., Pereira L., Campadelli-Fiume G. Comparative study on O-linked oligosaccharides of glycoprotein D of herpes simplex virus types 1 and 2. J Gen Virol. 1988 Apr;69(Pt 4):869–877. doi: 10.1099/0022-1317-69-4-869. [DOI] [PubMed] [Google Scholar]
- Serafini-Cessi F., Dall'Olio F., Pereira L., Campadelli-Fiume G. Processing of N-linked oligosaccharides from precursor- to mature-form herpes simplex virus type 1 glycoprotein gC. J Virol. 1984 Sep;51(3):838–844. doi: 10.1128/jvi.51.3.838-844.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini-Cessi F., Malagolini N., Dall'Olio F., Pereira L., Campadelli-Fiume G. Oligosaccharide chains of herpes simplex virus type 2 glycoprotein gG.2. Arch Biochem Biophys. 1985 Aug 1;240(2):866–876. doi: 10.1016/0003-9861(85)90097-9. [DOI] [PubMed] [Google Scholar]
- Sharon N., Lis H. Lectins: cell-agglutinating and sugar-specific proteins. Science. 1972 Sep 15;177(4053):949–959. doi: 10.1126/science.177.4053.949. [DOI] [PubMed] [Google Scholar]
- Spear P. G. Membrane proteins specified by herpes simplex viruses. I. Identification of four glycoprotein precursors and their products in type 1-infected cells. J Virol. 1976 Mar;17(3):991–1008. doi: 10.1128/jvi.17.3.991-1008.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear P. G., Roizman B. Proteins specified by herpes simplex virus. V. Purification and structural proteins of the herpesvirion. J Virol. 1972 Jan;9(1):143–159. doi: 10.1128/jvi.9.1.143-159.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takizawa P. A., Yucel J. K., Veit B., Faulkner D. J., Deerinck T., Soto G., Ellisman M., Malhotra V. Complete vesiculation of Golgi membranes and inhibition of protein transport by a novel sea sponge metabolite, ilimaquinone. Cell. 1993 Jun 18;73(6):1079–1090. doi: 10.1016/0092-8674(93)90638-7. [DOI] [PubMed] [Google Scholar]
- Torrisi M. R., Di Lazzaro C., Pavan A., Pereira L., Campadelli-Fiume G. Herpes simplex virus envelopment and maturation studied by fracture label. J Virol. 1992 Jan;66(1):554–561. doi: 10.1128/jvi.66.1.554-561.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward P. L., Campadelli-Fiume G., Avitabile E., Roizman B. Localization and putative function of the UL20 membrane protein in cells infected with herpes simplex virus 1. J Virol. 1994 Nov;68(11):7406–7417. doi: 10.1128/jvi.68.11.7406-7417.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren G. Membrane partitioning during cell division. Annu Rev Biochem. 1993;62:323–348. doi: 10.1146/annurev.bi.62.070193.001543. [DOI] [PubMed] [Google Scholar]
- Wenske E. A., Bratton M. W., Courtney R. J. Endo-beta-N-acetylglucosaminidase H sensitivity of precursors to herpes simplex virus type 1 glycoproteins gB and gC. J Virol. 1982 Oct;44(1):241–248. doi: 10.1128/jvi.44.1.241-248.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whealy M. E., Card J. P., Meade R. P., Robbins A. K., Enquist L. W. Effect of brefeldin A on alphaherpesvirus membrane protein glycosylation and virus egress. J Virol. 1991 Mar;65(3):1066–1081. doi: 10.1128/jvi.65.3.1066-1081.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]