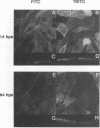
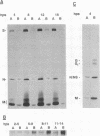
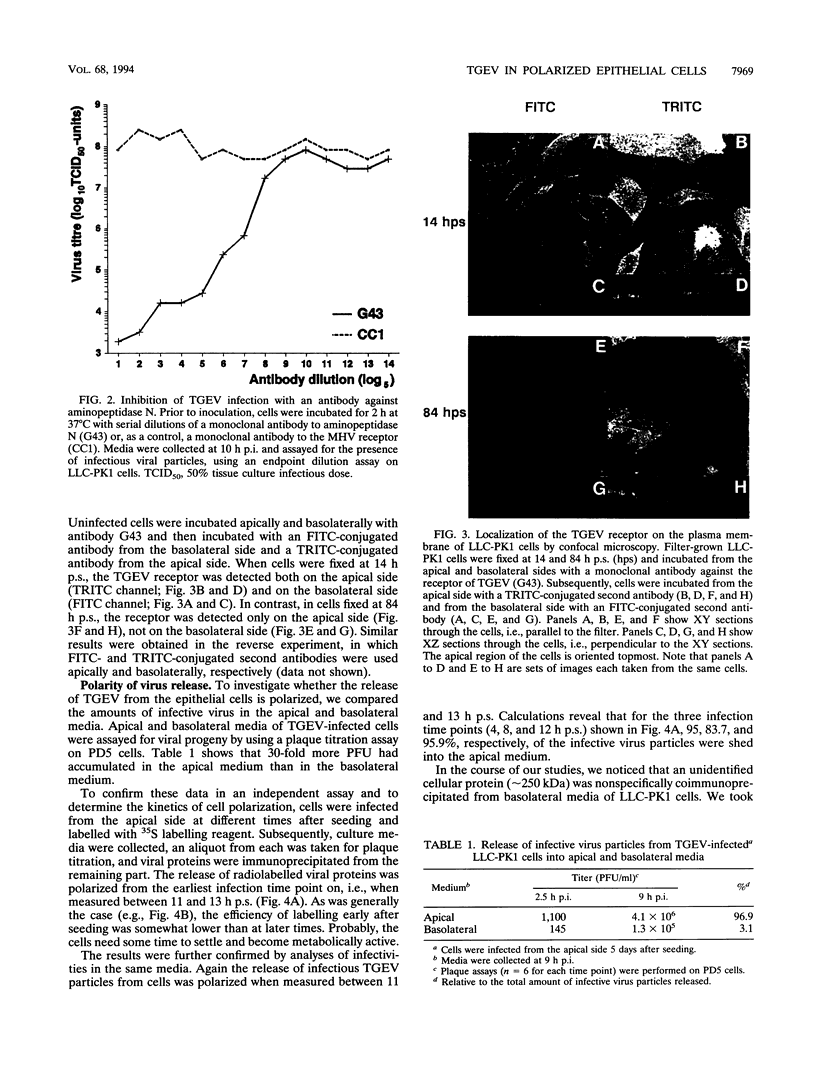
Abstract
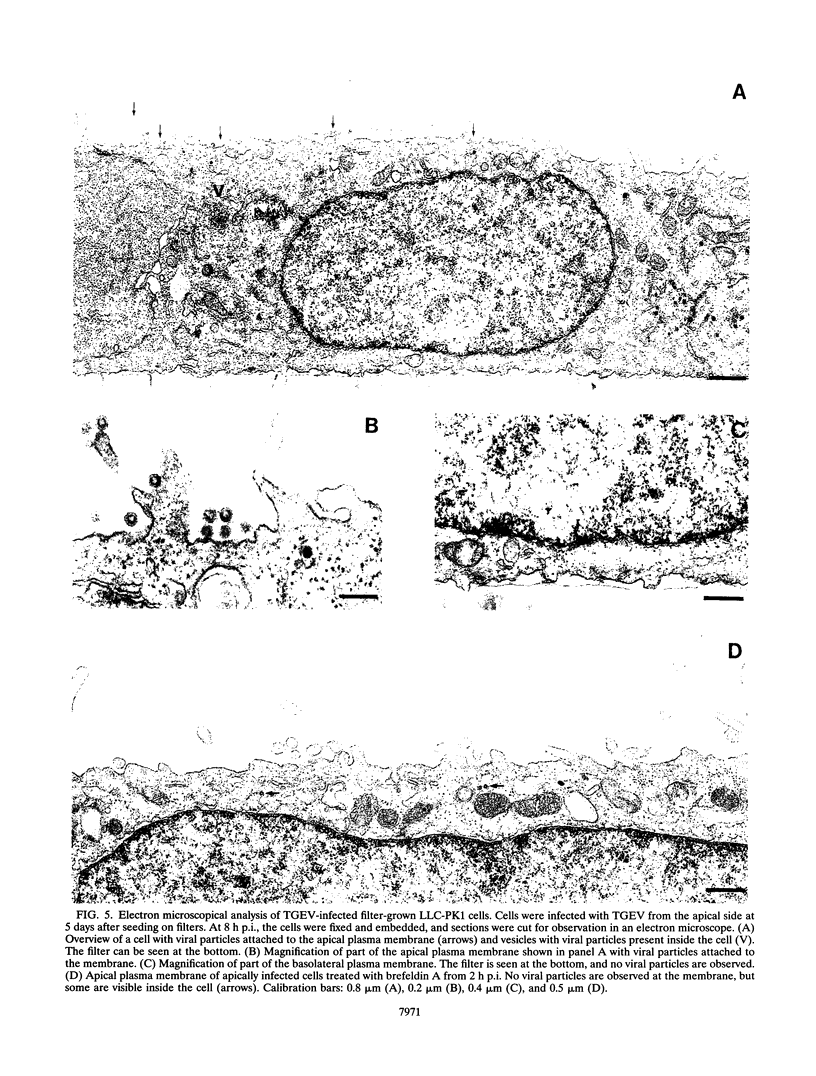
The transmissible gastroenteritis coronavirus (TGEV) infects the epithelial cells of the intestinal tract of pigs, resulting in a high mortality rate in piglets. This study shows the interaction of TGEV with a porcine epithelial cell line. To determine the site of viral entry, LLC-PK1 cells were grown on permeable filter supports and infected with TGEV from the apical or basolateral side. Initially after plating, the virus was found to enter the cells from both sides. During further development of cell polarity, however, the entry became restricted to the apical membrane. Viral entry could be blocked by a monoclonal antibody to the viral receptor aminopeptidase N. Confocal laser scanning microscopy showed that this receptor protein was present at both the apical and basolateral plasma membrane domains just after plating of the cells but that it became restricted to the apical plasma membrane during culture. To establish the site of viral release, the viral content of the apical and basolateral media of apically infected LLC-PK1 cells was measured by determining the amount of radioactively labelled viral proteins and infectious viral particles. We found that TGEV was preferentially released from the apical plasma membrane. This conclusion was confirmed by electron microscopy, which demonstrated that newly synthesized viral particles attached to the apical membrane. The results support the idea that the rapid lateral spread of TGEV infection over the intestinal epithelia occurs by the preferential release of virus from infected epithelial cells into the gut lumen followed by efficient infection of nearby cells through the apical domain.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambali A. G., Jones R. C. Early pathogenesis in chicks of infection with an enterotropic strain of infectious bronchitis virus. Avian Dis. 1990 Oct-Dec;34(4):809–817. [PubMed] [Google Scholar]
- Anderson G. W., Jr, Smith J. F. Immunoelectron microscopy of Rift Valley fever viral morphogenesis in primary rat hepatocytes. Virology. 1987 Nov;161(1):91–100. doi: 10.1016/0042-6822(87)90174-7. [DOI] [PubMed] [Google Scholar]
- Baron R., Neff L., Brown W., Courtoy P. J., Louvard D., Farquhar M. G. Polarized secretion of lysosomal enzymes: co-distribution of cation-independent mannose-6-phosphate receptors and lysosomal enzymes along the osteoclast exocytic pathway. J Cell Biol. 1988 Jun;106(6):1863–1872. doi: 10.1083/jcb.106.6.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereijido M., Contreras R. G., Gonzalez-Mariscal L. Development and alteration of polarity. Annu Rev Physiol. 1989;51:785–795. doi: 10.1146/annurev.ph.51.030189.004033. [DOI] [PubMed] [Google Scholar]
- Cerneus D. P., Strous G. J., van der Ende A. Bidirectional transcytosis determines the steady state distribution of the transferrin receptor at opposite plasma membrane domains of BeWo cells. J Cell Biol. 1993 Sep;122(6):1223–1230. doi: 10.1083/jcb.122.6.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. Y., Matsuoka Y., Compans R. W. Assembly and polarized release of Punta Toro virus and effects of brefeldin A. J Virol. 1991 Mar;65(3):1427–1439. doi: 10.1128/jvi.65.3.1427-1439.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayson E. T., Compans R. W. Entry of simian virus 40 is restricted to apical surfaces of polarized epithelial cells. Mol Cell Biol. 1988 Aug;8(8):3391–3396. doi: 10.1128/mcb.8.8.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compans R. W., Srinivas R. V. Protein sorting in polarized epithelial cells. Curr Top Microbiol Immunol. 1991;170:141–181. doi: 10.1007/978-3-642-76389-2_5. [DOI] [PubMed] [Google Scholar]
- Condron R. J., Marshall A. T. Pathogenesis of infectious bronchitis nephritis. 1. Morphometric analysis of kidney proximal tubular epithelium in chickens. J Comp Pathol. 1986 Jan;96(1):47–61. doi: 10.1016/0021-9975(86)90022-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas B., Gelfi J., L'Haridon R., Vogel L. K., Sjöström H., Norén O., Laude H. Aminopeptidase N is a major receptor for the entero-pathogenic coronavirus TGEV. Nature. 1992 Jun 4;357(6377):417–420. doi: 10.1038/357417a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller S. D., von Bonsdorff C. H., Simons K. Cell surface influenza haemagglutinin can mediate infection by other animal viruses. EMBO J. 1985 Oct;4(10):2475–2485. doi: 10.1002/j.1460-2075.1985.tb03959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller S., von Bonsdorff C. H., Simons K. Vesicular stomatitis virus infects and matures only through the basolateral surface of the polarized epithelial cell line, MDCK. Cell. 1984 Aug;38(1):65–77. doi: 10.1016/0092-8674(84)90527-0. [DOI] [PubMed] [Google Scholar]
- Griffiths G., Rottier P. Cell biology of viruses that assemble along the biosynthetic pathway. Semin Cell Biol. 1992 Oct;3(5):367–381. doi: 10.1016/1043-4682(92)90022-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gstraunthaler G. J. Epithelial cells in tissue culture. Ren Physiol Biochem. 1988 Jan-Feb;11(1-2):1–42. doi: 10.1159/000173147. [DOI] [PubMed] [Google Scholar]
- Hull R. N., Cherry W. R., Weaver G. W. The origin and characteristics of a pig kidney cell strain, LLC-PK. In Vitro. 1976 Oct;12(10):670–677. doi: 10.1007/BF02797469. [DOI] [PubMed] [Google Scholar]
- Klumperman J., Locker J. K., Meijer A., Horzinek M. C., Geuze H. J., Rottier P. J. Coronavirus M proteins accumulate in the Golgi complex beyond the site of virion budding. J Virol. 1994 Oct;68(10):6523–6534. doi: 10.1128/jvi.68.10.6523-6534.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pensaert M., Haelterman E. O., Burnstein T. Transmissible gastroenteritis of swine: virus-intestinal cell interactions. I. Immunofluorescence, histopathology and virus production in the small intestine through the course of infection. Arch Gesamte Virusforsch. 1970;31(3):321–334. doi: 10.1007/BF01253767. [DOI] [PubMed] [Google Scholar]
- Pensaert M., Haelterman E. O., Hinsman E. J. Transmissible gastroenteritis of swine: virus-intestinal cell interactions. II. Electron microscopy of the epithelium in isolated jejunal loops. Arch Gesamte Virusforsch. 1970;31(3):335–351. doi: 10.1007/BF01253768. [DOI] [PubMed] [Google Scholar]
- Pfeffer S. R. Mannose 6-phosphate receptors and their role in targeting proteins to lysosomes. J Membr Biol. 1988 Jul;103(1):7–16. doi: 10.1007/BF01871928. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabito C. A., Kreisberg J. I., Wight D. Alkaline phosphatase and gamma-glutamyl transpeptidase as polarization markers during the organization of LLC-PK1 cells into an epithelial membrane. J Biol Chem. 1984 Jan 10;259(1):574–582. [PubMed] [Google Scholar]
- Rodriguez Boulan E., Sabatini D. D. Asymmetric budding of viruses in epithelial monlayers: a model system for study of epithelial polarity. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5071–5075. doi: 10.1073/pnas.75.10.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Boulan E., Nelson W. J. Morphogenesis of the polarized epithelial cell phenotype. Science. 1989 Aug 18;245(4919):718–725. doi: 10.1126/science.2672330. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan E., Paskiet K. T., Sabatini D. D. Assembly of enveloped viruses in Madin-Darby canine kidney cells: polarized budding from single attached cells and from clusters of cells in suspension. J Cell Biol. 1983 Mar;96(3):866–874. doi: 10.1083/jcb.96.3.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See H., Reithmeier R. A. Identification and characterization of the major stilbene-disulphonate- and concanavalin A-binding protein of the porcine renal brush-border membrane as aminopeptidase N. Biochem J. 1990 Oct 1;271(1):147–155. doi: 10.1042/bj2710147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K., Wandinger-Ness A. Polarized sorting in epithelia. Cell. 1990 Jul 27;62(2):207–210. doi: 10.1016/0092-8674(90)90357-k. [DOI] [PubMed] [Google Scholar]
- Simons K., van Meer G. Lipid sorting in epithelial cells. Biochemistry. 1988 Aug 23;27(17):6197–6202. doi: 10.1021/bi00417a001. [DOI] [PubMed] [Google Scholar]
- Srinivas R. V., Balachandran N., Alonso-Caplen F. V., Compans R. W. Expression of herpes simplex virus glycoproteins in polarized epithelial cells. J Virol. 1986 May;58(2):689–693. doi: 10.1128/jvi.58.2.689-693.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro M., Pritzer E., Khoshnan M. A., Yamakawa M., Kuroda K., Klenk H. D., Rott R., Seto J. T. Characterization of a pantropic variant of Sendai virus derived from a host range mutant. Virology. 1988 Aug;165(2):577–583. doi: 10.1016/0042-6822(88)90601-0. [DOI] [PubMed] [Google Scholar]
- Tashiro M., Yamakawa M., Tobita K., Seto J. T., Klenk H. D., Rott R. Altered budding site of a pantropic mutant of Sendai virus, F1-R, in polarized epithelial cells. J Virol. 1990 Oct;64(10):4672–4677. doi: 10.1128/jvi.64.10.4672-4677.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze J., Tooze S. A., Fuller S. D. Sorting of progeny coronavirus from condensed secretory proteins at the exit from the trans-Golgi network of AtT20 cells. J Cell Biol. 1987 Sep;105(3):1215–1226. doi: 10.1083/jcb.105.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze J., Tooze S., Warren G. Replication of coronavirus MHV-A59 in sac- cells: determination of the first site of budding of progeny virions. Eur J Cell Biol. 1984 Mar;33(2):281–293. [PubMed] [Google Scholar]
- Tucker S. P., Compans R. W. Virus infection of polarized epithelial cells. Adv Virus Res. 1993;42:187–247. doi: 10.1016/S0065-3527(08)60086-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels H. P., Hansen G. H., Fuhrer C., Look A. T., Sjöström H., Norén O., Spiess M. Aminopeptidase N is directly sorted to the apical domain in MDCK cells. J Cell Biol. 1990 Dec;111(6 Pt 2):2923–2930. doi: 10.1083/jcb.111.6.2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. K., Jiang G. S., Snyder S. W., Frana M. F., Holmes K. V. Purification of the 110-kilodalton glycoprotein receptor for mouse hepatitis virus (MHV)-A59 from mouse liver and identification of a nonfunctional, homologous protein in MHV-resistant SJL/J mice. J Virol. 1990 Aug;64(8):3817–3823. doi: 10.1128/jvi.64.8.3817-3823.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot R. J., ter Haar R. J., Horzinek M. C., van der Zeijst B. A. Intracellular RNAs of the feline infectious peritonitis coronavirus strain 79-1146. J Gen Virol. 1987 Apr;68(Pt 4):995–1002. doi: 10.1099/0022-1317-68-4-995. [DOI] [PubMed] [Google Scholar]
- van Meer G., Simons K. Viruses budding from either the apical or the basolateral plasma membrane domain of MDCK cells have unique phospholipid compositions. EMBO J. 1982;1(7):847–852. doi: 10.1002/j.1460-2075.1982.tb01258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]