Abstract
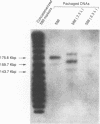
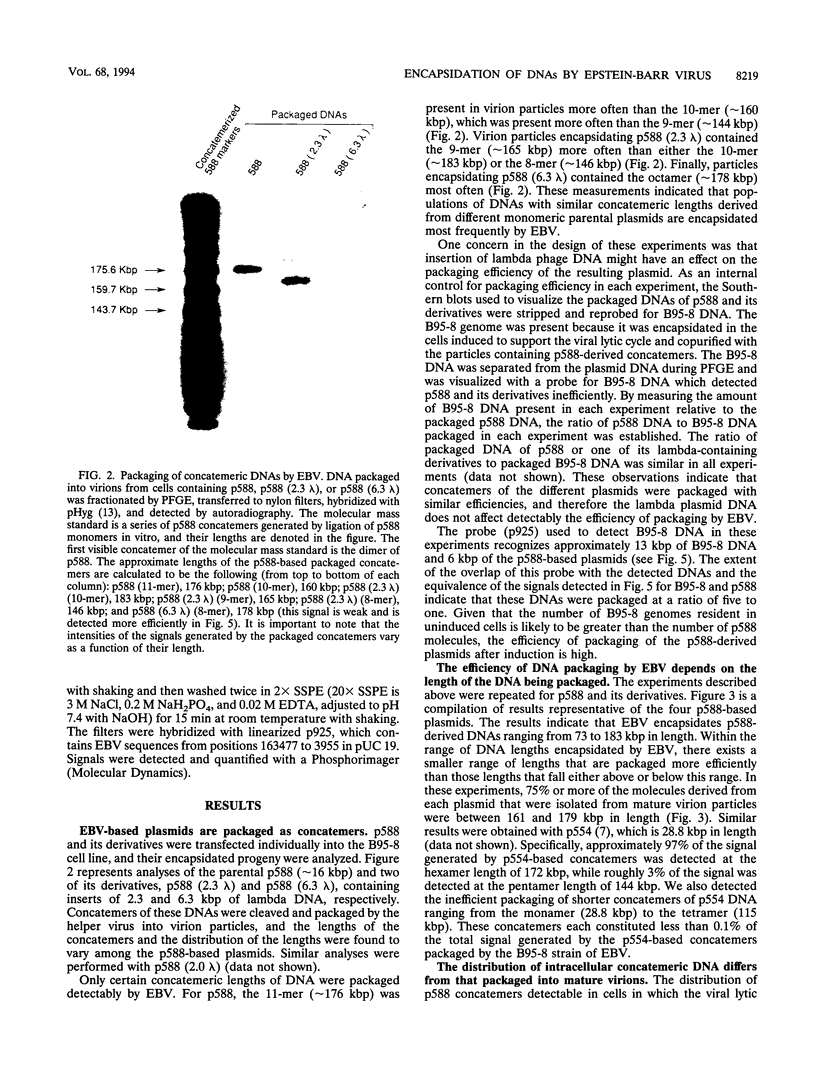
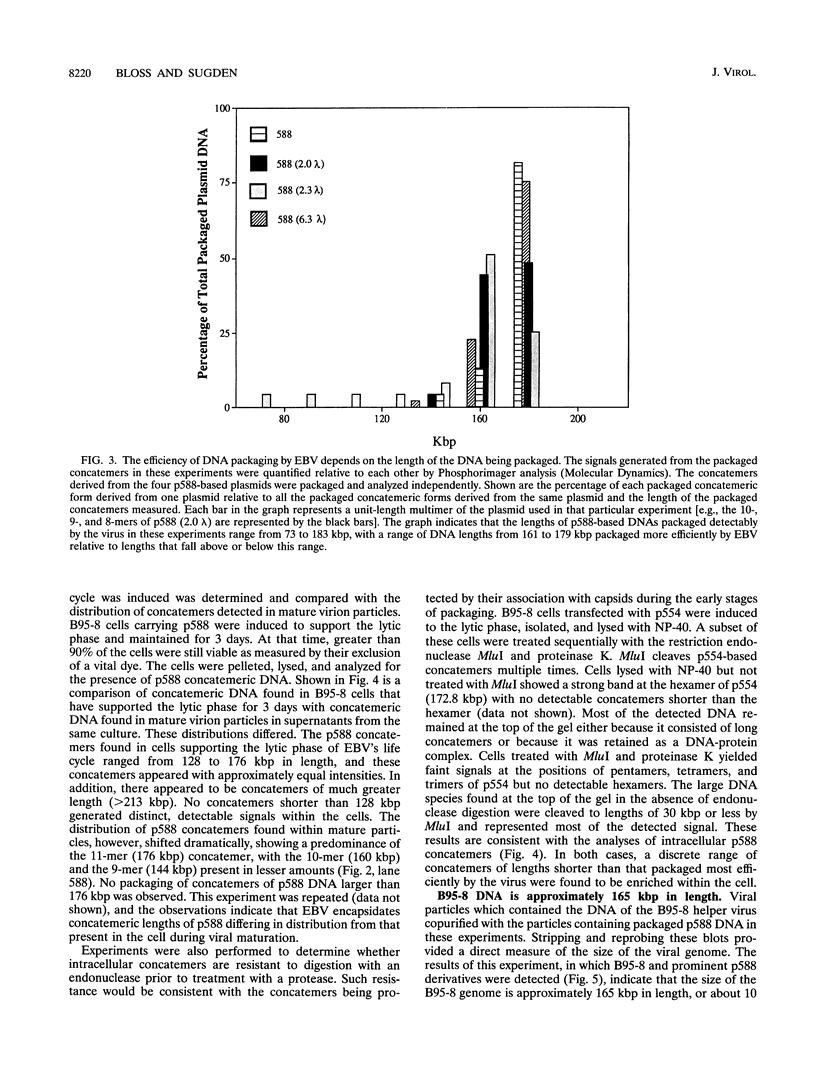
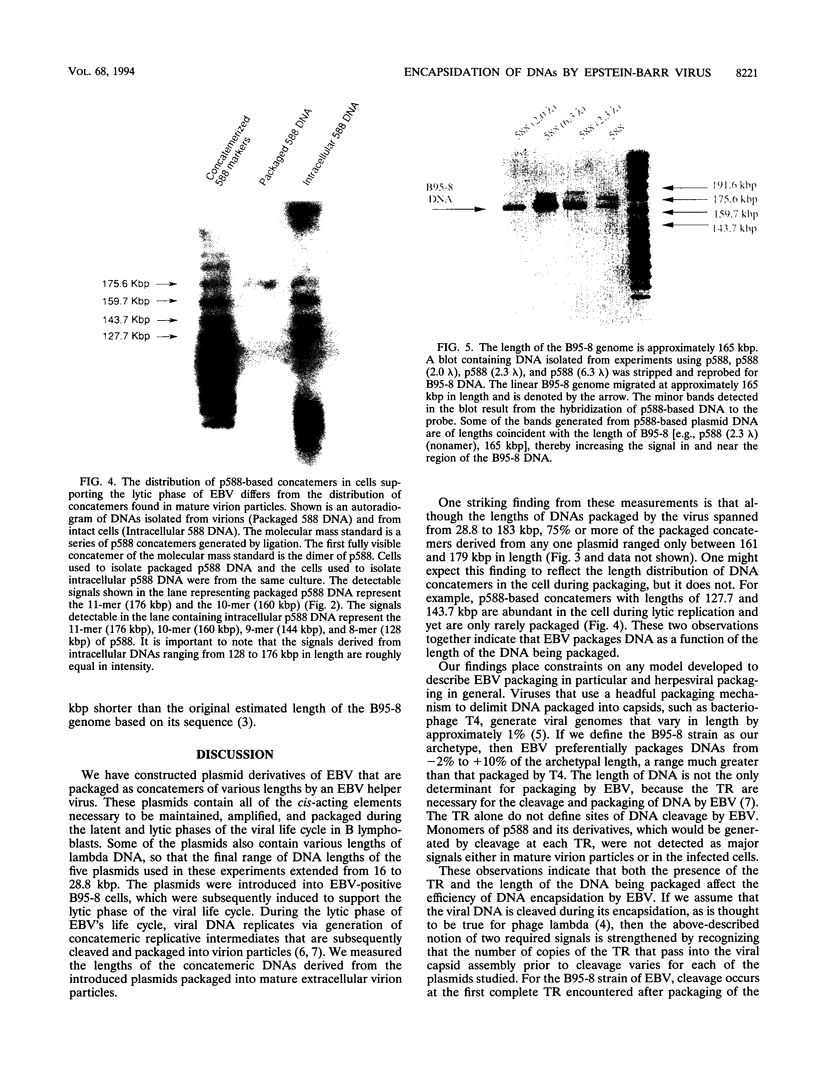
We measured the efficiency of DNA packaging by Epstein-Barr virus (EBV) as a function of the length of the DNA being packaged. Plasmids that contain oriP (the origin of latent EBV DNA replication), oriLyt (the origin of lytic EBV DNA replication), the viral terminal repeats (necessary for cleavage and packaging by EBV), and various lengths of bacteriophage lambda DNA were introduced into EBV-positive cells. Upon induction of the resident EBV's lytic phase, introduced plasmids replicated as concatemers and were packaged. Plasmid-derived concatemers of DNA with certain lengths were found to predominate in isolated virion particles. We measured the distribution of lengths of plasmid concatemers found within cells supporting the lytic phase of the viral life cycle and found that this distribution differed from the distribution of lengths of concatemers found in mature virion particles. This finding indicates that the DNA packaged into mature virions represents a selected subset of those present in the cell during packaging. These observations together indicate that the length of DNA affects the efficiency with which that DNA is packaged by EBV. Finally, we measured the length of the packaged B95-8 viral DNA and found it to be approximately 165 kbp, or 10 kbp shorter than the originally predicted size for B95-8 based on its sequence. Together with the results of other studies, these findings indicate that the packaging of DNAs by EBV is dependent on two imprecisely recognized elements: the viral terminal repeats and the length of the DNA being packaged by the virus.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams A. Replication of latent Epstein-Barr virus genomes in Raji cells. J Virol. 1987 May;61(5):1743–1746. doi: 10.1128/jvi.61.5.1743-1746.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan G. J., Rowe D. T. Size and stability of the Epstein-Barr virus major internal repeat (IR-1) in Burkitt's lymphoma and lymphoblastoid cell lines. Virology. 1989 Dec;173(2):489–498. doi: 10.1016/0042-6822(89)90561-8. [DOI] [PubMed] [Google Scholar]
- Baer R., Bankier A. T., Biggin M. D., Deininger P. L., Farrell P. J., Gibson T. J., Hatfull G., Hudson G. S., Satchwell S. C., Séguin C. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984 Jul 19;310(5974):207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- Becker A., Murialdo H. Bacteriophage lambda DNA: the beginning of the end. J Bacteriol. 1990 Jun;172(6):2819–2824. doi: 10.1128/jb.172.6.2819-2824.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt W., Sugden B. Genetic analysis of immortalizing functions of Epstein-Barr virus in human B lymphocytes. Nature. 1989 Aug 3;340(6232):393–397. doi: 10.1038/340393a0. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt W., Sugden B. Identification and characterization of oriLyt, a lytic origin of DNA replication of Epstein-Barr virus. Cell. 1988 Nov 4;55(3):427–433. doi: 10.1016/0092-8674(88)90028-1. [DOI] [PubMed] [Google Scholar]
- Knutson J. C., Yee D. Electroporation: parameters affecting transfer of DNA into mammalian cells. Anal Biochem. 1987 Jul;164(1):44–52. doi: 10.1016/0003-2697(87)90365-4. [DOI] [PubMed] [Google Scholar]
- Middleton T., Gahn T. A., Martin J. M., Sugden B. Immortalizing genes of Epstein-Barr virus. Adv Virus Res. 1991;40:19–55. doi: 10.1016/s0065-3527(08)60276-6. [DOI] [PubMed] [Google Scholar]
- Miller G., Shope T., Lisco H., Stitt D., Lipman M. Epstein-Barr virus: transformation, cytopathic changes, and viral antigens in squirrel monkey and marmoset leukocytes. Proc Natl Acad Sci U S A. 1972 Feb;69(2):383–387. doi: 10.1073/pnas.69.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sugden B., Marsh K., Yates J. A vector that replicates as a plasmid and can be efficiently selected in B-lymphoblasts transformed by Epstein-Barr virus. Mol Cell Biol. 1985 Feb;5(2):410–413. doi: 10.1128/mcb.5.2.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates J. L., Guan N. Epstein-Barr virus-derived plasmids replicate only once per cell cycle and are not amplified after entry into cells. J Virol. 1991 Jan;65(1):483–488. doi: 10.1128/jvi.65.1.483-488.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates J., Warren N., Reisman D., Sugden B. A cis-acting element from the Epstein-Barr viral genome that permits stable replication of recombinant plasmids in latently infected cells. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3806–3810. doi: 10.1073/pnas.81.12.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]