Abstract
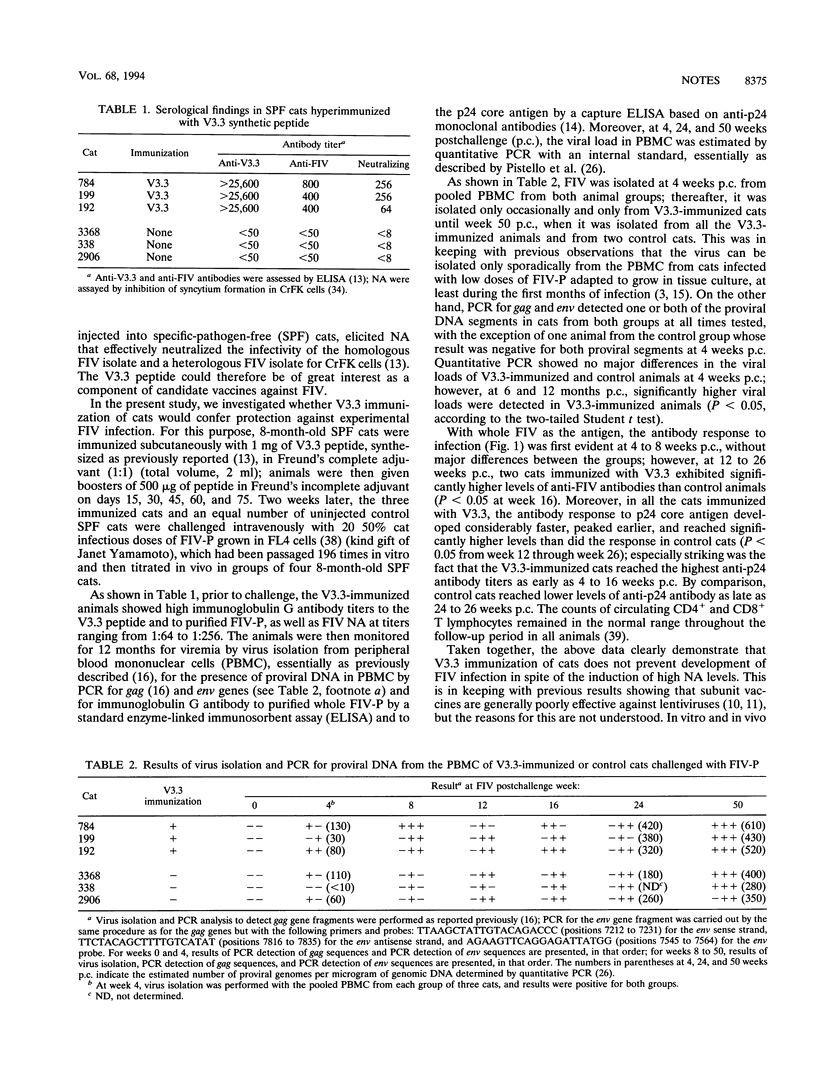
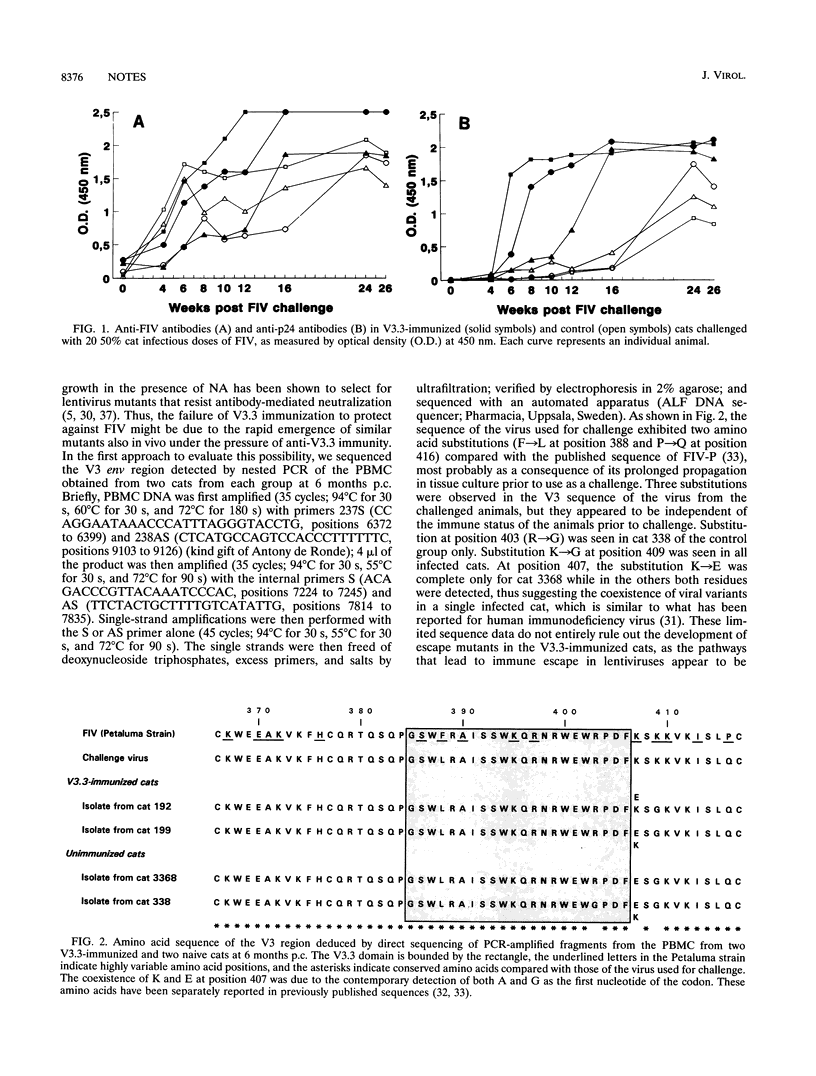
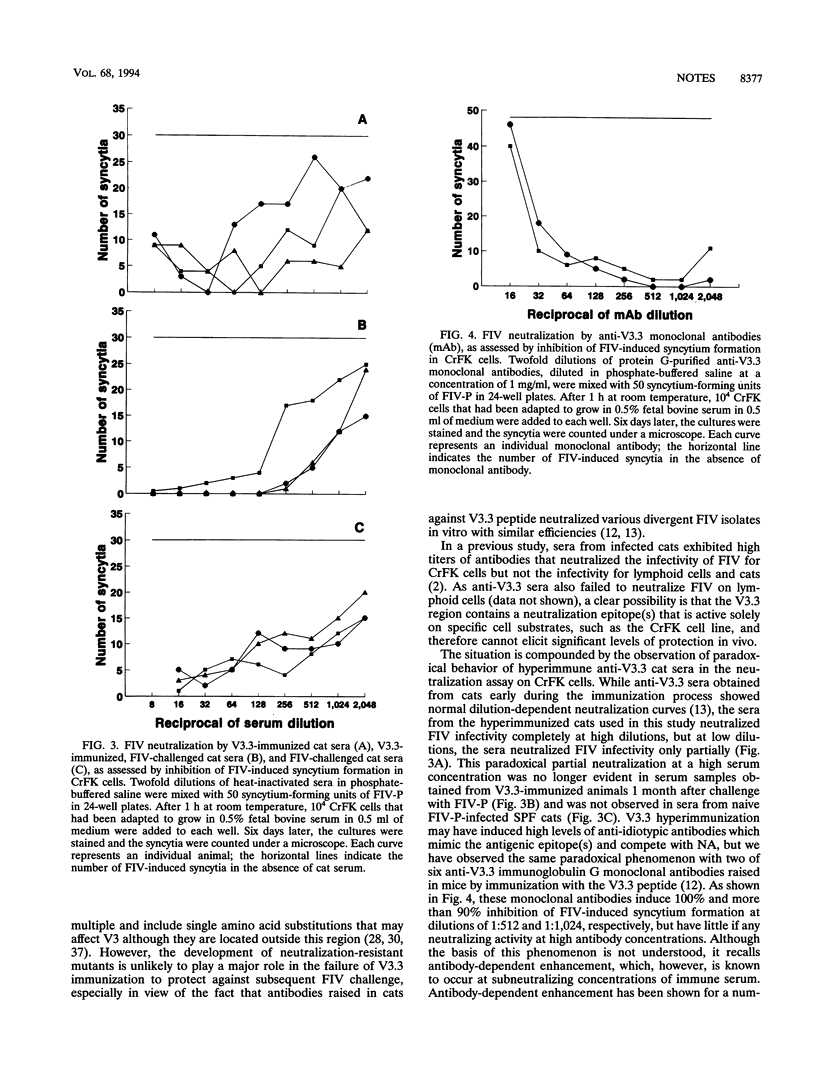
Specific-pathogen-free cats, immunized with a 22-amino-acid synthetic peptide designated V3.3 and derived from the third variable region of the envelope glycoprotein of the Petaluma isolate of feline immunodeficiency virus (FIV), developed high antibody titers to the V3.3 peptide and to purified virus, as assayed by enzyme-linked immunoassays, as well as neutralizing antibodies, as assayed by the inhibition of syncytium formation in Crandell feline kidney cells. V3.3-immunized animals and control cats were challenged with FIV and then monitored for 12 months; V3.3 immunization failed to prevent FIV infection, as shown by virus isolation, anti-whole virus and anti-p24 immunoglobulin G antibody responses, and positive PCRs for gag and env gene fragments. Sequence analysis of the V3 region showed no evidence for the emergence of escape mutants that might have contributed to the lack of protection. The sera of the V3.3-hyperimmunized cats and two anti-V3.3 monoclonal antibodies neutralized FIV infectivity for Crandell feline kidney cells at high antibody dilutions but paradoxically failed to completely neutralize FIV infectivity at low dilutions. Moreover, following FIV challenge, V3.3-immunized animals developed a faster and higher antiviral antibody response than control cats. This was probably due to enhanced virus replication, as also suggested by quantitative PCR data.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avrameas A., Guillet J. G., Chouchane L., Moraillon A., Sonigo P., Strosberg A. D. Localisation of three epitopes of the env protein of feline immunodeficiency virus. Mol Immunol. 1992 May;29(5):565–572. doi: 10.1016/0161-5890(92)90192-z. [DOI] [PubMed] [Google Scholar]
- Baldinotti F., Matteucci D., Mazzetti P., Giannelli C., Bandecchi P., Tozzini F., Bendinelli M. Serum neutralization of feline immunodeficiency virus is markedly dependent on passage history of the virus and host system. J Virol. 1994 Jul;68(7):4572–4579. doi: 10.1128/jvi.68.7.4572-4579.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlough J. E., North T. W., Oxford C. L., Remington K. M., Dandekar S., Ellis M. N., Pedersen N. C. Feline immunodeficiency virus infection of cats as a model to test the effect of certain in vitro selection pressures on the infectivity and virulence of resultant lentivirus variants. Antiviral Res. 1993 Dec;22(4):259–272. doi: 10.1016/0166-3542(93)90036-i. [DOI] [PubMed] [Google Scholar]
- Battegay M., Kyburz D., Hengartner H., Zinkernagel R. M. Enhancement of disease by neutralizing antiviral antibodies in the absence of primed antiviral cytotoxic T cells. Eur J Immunol. 1993 Dec;23(12):3236–3241. doi: 10.1002/eji.1830231229. [DOI] [PubMed] [Google Scholar]
- Burns D. P., Collignon C., Desrosiers R. C. Simian immunodeficiency virus mutants resistant to serum neutralization arise during persistent infection of rhesus monkeys. J Virol. 1993 Jul;67(7):4104–4113. doi: 10.1128/jvi.67.7.4104-4113.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egberink H. F., Ederveen J., Montelaro R. C., Pedersen N. C., Horzinek M. C., Koolen M. J. Intracellular proteins of feline immunodeficiency virus and their antigenic relationship with equine infectious anaemia virus proteins. J Gen Virol. 1990 Mar;71(Pt 3):739–743. doi: 10.1099/0022-1317-71-3-739. [DOI] [PubMed] [Google Scholar]
- Fevereiro M., Roneker C., Laufs A., Tavares L., de Noronha F. Characterization of two monoclonal antibodies against feline immunodeficiency virus gag gene products and their application in an assay to evaluate neutralizing antibody activity. J Gen Virol. 1991 Mar;72(Pt 3):617–622. doi: 10.1099/0022-1317-72-3-617. [DOI] [PubMed] [Google Scholar]
- Fontenot J. D., Hoover E. A., Elder J. H., Montelaro R. C. Evaluation of feline immunodeficiency virus and feline leukemia virus transmembrane peptides for serological diagnosis. J Clin Microbiol. 1992 Jul;30(7):1885–1890. doi: 10.1128/jcm.30.7.1885-1890.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosie M. J., Osborne R., Reid G., Neil J. C., Jarrett O. Enhancement after feline immunodeficiency virus vaccination. Vet Immunol Immunopathol. 1992 Dec;35(1-2):191–197. doi: 10.1016/0165-2427(93)90149-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel Z. R., Edmonson P. F., Maul D. H., O'Neil S. P., Mossman S. P., Thiriart C., Fabry L., Van Opstal O., Bruck C., Bex F. Incomplete protection, but suppression of virus burden, elicited by subunit simian immunodeficiency virus vaccines. J Virol. 1994 Mar;68(3):1843–1853. doi: 10.1128/jvi.68.3.1843-1853.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy J. A. Pathogenesis of human immunodeficiency virus infection. Microbiol Rev. 1993 Mar;57(1):183–289. doi: 10.1128/mr.57.1.183-289.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi S., Garzelli C., La Rosa C., Zaccaro L., Specter S., Malvaldi G., Tozzini F., Esposito F., Bendinelli M. Identification of a linear neutralization site within the third variable region of the feline immunodeficiency virus envelope. J Virol. 1993 Aug;67(8):4742–4749. doi: 10.1128/jvi.67.8.4742-4749.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi S., Poli A., Massi C., Abramo F., Zaccaro L., Bazzichi A., Malvaldi G., Bendinelli M., Garzelli C. Detection of feline immunodeficiency virus p24 antigen and p24-specific antibodies by monoclonal antibody-based assays. J Virol Methods. 1994 Mar;46(3):287–301. doi: 10.1016/0166-0934(94)90001-9. [DOI] [PubMed] [Google Scholar]
- Matteucci D., Baldinotti F., Mazzetti P., Pistello M., Bandecchi P., Ghilarducci R., Poli A., Tozzini F., Bendinelli M. Detection of feline immunodeficiency virus in saliva and plasma by cultivation and polymerase chain reaction. J Clin Microbiol. 1993 Mar;31(3):494–501. doi: 10.1128/jcm.31.3.494-501.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire T. C., Adams D. S., Johnson G. C., Klevjer-Anderson P., Barbee D. D., Gorham J. R. Acute arthritis in caprine arthritis-encephalitis virus challenge exposure of vaccinated or persistently infected goats. Am J Vet Res. 1986 Mar;47(3):537–540. [PubMed] [Google Scholar]
- Montefiori D. C., Robinson W. E., Jr, Hirsch V. M., Modliszewski A., Mitchell W. M., Johnson P. R. Antibody-dependent enhancement of simian immunodeficiency virus (SIV) infection in vitro by plasma from SIV-infected rhesus macaques. J Virol. 1990 Jan;64(1):113–119. doi: 10.1128/jvi.64.1.113-119.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa S., Lutz H., Aubert A., Bishop D. H. Identification of conserved and variable regions in the envelope glycoprotein sequences of two feline immunodeficiency viruses isolated in Zurich, Switzerland. Virus Res. 1991 Sep;21(1):53–63. doi: 10.1016/0168-1702(91)90071-3. [DOI] [PubMed] [Google Scholar]
- Pancino G., Chappey C., Saurin W., Sonigo P. B epitopes and selection pressures in feline immunodeficiency virus envelope glycoproteins. J Virol. 1993 Feb;67(2):664–672. doi: 10.1128/jvi.67.2.664-672.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pancino G., Fossati I., Chappey C., Castelot S., Hurtrel B., Moraillon A., Klatzmann D., Sonigo P. Structure and variations of feline immunodeficiency virus envelope glycoproteins. Virology. 1993 Feb;192(2):659–662. doi: 10.1006/viro.1993.1083. [DOI] [PubMed] [Google Scholar]
- Pedersen N. C., Johnson L., Birch D., Theilen G. H. Possible immunoenhancement of persistent viremia by feline leukemia virus envelope glycoprotein vaccines in challenge-exposure situations where whole inactivated virus vaccines were protective. Vet Immunol Immunopathol. 1986 Feb;11(2):123–148. doi: 10.1016/0165-2427(86)90093-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips T. R., Talbott R. L., Lamont C., Muir S., Lovelace K., Elder J. H. Comparison of two host cell range variants of feline immunodeficiency virus. J Virol. 1990 Oct;64(10):4605–4613. doi: 10.1128/jvi.64.10.4605-4613.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistello M., Menzo S., Giorgi M., Da Prato L., Cammarota G., Clementi M., Bendinelli M. Competitive polymerase chain reaction for quantitating feline immunodeficiency virus load in infected cat tissues. Mol Cell Probes. 1994 Jun;8(3):229–234. doi: 10.1006/mcpr.1994.1032. [DOI] [PubMed] [Google Scholar]
- Robinson W. E., Jr, Montefiori D. C., Mitchell W. M., Prince A. M., Alter H. J., Dreesman G. R., Eichberg J. W. Antibody-dependent enhancement of human immunodeficiency virus type 1 (HIV-1) infection in vitro by serum from HIV-1-infected and passively immunized chimpanzees. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4710–4714. doi: 10.1073/pnas.86.12.4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebelink K. H., Chu I. H., Rimmelzwaan G. F., Weijer K., Osterhaus A. D., Bosch M. L. Isolation and partial characterization of infectious molecular clones of feline immunodeficiency virus obtained directly from bone marrow DNA of a naturally infected cat. J Virol. 1992 Feb;66(2):1091–1097. doi: 10.1128/jvi.66.2.1091-1097.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebelink K. H., Rimmelzwaan G. F., Bosch M. L., Meloen R. H., Osterhaus A. D. A single amino acid substitution in hypervariable region 5 of the envelope protein of feline immunodeficiency virus allows escape from virus neutralization. J Virol. 1993 Apr;67(4):2202–2208. doi: 10.1128/jvi.67.4.2202-2208.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds P., Zhang L. Q., McOmish F., Balfe P., Ludlam C. A., Brown A. J. Discontinuous sequence change of human immunodeficiency virus (HIV) type 1 env sequences in plasma viral and lymphocyte-associated proviral populations in vivo: implications for models of HIV pathogenesis. J Virol. 1991 Nov;65(11):6266–6276. doi: 10.1128/jvi.65.11.6266-6276.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodora D. L., Shpaer E. G., Kitchell B. E., Dow S. W., Hoover E. A., Mullins J. I. Identification of three feline immunodeficiency virus (FIV) env gene subtypes and comparison of the FIV and human immunodeficiency virus type 1 evolutionary patterns. J Virol. 1994 Apr;68(4):2230–2238. doi: 10.1128/jvi.68.4.2230-2238.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbott R. L., Sparger E. E., Lovelace K. M., Fitch W. M., Pedersen N. C., Luciw P. A., Elder J. H. Nucleotide sequence and genomic organization of feline immunodeficiency virus. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5743–5747. doi: 10.1073/pnas.86.15.5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozzini F., Matteucci D., Bandecchi P., Baldinotti F., Poli A., Pistello M., Siebelink K. H., Ceccherini-Nelli L., Bendinelli M. Simple in vitro methods for titrating feline immunodeficiency virus (FIV) and FIV neutralizing antibodies. J Virol Methods. 1992 Jun;37(3):241–252. doi: 10.1016/0166-0934(92)90026-a. [DOI] [PubMed] [Google Scholar]
- Tozzini F., Matteucci D., Bandecchi P., Baldinotti F., Siebelink K., Osterhaus A., Bendinelli M. Neutralizing antibodies in cats infected with feline immunodeficiency virus. J Clin Microbiol. 1993 Jun;31(6):1626–1629. doi: 10.1128/jcm.31.6.1626-1629.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S. Z., Rushlow K. E., Issel C. J., Cook R. F., Cook S. J., Raabe M. L., Chong Y. H., Costa L., Montelaro R. C. Enhancement of EIAV replication and disease by immunization with a baculovirus-expressed recombinant envelope surface glycoprotein. Virology. 1994 Feb 15;199(1):247–251. doi: 10.1006/viro.1994.1120. [DOI] [PubMed] [Google Scholar]
- Watkins B. A., Reitz M. S., Jr, Wilson C. A., Aldrich K., Davis A. E., Robert-Guroff M. Immune escape by human immunodeficiency virus type 1 from neutralizing antibodies: evidence for multiple pathways. J Virol. 1993 Dec;67(12):7493–7500. doi: 10.1128/jvi.67.12.7493-7500.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto J. K., Ackley C. D., Zochlinski H., Louie H., Pembroke E., Torten M., Hansen H., Munn R., Okuda T. Development of IL-2-independent feline lymphoid cell lines chronically infected with feline immunodeficiency virus: importance for diagnostic reagents and vaccines. Intervirology. 1991;32(6):361–375. doi: 10.1159/000150220. [DOI] [PubMed] [Google Scholar]


