Abstract
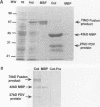
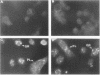
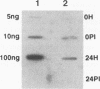
Polydnaviruses suppress the cellular immune response and inhibit growth and development in their lepidopteran host, allowing survival of their endoparasitic hymenopteran host. Characterization of genes disrupting insect physiological systems is a major objective in the study of polydnaviruses. Recently, a cysteine-rich gene family encoding a motif composed of invariable cysteine residues flanking hypervariable intercysteine amino acids was described (S.D. Dib-Hajj, B.A. Webb, and M.D. Summers, Proc. Natl. Acad. Sci. USA 90:3765-3769, 1993). They noted similarities to the positive selection pressure for mutations within the vertebrate major histocompatibility complex (MHC) class II genes and speculated that this class of polydnavirus genes may target and disrupt the insect immune system. To study the functional activity of this family of predicted cysteine-rich proteins, the VHv1.1 gene product was produced from bacterial and baculovirus expression systems. Polyclonal antiserum produced from the bacterial fusion protein reacted with a 30-kDa protein from hemocytes, cell-free plasma, and fat body of parasitized larvae. Immunofluorescence analysis of hemocytes from parasitized insects detected the 30-kDa protein bound to granulocytes and plasmacytes. To assay the functional activity of the 30-kDa VHv1.1 protein, a recombinant baculovirus was constructed allowing in vivo expression of the 30-kDa polydnavirus protein from infected insects. Expression of the VHv1.1 protein from the baculovirus system reduced the encapsulation response to washed wasp eggs relative to controls. The experimental evidence demonstrates that Campoletis sonorensis polydnavirus-infected cells secrete VHv1.1 into the hemolymph, where it binds to hemocytes and is associated with the inhibition of the cellular immune response.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bedwin O. The particulate basis of the resistance of a parasitoid to the defence reactions of its insect host. Proc R Soc Lond B Biol Sci. 1979 Aug 1;205(1159):267–270. doi: 10.1098/rspb.1979.0064. [DOI] [PubMed] [Google Scholar]
- Blissard G. W., Smith O. P., Summers M. D. Two related viral genes are located on a single superhelical DNA segment of the multipartite Campoletis sonorensis virus genome. Virology. 1987 Sep;160(1):120–134. doi: 10.1016/0042-6822(87)90052-3. [DOI] [PubMed] [Google Scholar]
- Blissard G. W., Theilmann D. A., Summers M. D. Segment W of Campoletis sonorensis virus: expression, gene products, and organization. Virology. 1989 Mar;169(1):78–89. doi: 10.1016/0042-6822(89)90043-3. [DOI] [PubMed] [Google Scholar]
- Blissard G. W., Vinson S. B., Summers M. D. Identification, Mapping, and In Vitro Translation of Campoletis sonorensis Virus mRNAs from Parasitized Heliothis virescens Larvae. J Virol. 1986 Jan;57(1):318–327. doi: 10.1128/jvi.57.1.318-327.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontems F., Roumestand C., Gilquin B., Ménez A., Toma F. Refined structure of charybdotoxin: common motifs in scorpion toxins and insect defensins. Science. 1991 Dec 6;254(5037):1521–1523. doi: 10.1126/science.1720574. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Dib-Hajj S. D., Webb B. A., Summers M. D. Structure and evolutionary implications of a "cysteine-rich" Campoletis sonorensis polydnavirus gene family. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3765–3769. doi: 10.1073/pnas.90.8.3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edson K. M., Vinson S. B., Stoltz D. B., Summers M. D. Virus in a parasitoid wasp: suppression of the cellular immune response in the parasitoid's host. Science. 1981 Feb 6;211(4482):582–583. doi: 10.1126/science.7455695. [DOI] [PubMed] [Google Scholar]
- Fleming J. A., Blissard G. W., Summers M. D., Vinson S. B. Expression of Campoletis sonorensis Virus in the Parasitized Host, Heliothis virescens. J Virol. 1983 Oct;48(1):74–78. doi: 10.1128/jvi.48.1.74-78.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming J. A. Polydnaviruses: mutualists and pathogens. Annu Rev Entomol. 1992;37:401–425. doi: 10.1146/annurev.en.37.010192.002153. [DOI] [PubMed] [Google Scholar]
- Fleming J. A., Summers M. D. Campoletis sonorensis Endoparasitic Wasps Contain Forms of C. sonorensis Virus DNA Suggestive of Integrated and Extrachromosomal Polydnavirus DNAs. J Virol. 1986 Feb;57(2):552–562. doi: 10.1128/jvi.57.2.552-562.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa Y. Cellular immunosuppressive protein in the plasma of parasitized insect larvae. J Biol Chem. 1994 May 20;269(20):14536–14540. [PubMed] [Google Scholar]
- Huebers H. A., Huebers E., Finch C. A., Webb B. A., Truman J. W., Riddiford L. M., Martin A. W., Massover W. H. Iron binding proteins and their roles in the tobacco hornworm, Manduca sexta (L.). J Comp Physiol B. 1988;158(3):291–300. doi: 10.1007/BF00695327. [DOI] [PubMed] [Google Scholar]
- Kitano Y., Okada N., Adachi J. TPA-induced alteration of actin organization in cultured human keratinocytes. Exp Cell Res. 1986 Dec;167(2):369–375. doi: 10.1016/0014-4827(86)90177-1. [DOI] [PubMed] [Google Scholar]
- Krell P. J., Summers M. D., Vinson S. B. Virus with a Multipartite Superhelical DNA Genome from the Ichneumonid Parasitoid Campoletis sonorensis. J Virol. 1982 Sep;43(3):859–870. doi: 10.1128/jvi.43.3.859-870.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivera B. M., Rivier J., Clark C., Ramilo C. A., Corpuz G. P., Abogadie F. C., Mena E. E., Woodward S. R., Hillyard D. R., Cruz L. J. Diversity of Conus neuropeptides. Science. 1990 Jul 20;249(4966):257–263. doi: 10.1126/science.2165278. [DOI] [PubMed] [Google Scholar]
- Rizki R. M., Rizki T. M. Effects of lamellolysin from a parasitoid wasp on Drosophila blood cells in vitro. J Exp Zool. 1991 Feb;257(2):236–244. doi: 10.1002/jez.1402570214. [DOI] [PubMed] [Google Scholar]
- Stoltz D. B. Evidence for chromosomal transmission of polydnavirus DNA. J Gen Virol. 1990 May;71(Pt 5):1051–1056. doi: 10.1099/0022-1317-71-5-1051. [DOI] [PubMed] [Google Scholar]
- Stoltz D. B., Krell P., Summers M. D., Vinson S. B. Polydnaviridae - a proposed family of insect viruses with segmented, double-stranded, circular DNA genomes. Intervirology. 1984;21(1):1–4. doi: 10.1159/000149497. [DOI] [PubMed] [Google Scholar]
- Stoltz D. B., Vinson S. B. Viruses and parasitism in insects. Adv Virus Res. 1979;24:125–171. doi: 10.1016/s0065-3527(08)60393-0. [DOI] [PubMed] [Google Scholar]
- Theilmann D. A., Summers M. D. Molecular analysis of Campoletis sonorensis virus DNA in the lepidopteran host Heliothis virescens. J Gen Virol. 1986 Sep;67(Pt 9):1961–1969. doi: 10.1099/0022-1317-67-9-1961. [DOI] [PubMed] [Google Scholar]
- Webb B. A., Luckhart S. Evidence for an early immunosuppressive role for related Campoletis sonorensis venom and ovarian proteins in Heliothis virescens. Arch Insect Biochem Physiol. 1994;26(2-3):147–163. doi: 10.1002/arch.940260208. [DOI] [PubMed] [Google Scholar]
- Webb B. A., Riddiford L. M. Regulation of expression of arylphorin and female-specific protein mRNAs in the tobacco hornworm, Manduca sexta. Dev Biol. 1988 Dec;130(2):682–692. doi: 10.1016/0012-1606(88)90360-0. [DOI] [PubMed] [Google Scholar]
- Webb B. A., Riddiford L. M. Synthesis of two storage proteins during larval development of the tobacco hornworm, Manduca sexta. Dev Biol. 1988 Dec;130(2):671–681. doi: 10.1016/0012-1606(88)90359-4. [DOI] [PubMed] [Google Scholar]
- Webb B. A., Summers M. D. Venom and viral expression products of the endoparasitic wasp Campoletis sonorensis share epitopes and related sequences. Proc Natl Acad Sci U S A. 1990 Jul;87(13):4961–4965. doi: 10.1073/pnas.87.13.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D. M., Stoltz D. Evidence for a chromosomal location of polydnavirus DNA in the ichneumonid parasitoid Hyposoter fugitivus. J Virol. 1991 Dec;65(12):6693–6704. doi: 10.1128/jvi.65.12.6693-6704.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]