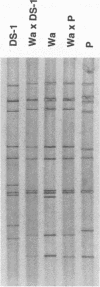
Abstract
Group A human rotavirus strains D, Wa, DS-1, and P were originally recovered from children with diarrhea. In an attempt to attenuate virulent, wild-type human rotaviruses of major epidemiological importance for use in a live oral vaccine, two reference rotavirus strains, D and DS-1, and two laboratory-generated reassortants, Wa x DS-1 and Wa x P, were subjected to cold adaptation. Collectively, these viruses provide antigenic coverage for both of the clinically important rotavirus VP4 antigens and three of the four important rotavirus VP7 antigens. Mutants of each of these rotaviruses were selected during successive serial passage in primary African green monkey kidney cells at progressively lower suboptimal temperatures (30, 28, and 26 degrees C). The genotype of each mutant appeared to be indistinguishable from that of its wild-type, parental virus. The mutants recovered after 10 serial passages at 30 degrees C exhibited both temperature sensitivity of plaque formation (i.e., a ts phenotype) and the ability to form plaques efficiently at suboptimal temperature (i.e., a cold adaptation [ca] phenotype), in contrast to parental wild-type rotavirus. The succeeding set of 10 serial passages at 28 degrees C selected mutants that exhibited an increased degree of cold adaptation, and three of the mutants exhibited an associated increase in temperature sensitivity. Finally, in the case of three of the strains, the third successive serial passage series, which was performed at 26 degrees C, selected for mutants with an even greater degree of cold adaptation than the previous series and was associated with greater temperature sensitivity in one instance. It appeared that each of the viruses sustained a minimum of four to five mutations during the total selection procedure. The ultimate identification of candidate vaccine viruses that exhibit the desired level of attenuation, immunogenicity, and protective efficacy needed for immunoprophylaxis will require evaluation of these mutants in susceptible humans.
Full text
PDF
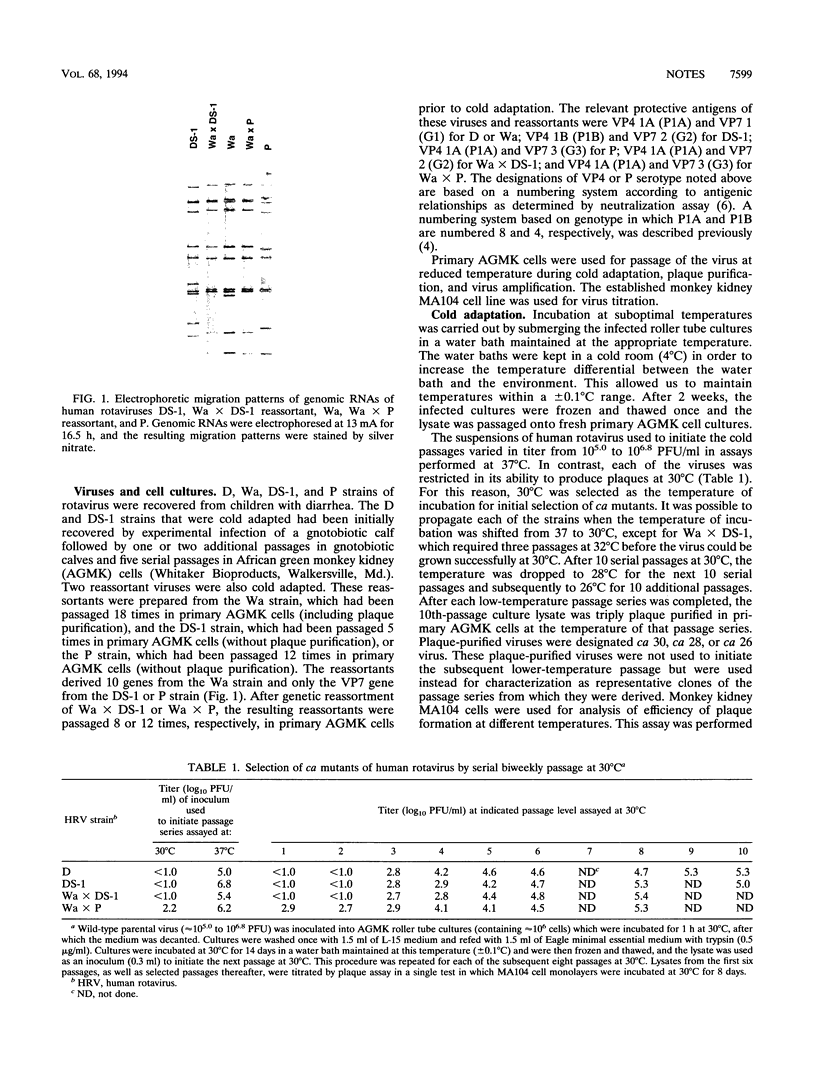


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernstein D. I., Smith V. E., Sander D. S., Pax K. A., Schiff G. M., Ward R. L. Evaluation of WC3 rotavirus vaccine and correlates of protection in healthy infants. J Infect Dis. 1990 Nov;162(5):1055–1062. doi: 10.1093/infdis/162.5.1055. [DOI] [PubMed] [Google Scholar]
- Clark H. F., Offit P. A., Dolan K. T., Tezza A., Gogalin K., Twist E. M., Plotkin S. A. Response of adult human volunteers to oral administration of bovine and bovine/human reassortant rotaviruses. Vaccine. 1986 Mar;4(1):25–31. doi: 10.1016/0264-410x(86)90094-0. [DOI] [PubMed] [Google Scholar]
- Estes M. K., Cohen J. Rotavirus gene structure and function. Microbiol Rev. 1989 Dec;53(4):410–449. doi: 10.1128/mr.53.4.410-449.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores J., Perez-Schael I., Blanco M., Vilar M., Garcia D., Perez M., Daoud N., Midthun K., Kapikian A. Z. Reactions to and antigenicity of two human-rhesus rotavirus reassortant vaccine candidates of serotypes 1 and 2 in Venezuelan infants. J Clin Microbiol. 1989 Mar;27(3):512–518. doi: 10.1128/jcm.27.3.512-518.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorziglia M., Larralde G., Kapikian A. Z., Chanock R. M. Antigenic relationships among human rotaviruses as determined by outer capsid protein VP4. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7155–7159. doi: 10.1073/pnas.87.18.7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green K. Y., Taniguchi K., Mackow E. R., Kapikian A. Z. Homotypic and heterotypic epitope-specific antibody responses in adult and infant rotavirus vaccinees: implications for vaccine development. J Infect Dis. 1990 Apr;161(4):667–679. doi: 10.1093/infdis/161.4.667. [DOI] [PubMed] [Google Scholar]
- Greenberg H. B., Valdesuso J., van Wyke K., Midthun K., Walsh M., McAuliffe V., Wyatt R. G., Kalica A. R., Flores J., Hoshino Y. Production and preliminary characterization of monoclonal antibodies directed at two surface proteins of rhesus rotavirus. J Virol. 1983 Aug;47(2):267–275. doi: 10.1128/jvi.47.2.267-275.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Saif L. J., Sereno M. M., Chanock R. M., Kapikian A. Z. Infection immunity of piglets to either VP3 or VP7 outer capsid protein confers resistance to challenge with a virulent rotavirus bearing the corresponding antigen. J Virol. 1988 Mar;62(3):744–748. doi: 10.1128/jvi.62.3.744-748.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Sereno M. M., Midthun K., Flores J., Kapikian A. Z., Chanock R. M. Independent segregation of two antigenic specificities (VP3 and VP7) involved in neutralization of rotavirus infectivity. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8701–8704. doi: 10.1073/pnas.82.24.8701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapikian A. Z., Vesikari T., Ruuska T., Madore H. P., Christy C., Dolin R., Flores J., Green K. Y., Davidson B. L., Gorziglia M. An update on the "Jennerian" and modified "Jennerian" approach to vaccination of infants and young children against rotavirus diarrhea. Adv Exp Med Biol. 1992;327:59–69. doi: 10.1007/978-1-4615-3410-5_8. [DOI] [PubMed] [Google Scholar]
- Matsuno S., Murakami S., Takagi M., Hayashi M., Inouye S., Hasegawa A., Fukai K. Cold-adaptation of human rotavirus. Virus Res. 1987 May;7(3):273–280. doi: 10.1016/0168-1702(87)90033-5. [DOI] [PubMed] [Google Scholar]
- Midthun K., Greenberg H. B., Hoshino Y., Kapikian A. Z., Wyatt R. G., Chanock R. M. Reassortant rotaviruses as potential live rotavirus vaccine candidates. J Virol. 1985 Mar;53(3):949–954. doi: 10.1128/jvi.53.3.949-954.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midthun K., Hoshino Y., Kapikian A. Z., Chanock R. M. Single gene substitution rotavirus reassortants containing the major neutralization protein (VP7) of human rotavirus serotype 4. J Clin Microbiol. 1986 Nov;24(5):822–826. doi: 10.1128/jcm.24.5.822-826.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy B. R., Hall S. L., Kulkarni A. B., Crowe J. E., Jr, Collins P. L., Connors M., Karron R. A., Chanock R. M. An update on approaches to the development of respiratory syncytial virus (RSV) and parainfluenza virus type 3 (PIV3) vaccines. Virus Res. 1994 Apr;32(1):13–36. doi: 10.1016/0168-1702(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Offit P. A., Blavat G. Identification of the two rotavirus genes determining neutralization specificities. J Virol. 1986 Jan;57(1):376–378. doi: 10.1128/jvi.57.1.376-378.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla-Noriega L., Arias C. F., López S., Puerto F., Snodgrass D. R., Taniguchi K., Greenberg H. B. Diversity of rotavirus serotypes in Mexican infants with gastroenteritis. J Clin Microbiol. 1990 Jun;28(6):1114–1119. doi: 10.1128/jcm.28.6.1114-1119.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman D. D., Murphy B. R. The association of the temperature-sensitive phenotype with viral attenuation in animals and humans: implications for the development and use of live virus vaccines. Rev Infect Dis. 1979 May-Jun;1(3):413–433. doi: 10.1093/clinids/1.3.413. [DOI] [PubMed] [Google Scholar]
- Shaw R. D., Stoner-Ma D. L., Estes M. K., Greenberg H. B. Specific enzyme-linked immunoassay for rotavirus serotypes 1 and 3. J Clin Microbiol. 1985 Aug;22(2):286–291. doi: 10.1128/jcm.22.2.286-291.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K., Urasawa T., Morita Y., Greenberg H. B., Urasawa S. Direct serotyping of human rotavirus in stools by an enzyme-linked immunosorbent assay using serotype 1-, 2-, 3-, and 4-specific monoclonal antibodies to VP7. J Infect Dis. 1987 Jun;155(6):1159–1166. doi: 10.1093/infdis/155.6.1159. [DOI] [PubMed] [Google Scholar]
- Vesikari T. Clinical trials of live oral rotavirus vaccines: the Finnish experience. Vaccine. 1993;11(2):255–261. doi: 10.1016/0264-410x(93)90026-t. [DOI] [PubMed] [Google Scholar]
- Vesikari T., Isolauri E., Delem A., D'Hondt E., André F. E., Zissis G. Immunogenicity and safety of live oral attenuated bovine rotavirus vaccine strain RIT 4237 in adults and young children. Lancet. 1983 Oct 8;2(8354):807–811. doi: 10.1016/s0140-6736(83)90734-1. [DOI] [PubMed] [Google Scholar]