Abstract
By using a microarray screen to compare gene responses after sterile laser wounding of wild-type and ‘macrophageless' serpent mutant Drosophila embryos, we show the wound-induced programmes that are independent of a pathogenic response and distinguish which of the genes are macrophage dependent. The evolutionarily conserved nature of this response is highlighted by our finding that one such new inflammation-associated gene, growth arrest and DNA damage-inducible gene 45 (GADD45), is upregulated in both Drosophila and murine repair models. Comparison of unwounded wild-type and serpent mutant embryos also shows a portfolio of ‘macrophage-specific' genes, which suggest analogous functions with vertebrate inflammatory cells. Besides identifying the various classes of wound- and macrophage-related genes, our data indicate that sterile injury per se, in the absence of pathogens, triggers induction of a ‘pathogen response', which might prime the organism for what is likely to be an increased risk of infection.
Keywords: repair, inflammation, hemocyte, antimicrobial, GADD45
Introduction
Similar to vertebrates, the survival of insects after tissue damage is critically dependent on a rapid repair response to seal the injured tissue layers and on an immune response that is necessary to prevent microbial invasion and subsequent sepsis. In vertebrates, a series of leukocytic lineages diapedese from nearby blood vessels and migrate to sites of tissue damage where they kill microbes, clear away cell and matrix debris, and release a plethora of signals that act on local cells at the wound margin. In the Drosophila embryo, haemocytes (Drosophila macrophages) show a similar rapid response to wounding. This intimate association between tissue damage and an immediate ‘inflammatory' response has made it difficult to distinguish which of the vast array of genes upregulated at a wound site are triggered directly by wounding and which are an indirect consequence of the wound-associated inflammatory response.
Numerous studies have examined the genome-wide Drosophila response to septic injury and these have been instrumental in our understanding of the genetics of innate immunity, showing, for example, the crucial role of Toll receptor signalling in the immune response (De Gregorio et al, 2002). However, these studies do not differentiate between the response to tissue injury and the response to infection. Furthermore, without spatial resolution, array studies cannot determine whether increased gene expression is due to upregulation by local wound tissues, by activated haemocytes or by other immunosensitive tissues such as the fat body.
By using mutant Drosophila embryos that lack haemocytes and a laser wounding model that creates a sterile epithelial wound, we have recently disassociated inflammation from tissue repair and shown that in flies, as in mice that are genetically impaired in their inflammatory response (Martin et al, 2003), wound healing does not seem to be absolutely dependent on a cellular immune response (Stramer et al, 2005).
Here, we used microarray analysis to compare the transcriptional profiles of unwounded and wounded wild-type and haemocyte-null embryos. Besides identifying new haemocyte and wound-specific genes, this approach distinguishes which of the wound-induced genes are triggered by inflammatory signals and which are independent of haemocytes. Furthermore, this screen shows that in the absence of pathogenic infection, mechanical wounding is able to induce an antibacterial response that might prime the organism to fight what is perceived to be an increased likelihood of infection.
Results And Discussion
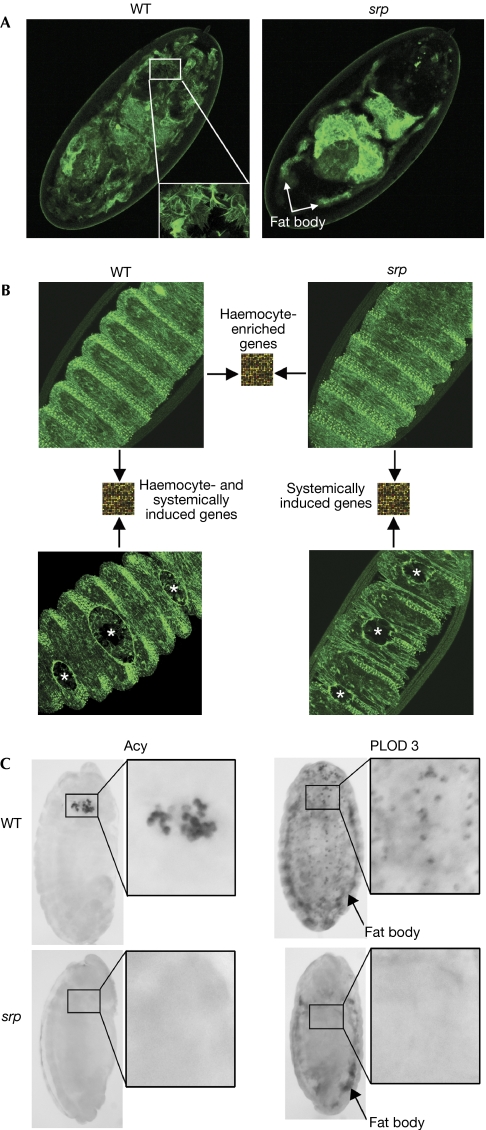
To investigate haemocyte-specific genes and the macrophage-dependent and macrophage-independent gene induction after wounding of Drosophila embryos, we used a microarray approach to compare unwounded and wounded embryos that were either wild-type or serpent (srp) mutant and lacked the haemocyte lineage (Fig 1A). Homozygous mutant embryos of the strong srp3 allele die during embryogenesis as a result of a failure in germ band retraction, whereas srpAS/srp3 embryos maintain srp expression in all the wild-type embryonic expression zones except for the head mesoderm from which the haemocyte primordia are derived (Rehorn et al, 1996). This results in an embryo that seems to be morphologically normal, with the majority of srp-dependent tissues, including the fat body, present, but which lacks haemocytes entirely (Fig 1A).
Figure 1.
Microarray schematic to elucidate haemocyte-specific, wound-induced and inflammation-dependent genes. (A) To illustrate the absence of macrophages without loss of other srp-dependent tissues, we used an srp-gal4 driver to drive GFP in wild-type (WT) and srpAS/srp3 mutants. Wild-type embryos show a labelled fat body and large numbers of haemocytes (wild-type inset), whereas srp mutants have only a labelled fat body. (B) RNA from unwounded wild-type and srp mutant embryos was compared by using microarray analysis to uncover haemocyte-enriched genes. RNA from unwounded wild-type embryos and embryos with three wounds (indicated by asterisks) was compared to elucidate haemocyte-induced and systemically induced wound genes, whereas RNA from wounded and unwounded srp mutant embryos was compared to show only inflammation-independent genes. All embryos in this figure constitutively express GFP-moesin, which shows both the wounded epithelium and recruited haemocytes. Note the absence of Drosophila macrophages in srp wounds. (C) Procollagen-lysine dioxygenase 3 (PLOD3) was expressed in wild-type embryos by the fat body and plasmatocytes, whereas in srp mutants it was expressed only by the fat body. Aminoacylase (Acy) was expressed by the crystal cell haemocyte subpopulation in wild-type embryos and was absent in srp mutants. GFP, green fluorescent protein; srp, serpent.
Many wild-type enriched genes are expressed by haemocytes
We began our microarray analysis by comparing the RNA profiles of unwounded wild-type versus srp mutant embryos (Fig 1B). Despite the fact that haemocytes make up only a small percentage of the total population of cells in a Drosophila embryo, our array comparison seemed to be an efficient filter for identifying haemocyte-specific genes. Approximately 20% of the transcripts we found to be expressed ⩾1.5-fold higher in wild-type versus srp unwounded embryos have previously been shown to be enriched in haemocytes (supplementary Table 1 online). Furthermore, approximately 8% of the differentially expressed genes encode proteins with a likely role in haemocyte function or immunity in larvae and adult flies (supplementary Table 2 online). Others with no previous link to haemocytes or infection are shown here to be expressed by either plasmatocytes or crystal cells, two subpopulations of haemocytes (Fig 1C).
Of those genes not previously linked to haemocytes, several encode proteins with likely roles in the normal functioning of these cells during embryonic development. For example, haemocytes are known to be the principal synthesizers of extracellular matrix (ECM) and to lay down embryonic basement membranes (Olofsson & Page, 2005); therefore, it is not surprising that one of our haemocyte-enriched genes is an orthologue of human procollagen-lysine dioxygenase 3 (PLOD3), which is involved in post-translational modification of collagens for normal basement membrane maturation (Myllyla et al, 2007). For other genes suggested to be haemocyte enriched by our microarray analysis, mutant studies showed reduced haemocyte numbers during embryogenesis; for example, the cell-cycle regulator string (cdc25; Milchanowski et al, 2004). The wild-type versus srp comparison also identified genes that indicate likely functional parallels between Drosophila immune cells and vertebrate leukocytes. For example, several glutathione-S-transferases (supplementary Table 3 online) were shown to be enriched in Drosophila macrophages, reflecting the high glutathione levels of haemocytes (Tirouvanziam et al, 2004), and this parallels the importance of glutathione for vertebrate immune function (Droge & Breitkreutz, 2000).
Identifying wound-induced genes
Next we used microarray analysis to compare the RNA profiles of wounded versus unwounded wild-type and srp mutant embryos. To increase the number of wound-activated cells per embryo, and thus reduce the dilution of wound-induced genes, we generated three large laser wounds in each embryo. Despite their large size, each of these wounds healed as efficiently as smaller wounds (Stramer et al, 2005). Even with three wounds, there are inevitable effects of dilution and, as a result, this microarray comparison yielded few significantly upregulated or downregulated genes. However, this strategy does enrich for wound-induced genes, and we have gone on to screen and elucidate the tissue-specific induction of several candidates identified in this way by using in situ hybridization. These studies showed several genes that were expressed by haemocytes at the wound site. Examples of such wound-activated haemocyte genes are secreted phospholipase A2 (sPLA2; CG14507) and the antimicrobial peptide drosomycin (Drs; Fig 2A–C). Induction of genes such as phospholipase A2 suggests the existence of evolutionarily conserved inflammatory signals, as the phospholipase A2 gene family is pivotal in the production of eicosanoids during the mammalian repair response and, in this way, regulates several aspects of leukocyte behaviour (Ninnemann, 1988). Interestingly, there is also evidence that eicosanoids might be involved in insect haemocyte immune responses (Stanley-Samuelson et al, 1991).
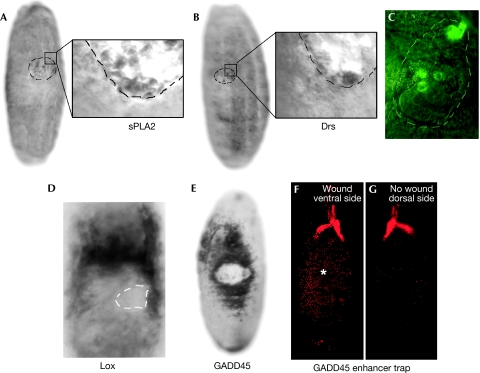
Figure 2.
In situ hybridization showing the expression domains of wound-induced genes 90 min after injury. Wound-activated haemocytes express (A) phospholipase A2 (sPLA2) and (B) the antimicrobial peptide drosomycin (Drs). (C) Drs induction by haemocytes is confirmed by wounding of the Drs-GFP reporter line. (D) Lox induction is seen in the wound epithelium and the underlying fat body. (E) In situ hybridization shows that GADD45 is induced in a broad band of epithelium spreading back from the wound edge. (F,G) A GADD45 enhancer trap line confirms GADD45 expression extending from the wound site (F), but this inductive wave fails to spread to the unwounded side of the embryo (G). The asterisk indicates a closed wound and the dotted lines indicate the wound site. GADD45, growth arrest and DNA damage-inducible gene 45; GFP, green fluorescent protein.
Our screen also showed several genes induced in the wounded epithelium or systemically in other distant tissues. A lysyl oxidase-like gene (CG11335) was induced by epithelial cells as well as other tissues during the repair process (Fig 2D). Lysyl oxidases are involved in crosslinking elastin and collagen molecules and this might be important in repairing ECM damage (Kagan & Li, 2003). Also upregulated by the wound epidermal cells was the Drosophila orthologue of the growth arrest and DNA damage-inducible gene 45 (GADD45; CG11086; Fig 2E–G), which is a known stress response gene with an ability to regulate MAP-kinase signalling (Takekawa & Saito, 1998) and thus affect, in several crucial ways, the repair process. It is intriguing that, in mammalian cells, GADD45 has been shown to ‘unsilence' genes by excisional repair-dependent DNA hypomethylation (Barreto et al, 2007) and, although it is generally believed that the Drosophila genome is not regulated by methylation, it might be the case that GADD45 has some role in the epigenetic regulation of wound target genes in migrating epidermal cells.
Drs, which is expressed by haemocytes at the wound site, was also systemically induced in the yolk after wounding (Fig 3A–E). An immune role for the embryonic yolk nuclei is consistent with the finding that cecropin, another antibacterial gene, is similarly expressed in this tissue during embryonic infection (Tingvall et al, 2001). We found that in older embryos (stage 17), the fat body, which is the main immune responsive tissue in the adult fly, also gained the ability to upregulate Drs after laser wounding (Fig 3F,G) in a haemocyte-independent manner (Fig 3C,H). However, diptericin, an antimicrobial peptide that is regulated by a different immune response pathway (De Gregorio et al, 2002), was not induced in the fat body after wounding in a diptericin-green fluorescent protein (GFP) reporter line (data not shown).
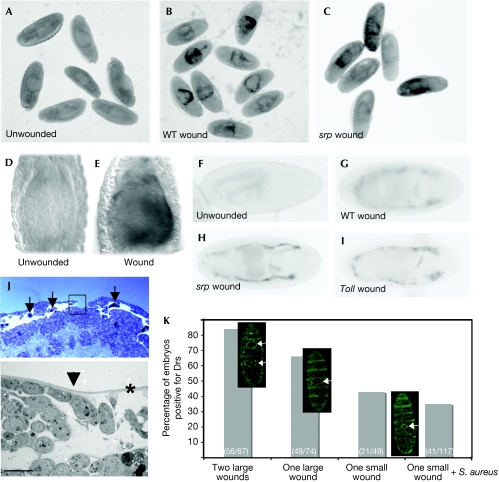
Figure 3.
Systemic induction of drosomycin after sterile wounding. In situ hybridization of (A) unwounded, (B) wounded wild-type (WT) and (C) srp mutant embryos shows that Drs is upregulated in the yolk after injury independent of haemocytes. (D,E) High-magnification view to compare Drs expression in the yolk of an unwounded and wounded embryo. (F–I) A Drs-GFP reporter line (colour inverted) shows that from stage 17, the fat body also upregulates Drs after laser wounding of (G) wild-type, (H) srp and (I) Toll mutants. (J) A methylene blue-stained resin section through a 90 min wound with recruited haemocytes indicated by arrows (top panel). The inset corresponds to an electron microscopic view (lower panel) illustrating how the vitelline membrane (indicated by the asterisk) remains intact beyond the epithelial wound edge (arrowhead). Scale bar, 5 μm. (K) Wound severity correlates with fat body expression of Drs. Two large wounds induced the Drs reporter in 84% of embryos, one large wound induced 66%, one small wound 43% and one small wound+Staphylococcus aureus 35%. GFP-moesin embryos were wounded to highlight wound sizes. Drs, drosomycin; GFP, green fluorescent protein; srp, serpent.
Although we believe that our laser wounds were sterile and did not seem to breach the vitelline membrane (Fig 3J), we cannot completely exclude the possibility that some pathogens were present on or within the vitelline membrane, thus inducing Drs after wounding. However, Drs induction after wounding was, at the very least, independent of any present Gram-positive or fungal infection, as Toll mutant embryos also expressed Drs following wounding (Fig 3I). We also found an increase in susceptibility to induce Drs within the fat body of embryos with increasing wound severity and number, but preincubation of embryos with a Gram-positive bacteria before wounding failed to increase the Drs response (Fig 3K), showing that the vitelline membrane was still a competent barrier to infection after laser ablation. These data support the idea that tissue damage per se might be a crucial component in triggering a pathogen response. Braun et al (1998) showed that although infection without injury was able to induce an immune response, the level of induction was significantly greater when infection was accompanied by injury. A more recent study has shown that survival rates are enhanced by previous wounding, indicating that tissue damage might prime the host's defence to future infections (Apidianakis et al, 2005).
‘Inflammation'-dependent and -independent wound genes
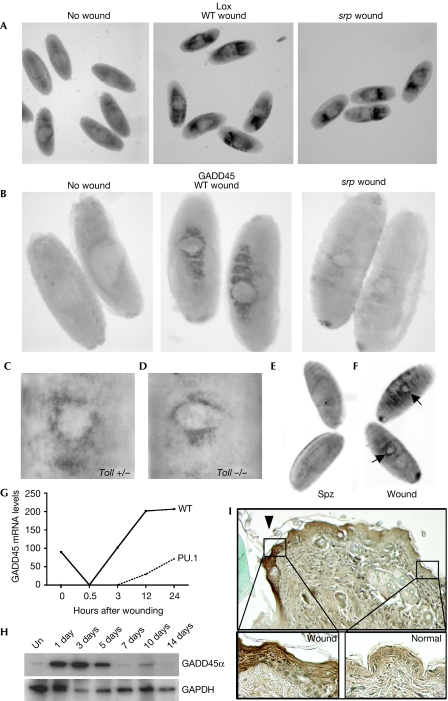
Microarray comparison of wounded srp and wild-type embryos also allowed us to screen which of the wound-induced genes was dependent on an inflammatory response. For example, our array analysis suggested that Lox was equivalently upregulated after wounding of both wild-type and srp embryos, and our in situ hybridization data confirmed similar expression levels in both genotypes after wounding (Fig 4A). However, it seemed that Drosophila GADD45 was much more robustly induced by wounding of wild-type embryos, and in situ hybridization showed that this gene was strongly inflammation dependent (Fig 4B). Our data suggest that Drosophila macrophages might secrete signals that are necessary for full GADD45 induction in the wounded epithelium. There is precedent for a paracrine signalling role for haemocytes during an immune response; after septic injury, an unpaired (Upd)-like cytokine is secreted by haemocytes and is necessary for Jak/Stat signalling in the fat body (Agaisse et al, 2003). Although GADD45 is not a known Jak/Stat target, it is responsive to Toll signalling (De Gregorio et al, 2002). However, Toll mutants showed a similar epithelial wound induction of GADD45 (Fig 4C,D), and expression of an activated form of the Toll ligand, spatzle (spz), in the epithelium of Drosophila embryos failed to induce GADD45 expression (Fig 4E,F), suggesting that GADD45 induction following wounding is Toll independent.
Figure 4.
Inflammation-dependent and inflammation-independent induced genes. (A) In situ hybridization studies show that Lox is induced by wounding, independent of a haemocyte response. (B) By contrast, GADD45 is robustly induced at wounds only in wild-type (WT) but not in srp mutant embryos. (C) Toll heterozygotes and (D) Toll mutants were wounded to show similar levels of GADD45 induction. (E) Ectopic expression of an activated form of spz in the epithelium failed to induce GADD45 expression, (F) unless wounded (arrows). (G) Temporal GADD45α messenger RNA expression following wounding of wild-type (solid line) and PU.1 mutant (dashed line) murine skin. (H) Western blot analysis shows transient GADD45α protein levels following excisional wounding of murine skin. (I) Immunostaining of GADD45α in 3-day murine wounds shows induction at the epithelial wound margin (arrowhead). The insets show a high-magnification view of increased GADD45α expression in wounded compared with normal epithelium. GADD45, growth arrest and DNA damage-inducible gene 45; spz, spatzle; srp, serpent; Un, unwounded.
Our data suggest that GADD45 is an ‘inflammation-associated' wound response gene in insects. To determine whether this response is conserved in mammals, we re-analysed microarray data from an analogous experiment in mice comparing the gene profiles of wounds in the presence and absence of an inflammatory response (Cooper et al, 2005). These data showed that a murine homologue of Drosophila GADD45 (Affymetrix MGU74A, 102292_at) was upregulated rapidly after wounding and that this response was much reduced in PU.1 null mice in which inflammatory cells were missing (Fig 4G). The expression of GADD45 protein after injury was examined by western blotting and showed rapid and transient induction by 1 day after wounding (Fig 4H). Furthermore, immunostaining showed that, as in Drosophila, murine GADD45 was induced in the wound epithelium (Fig 4I). This finding provided further evidence for an evolutionarily conserved repair response in flies and vertebrates, and highlights how useful Drosophila might be in elucidating new mechanisms regulating various aspects of vertebrate tissue repair.
Methods
Fly stocks. Mutant embryos for microarray analysis and for in situ hybridization were generated by using a heteroallelic combination of alleles srp3 and srpAS (Rehorn et al, 1996); wild-type embryos were rucuca strain (stock no. 576, Bloomington Stock Center). To visualize Drs (Drs-GFP) and diptericin expression, GFP reporter lines were wounded (Ferrandon et al, 1998; Tzou et al, 2000). To visualize epithelial wounds and haemocyte recruitment, a fly line expressing moesin fused to GFP was used (Kiehart et al, 2000). To examine Drs and GADD45 expression in srp and Toll mutants, lines containing Drs-GFP; srpAS/TTG (GFP balancer), Tlr4/TTG and Drs-GFP; Tlr4/TTG were created and mutant embryos were selected by their absence of fluorescence. For the GADD45 enhancer trap (stock no. 103594, Kyoto Stock Center, Kyoto, Japan), UAS-red stinger (stock no. 8546, Bloomington Stock Center, Bloomington, IN, USA) was recombined with this line before wounding. To express the spz ligand in the epithelium, an active form of spz (Ligoxygakis et al, 2002) was driven in the epithelium with the Engal4 driver.
Laser wounding and microarray analysis. Embryos were collected at stage 15 and wounded by using laser ablation (Wood et al, 2002). Embryos were collected after 90 min for in situ hybridization and microarray analysis, and total RNA was extracted from collections of 400 embryos for each time point by using Trizol reagent (Invitrogen, Carlsbad, CA, USA) and the RNeasy Cleanup Kit (Qiagen, Crawley, UK). Complementary DNA was labelled by using amino-allyl reverse transcription labelling. The samples were hybridized to an in-house cDNA array (Fred Hutchinson Cancer Center, Seattle, WA, USA) containing 12,144 probes derived from the following 3 collections: Dgcr1, Dgcr2 and the Northwest Drosophila Microarray Consortium.
For the unwounded wild type versus srp comparison, the experiment was conducted twice, yielding two independent replicate measurements. In addition, wounded versus unwounded wild-type embryos and wounded versus unwounded srp comparisons were made, and used to screen for inflammation-dependent and inflammation-independent transcripts. Analysis was performed by using the GeneSpring GX programme. Briefly, per spot and per chip intensity-dependent (Lowess) normalization was performed. Transcripts with unreliable scores were removed from the analysis on the basis of the cross-gene error model by using replicate scores. Genes were screened on the basis of t-test P-values and fold change. All raw microarray data are available in an MIAME-compliant format under accession number GSE10225 of the Gene Expression Omnibus (www.ncbi.nlm.nih.gov/geo/).
To examine Drs and diptericin expression by using GFP reporter lines, stage 17 embryos were wounded and examined 4 h later for fat body expression. To examine GADD45 induction with the enhancer trap line, stage 15 embryos were wounded and imaged after 12 h. For the Staphylococcus aureus experiment, embryos were preincubated in 1 × 107 CFU of S. aureus (reference strain ATCC 25923) before wounding.
In situ hybridization and electron microscopy. Immunohistochemical whole-mount in situ hybridization was carried out using standard methods (Lehmann & Tautz, 1994) with digoxigenin-substituted RNA probes generated by transcription of expressed-sequence tag clones from the Berkeley Drosophila Genome Project: LP04931 (Lox), RH27007 (CG14507), RE38345 (GADD45), LP03851 (Drs), LD37702 (PLOD3), RE63537 (aminoacylase). Embryos for transmission electron microscopy were frozen under high pressure, and then fixed and stained during the freeze substitution step with osmium tetroxide and uranyl acetate before embedding in resin. Thick sections were stained with methylene blue.
Murine histology, western blotting and immunostaining. Male, 8-week-old ICR mice were used according to UK Home Office regulations. Mice were anaesthetized by halothane inhalation and excisional wounds were made on the shaved back on either side of the dorsal midline with a 4-mm biopsy punch. Tissue was fixed in 10% formalin for embedding in paraffin. Wax sections (6 μm) were immunostained by using a GADD45α antibody, as described previously (Yamasawa et al, 2002). Protein samples were separated on Tris-glycine gels (Invitrogen) and blotted according to standard protocols by using GADD45α (Santa Cruz, Santa Cruz, CA, USA) and GAPDH (Abcam, Cambridge, UK) antibodies.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
supplementary tables
Acknowledgments
We thank P. Verkade, D. Carter and G. Tilly for help with transmission electron microscopy, B. Lemaitre for fly stocks, A. Hawrani for the bacteria and W. Wood for helpful discussions. We thank The Royal Society, The Medical Research Council, The Wellcome Trust and The Fred Hutchinson Cancer Center for funding this study.
Footnotes
The authors declare that they have no conflict of interest.
References
- Agaisse H, Petersen UM, Boutros M, Mathey-Prevot B, Perrimon N (2003) Signaling role of hemocytes in Drosophila JAK/STAT-dependent response to septic injury. Dev Cell 5: 441–450 [DOI] [PubMed] [Google Scholar]
- Apidianakis Y, Mindrinos MN, Xiao W, Lau GW, Baldini RL, Davis RW, Rahme LG (2005) Profiling early infection responses: Pseudomonas aeruginosa eludes host defenses by suppressing antimicrobial peptide gene expression. Proc Natl Acad Sci USA 102: 2573–2578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreto G et al. (2007) Gadd45a promotes epigenetic gene activation by repair-mediated DNA demethylation. Nature 445: 671–675 [DOI] [PubMed] [Google Scholar]
- Braun A, Hoffmann JA, Meister M (1998) Analysis of the Drosophila host defense in domino mutant larvae, which are devoid of hemocytes. Proc Natl Acad Sci USA 95: 14337–14342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper L, Johnson C, Burslem F, Martin P (2005) Wound healing and inflammation genes revealed by array analysis of ‘macrophageless' PU.1 null mice. Genome Biol 6: R5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gregorio E, Spellman PT, Tzou P, Rubin GM, Lemaitre B (2002) The Toll and Imd pathways are the major regulators of the immune response in Drosophila. EMBO J 21: 2568–2579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droge W, Breitkreutz R (2000) Glutathione and immune function. Proc Nutr Soc 59: 595–600 [DOI] [PubMed] [Google Scholar]
- Ferrandon D, Jung AC, Criqui M, Lemaitre B, Uttenweiler-Joseph S, Michaut L, Reichhart J, Hoffmann JA (1998) A drosomycin-GFP reporter transgene reveals a local immune response in Drosophila that is not dependent on the Toll pathway. EMBO J 17: 1217–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan HM, Li W (2003) Lysyl oxidase: properties, specificity, and biological roles inside and outside of the cell. J Cell Biochem 88: 660–672 [DOI] [PubMed] [Google Scholar]
- Kiehart DP, Galbraith CG, Edwards KA, Rickoll WL, Montague RA (2000) Multiple forces contribute to cell sheet morphogenesis for dorsal closure in Drosophila. J Cell Biol 149: 471–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann R, Tautz D (1994) In situ hybridization to RNA. Methods Cell Biol 44: 575–598 [DOI] [PubMed] [Google Scholar]
- Ligoxygakis P, Pelte N, Hoffmann JA, Reichhart JM (2002) Activation of Drosophila Toll during fungal infection by a blood serine protease. Science 297: 114–116 [DOI] [PubMed] [Google Scholar]
- Martin P, D'Souza D, Martin J, Grose R, Cooper L, Maki R, McKercher SR (2003) Wound healing in the PU.1 null mouse—tissue repair is not dependent on inflammatory cells. Curr Biol 13: 1122–1128 [DOI] [PubMed] [Google Scholar]
- Milchanowski AB, Henkenius AL, Narayanan M, Hartenstein V, Banerjee U (2004) Identification and characterization of genes involved in embryonic crystal cell formation during Drosophila hematopoiesis. Genetics 168: 325–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myllyla R, Wang C, Heikkinen J, Juffer A, Lampela O, Risteli M, Ruotsalainen H, Salo A, Sipila L (2007) Expanding the lysyl hydroxylase toolbox: new insights into the localization and activities of lysyl hydroxylase 3 (LH3). J Cell Physiol 212: 323–329 [DOI] [PubMed] [Google Scholar]
- Ninnemann J (1988) Prostaglandins, Leukotrienes, and the Immune Response. Cambridge, UK: Cambridge University Press [Google Scholar]
- Olofsson B, Page DT (2005) Condensation of the central nervous system in embryonic Drosophila is inhibited by blocking hemocyte migration or neural activity. Dev Biol 279: 233–243 [DOI] [PubMed] [Google Scholar]
- Rehorn KP, Thelen H, Michelson AM, Reuter R (1996) A molecular aspect of hematopoiesis and endoderm development common to vertebrates and Drosophila. Development 122: 4023–4031 [DOI] [PubMed] [Google Scholar]
- Stanley-Samuelson DW, Jensen E, Nickerson KW, Tiebel K, Ogg CL, Howard RW (1991) Insect immune response to bacterial infection is mediated by eicosanoids. Proc Natl Acad Sci USA 88: 1064–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stramer B, Wood W, Galko MJ, Redd MJ, Jacinto A, Parkhurst SM, Martin P (2005) Live imaging of wound inflammation in Drosophila embryos reveals key roles for small GTPases during in vivo cell migration. J Cell Biol 168: 567–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takekawa M, Saito H (1998) A family of stress-inducible GADD45-like proteins mediate activation of the stress-responsive MTK1/MEKK4 MAPKKK. Cell 95: 521–530 [DOI] [PubMed] [Google Scholar]
- Tingvall TO, Roos E, Engstrom Y (2001) The GATA factor Serpent is required for the onset of the humoral immune response in Drosophila embryos. Proc Natl Acad Sci USA 98: 3884–3888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirouvanziam R, Davidson CJ, Lipsick JS, Herzenberg LA (2004) Fluorescence-activated cell sorting (FACS) of Drosophila hemocytes reveals important functional similarities to mammalian leukocytes. Proc Natl Acad Sci USA 101: 2912–2917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W, Jacinto A, Grose R, Woolner S, Gale J, Wilson C, Martin P (2002) Wound healing recapitulates morphogenesis in Drosophila embryos. Nat Cell Biol 4: 907–912 [DOI] [PubMed] [Google Scholar]
- Yamasawa K, Nio Y, Dong M, Yamaguchi K, Itakura M (2002) Clinicopathological significance of abnormalities in Gadd45 expression and its relationship to p53 in human pancreatic cancer. Clin Cancer Res 8: 2563–2569 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplementary tables