Abstract
Several models have rationalized the use of antiviral drugs as an early control measure for delaying the progression and limiting the size of outbreaks during an influenza pandemic. However, the strategy for use of these drugs is still under debate. We evaluated the impact of prophylaxis of healthcare workers (HCWs) through a mathematical model that considers attack rates in a range of 25–35% in the general population and 25–50% among HCWs. Simulations and uncertainty analysis using the demographics of the province of Ontario, Canada show that increasing prophylaxis coverage of HCWs has little impact on reducing the reproduction number of disease transmission and may not prevent the occurrence of an outbreak if expected. However, it does enable a high level of treatment, which substantially reduces morbidity and mortality in the population as a whole. Therefore, prophylaxis of HCWs should be considered an important part of public health efforts for minimizing influenza pandemic burden and its socio-economic disruption.
Keywords: influenza pandemic, antivirals, prophylactic treatment, epidemic models
1. Introduction
Previous influenza pandemics occurred with significant rates of morbidity and mortality worldwide (Cox et al. 2003; Taubenberger & Morens 2006). Such a high mortality is often attributed to the lack of pre-existing immunity to a mutated virus that has transferred to the human populations (Nicholson et al. 2003; Hoft & Belshe 2004). Recent outbreaks of highly pathogenic avian influenza in poultry have raised the threat of an impending influenza pandemic through the mutation of the avian virus into one that readily infects humans (Gani et al. 2005).
Planning for the next influenza pandemic may be influenced by several public health intervention strategies that aim to reduce morbidity and mortality, as well as socio-economic devastation. Although vaccination has been a key strategy for interpandemic influenza epidemics (WHO 2000), it may not be the primary control measure early on in a pandemic (Ferguson et al. 2003). Other public health measures, including social distancing, school/border closure, travel restrictions and quarantine/isolation, may be considered; however, these measures, in general, have limited impact on mitigating the pandemic and, in some cases, may not even be feasible (Ferguson et al. 2006). Therefore, antiviral agents are widely considered to be an important control strategy for both treatment and prophylaxis during a pandemic (Ferguson et al. 2003, 2005; Longini et al. 2004, 2005; Gani et al. 2005).
One plausible treatment strategy would be to focus primarily on the treatment of hospitalized patients, healthcare workers (HCWs) and first responders. An extended programme could also include prophylaxis of HCWs, which would require stockpiling massive quantities of antivirals to be taken for the duration of a pandemic. These strategies lead to subtle, yet very significant, policy differences. While recognizing that the extended programme attempts to maintain high capacity in the healthcare system, it may entail a prohibitively expensive public health policy. Therefore, solid analysis and computer-assisted simulations should be undertaken to predict the trade-off between financial cost and overall healthcare benefit before implementing this strategy.
In the presence of antiviral agents, it is imperative to assess the relative impact of treatment of priority groups and prophylaxis of HCWs on preventing morbidity and mortality in the population as a whole. Assuming a viral strain with 25–35% clinical attack rate over the estimated 8–12 weeks of a pandemic wave (Longini et al. 2004, 2005; Gani et al. 2005), a sizable portion of HCWs may become infected early on, due to a higher level of exposure while providing care (Salgado et al. 2002; Bridges et al. 2003; Horcajada et al. 2003; Low & Wilder-Smith 2005). This will reduce the capacity of the healthcare system, which in turn could compromise the effectiveness of any treatment strategy. The prompt onset of treatment can substantially contribute to the containment of a pandemic, by reducing both the infectivity and the period of infectiousness of clinical cases. With a diminished healthcare work force in place and inadequate level of treatment, the disease can readily spread in the population. One would therefore expect that prophylaxis of HCWs may alleviate this situation by reducing the risk of infection and absenteeism due to illness as well as concern over exposure to infection in the workplace (Cinti et al. 2005).
This study undertakes to evaluate the relative importance of prophylaxis and treatment for a pandemic influenza, using population demographics of the province of Ontario, Canada as a case study. Here, we develop a mathematical model that incorporates parameters representing the effect of treatment and the functional relation describing the effect of prophylaxis of HCW on disease burden.
2. The model structure
Following previous work (Arino et al. 2006), we considered a relatively homogeneous population consisting of HCWs and the rest of the population as a general population (GP). We further divided each subpopulation into several compartments comprising susceptible (S), exposed but not infectious (E), asymptomatic and infectious (A), pre-symptomatic and infectious (P), symptomatic and highly infectious (stage 1, I), untreated symptomatic and infectious (stage 2, IU), treated symptomatic and infectious (IT), recovered (R) and dead (D) individuals (figure 1). These compartments are denoted by using subindex H (HP) for the subpopulation of HCWs without or with prophylaxis.
Figure 1.
A model diagram with treatment strategy.
We assumed that the transmission of infection can occur through contacts between the susceptible and infected individuals, in which mass action incidence is used for the sake of simplicity. Previous work (Arino et al. 2006) shows that this simplification still provides a good approximation to a more realistic situation in which other incidence functions may be used. Since the period of a pandemic is expected to be short, we ignore the effect of birth and natural death rates on the transmission dynamics of infection. It is assumed that infected individuals under treatment can contribute to disease transmission only through contacts with their healthcare providers. Therefore, under restricted infection control practices (Salgado et al. 2002), hospitalized patients are assumed to have contacts with only HCWs during their hospitalization. We considered only susceptible HCWs for a prophylactic treatment that reduces susceptibility, infectiousness if infection occurs and the probability of developing clinical symptoms. These assumptions, with the associated parameters given in table 1, lead to the following sets of deterministic equations:
Table 1.
Description of the model parameters with their values (ranges) for the GP and the HCWs (Salgado et al. 2002; Bridges et al. 2003; Ferguson et al. 2003, 2005; Horcajada et al. 2003; Longini et al. 2004, 2005; Gani et al. 2005; Low & Wilder-Smith 2005; Arino et al. 2006; Jefferson et al. 2006).
| parameter | description | value (range) |
|---|---|---|
| c1 | clinical attack rate in the GP | 25–35% |
| c2 | clinical attack rate among HCWs | 25–50% |
| 1/μE | incubation period | 1.25 days |
| 1/μP | infectious period of pre-symptomatic case | 0.25 day |
| 1/μI | infectious period of symptomatic case (stage 1) | 1 day |
| 1/μU | infectious period of untreated symptomatic case (stage 2) | 2.85 days |
| 1/μT | infectious period of treated symptomatic case | 1.35 days |
| 1/μA | infectious period of asymptomatic case | 4.1 days |
| dU | death rate of untreated symptomatic infection | 0.002 d−1 |
| dT | death rate of treated symptomatic infection | 0.001 d−1 |
| δP | reduction in infectiousness of pre-symptomatic case | 0.286 |
| δA | reduction in infectiousness of asymptomatic case | 0.071 |
| δU | reduction in infectiousness of untreated symptomatic case | 0.143 |
| δT | reduction in infectiousness of treated symptomatic case | 60% |
| αS | reduction in susceptibility due to prophylaxis | 25–35% |
| αP | reduction in infectiousness due to prophylaxis | 60% |
| p | probability of developing symptoms without prophylaxis | 0.67 |
| pP | probability of developing symptoms with prophylaxis | 0.35 |
2.1 General population
| (2.1) |
2.2 Healthcare workers without prophylaxis
| (2.2) |
2.3 Healthcare workers with prophylaxis
| (2.3) |
where the prime denotes the derivative with respect to the time, and the equations for the force of infection are given by
In comparison with Q, the force of infection term QH includes the infectious compartments IT, IHT and IHPT that correspond to the additional risk of HCWs infection from influenza patients under treatment. The term QH also involves AH, PH, AHP and PHP, as evidence shows that many affected HCWs with subclinical or even full clinical syndromes continue to report to work while unwell and become a source of viral shedding (Weingarten et al. 1989; Elder et al. 1996; Low & Wilder-Smith 2005). Therefore, susceptible HCWs may be exposed to even higher level of influenza transmission (Nguyen-Van-Tam et al. 1999; Low & Wilder-Smith 2005). More details of the model formulation and parameter assignment can be found in the electronic supplementary material.
The central role of HCWs in a pandemic response may be well represented through maintaining the quality of healthcare and providing high level of treatment. This is affected by several factors including the capacity of the healthcare system at the onset of a pandemic and, more importantly, the proportion of HCWs who are permitted (under the infection control protocol) to contribute to the treatment strategy. We defined the level of treatment (ρ) as the product of the fraction of clinical cases in the GP, which is effectively treated (ν), and the proportion of HCWs who provide care during a pandemic (θ). This variable proportion is given by
| (2.4) |
where SH(0)+SHP(0) is the population size of susceptible HCWs (without and with prophylaxis) at the onset of a pandemic. The numerator of this proportion includes susceptible, exposed, asymptomatic, pre-symptomatic and recovered HCWs, except those who are infected and clinically recognizable. At the beginning of a pandemic, θ(0)=1, representing the highest level of treatment afforded by the capacity of the healthcare system. However, the spread of infection increases the number of clinical cases among HCWs, thereby reducing their availability as expressed by θ. This in turn decreases the level of treatment ρ=νθ, and also contributes to the GP infection through regular contacts with HCWs outside the workplace.
3. Basic reproduction number and final size relation
The number of new infections produced by a single infected individual (R0) is the product of three parameters: the number of contacts with susceptible individuals per unit time; the probability of viral transmission; and the generation time. In the absence of antiviral treatment, we may follow a previous approach (Diekmann & Heesterbeek 2000; van den Driessche & Watmough 2002) to compute the basic reproduction number (see electronic supplementary material)
| (3.1) |
where β is the transmission rate; S0 is the initial size of the susceptible population; and other parameters are defined in table 1. Assuming that, at the beginning of a pandemic, the size of the infected population is small when compared with that of the susceptible population, there is a final size relation
| (3.2) |
where S∞ is the size of the susceptible population when the epidemic dies out. To estimate R0 from (3.2), we used the clinical attack rate defined as the fraction of the susceptible population that develops clinical symptoms. With the above notation, this rate is given by p(1−S∞/S0). For a range of 25–35% clinical attack rate in the GP, and assuming that 67% of the infected individuals develop clinical symptoms (Longini et al. 2004, 2005), the basic reproduction number ranges from 1.2516 to 1.4146 (table 3). However, if the probability of developing clinical symptoms is reduced to 50% (Ferguson et al. 2005), then for the same range of clinical attack rates R0 will be higher, varying between 1.3863 and 1.7200.
Table 3.
Variations in the mean value of Ra for different attack rates c1 and c2 in the GP and HCWs, as susceptibility to the disease due to prophylaxis changes (αS : 25–35%) and prophylaxis coverage of HCWs increases.
| attack rate among HCWs (%) | variations in the mean value of Ra | attack rate in the GP (%) | R0 | |||
|---|---|---|---|---|---|---|
| prophylaxis coverage | reduction (%) | |||||
| 0% | 50% | 100% | ||||
| 25–40 | 1.0627 | 1.0445 | 1.0170 | 4.3 | 25 | 1.2516 |
| 30–45 | 1.0973 | 1.0795 | 1.0522 | 4.11 | 30 | 1.3261 |
| 35–50 | 1.1488 | 1.1326 | 1.1112 | 3.27 | 35 | 1.4146 |
Given the clinical attack rate, the expression for R0 provides an estimation of the transmission rate β in the GP (see electronic supplementary material). The clinical attack rate of a pandemic strain may be as high as 60% among those caring for patients with influenza (Salgado et al. 2002; Bridges et al. 2003; Horcajada et al. 2003; Low & Wilder-Smith 2005), and we considered a range of 25–50% attack rate among HCWs. This is reflected in the transmission rate within healthcare settings, which is influenced by the transmission rate in the GP and calculated through the final size relation. With the variations in attack rates, we assessed the effect of changes in the treatment level on the time courses of infection and disease burden.
4. Results
We assumed that, at the onset of a pandemic, 90% of clinical cases in the GP (60% of the total GP infections) and 100% of clinical cases among HCWs are detected and effectively treated (Ferguson et al. 2005), corresponding to ν=0.9 and νH=1. The results are presented here using parameter values from recently published literature (table 1) and the population demographics of the province of Ontario, Canada (table 2).
Table 2.
(a) The number of HCWs confirmed by the Emergency Management Unit of the Ministry of Health and Long-Term Care in the province of Ontario, Canada, 2004. (b) Population size of Ontario, 2004, represented by Statistics Canada.
| number | |
|---|---|
| (a) healthcare workers | |
| LPNs (licensed practical nurses) | 23 705 |
| active physicians | 21 793 |
| RNs (registered nurses) | 87 329 |
| RRTs (registered respiratory therapists) | 2198 |
| (b) population size | 12 407 300 |
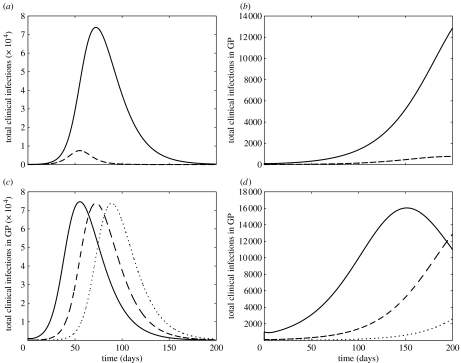
Figure 2a,b illustrates the profiles of clinical infections in the GP (solid curves) and HCWs (dashed curves), with an initial number of I(0)=100 infectives. With clinical attack rates of 30 and 45% in the GP and HCWs, respectively, these figures demonstrate that not only can prophylaxis of HCWs contribute to a significant delay in the progression of the epidemic, but it can also substantially reduce the magnitude of the disease outbreak (figure 2b). While the outbreak appears in a greatly milder form with prophylaxis of HCWs, it can be affected by the initial size of the infected population. Simulations presented in figure 2c,d show that although the initial number of infectives has relatively low impact on the magnitude of the epidemic, it can dramatically influence the progression and peaking time of the outbreak.
Figure 2.
The time courses of the total clinical infections in the GP (solid curves) and HCWs (dashed curves) with 30 and 45% attack rates, respectively, are illustrated in the following: (a) without prophylaxis of HCWs and (b) with 100% prophylaxis coverage of HCWs and 30% reduction in susceptibility due to prophylaxis (αS=0.3). An initial number of I(0)=100 infectives in the GP was assumed in (a) and (b). The time courses of the total clinical infections in the GP with different initial values of infectives are illustrated in the following: (c) without prophylaxis and (d) with 100% prophylaxis coverage of HCWs. The initial values of infectives are as follows: dotted curves, I(0)=10; dashed curves, I(0)=100; solid curves, I(0)=1000.
An important public health concern is whether the combined effect of the treatment of clinical cases and the prophylaxis of HCWs can result in containing a pandemic. We performed an uncertainty analysis by considering a sampling approach that allows for the simultaneous variations of clinical attack rates (c1, c2) in the GP and HCWs, and the reduction in susceptibility to infection (αS), as prophylaxis coverage increases. Using the Latin hypercube sampling (LHS) technique (McKay et al. 1979), we generated samples of size n=1000, in which each parameter is treated as a random variable and assigned a probability function. In this technique, the parameters are uniformly distributed and sampled within the ranges given in table 1.
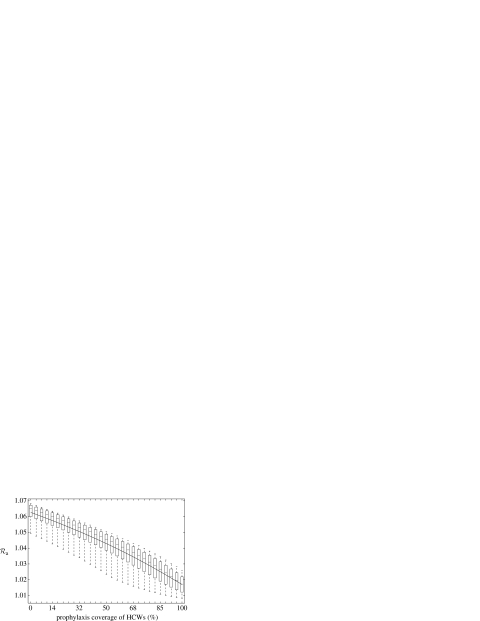
The results of this uncertainty analysis are illustrated in box plots (figure 3) for the variations of the averaged control reproduction number (Ra). The quantity Ra gives the average number of secondary infections generated by an infected individual (during the entire course of an outbreak) when control measures are implemented. In the presence of a treatment strategy, we ran the simulations for different prophylaxis coverage of HCWs to evaluate the final size of the susceptible population S∞, when the epidemic dies out. We then used the final size relation (see electronic supplementary material) to calculate the corresponding values of Ra. Figure 3 indicates that if an outbreak is expected to occur, prophylaxis of HCWs may not be able to prevent it. In this case, increasing prophylaxis coverage leads to a marginal reduction (4.3%) in the mean value of Ra from 1.0627 (without prophylaxis) to 1.017 (with 100% prophylaxis coverage). An important epidemiological consequence is that prophylaxis of HCWs may not be considered as a disease control measure if the treatment of clinical infections fails to prevent the occurrence of an outbreak. We observed similar results when attack rates in the GP and HCWs vary between 25–35% and 25–50%, respectively. These observations demonstrate a reduction of less than 5% in the mean values of Ra (table 3).
Figure 3.
Sensitivity analysis: box plots for the variations of Ra as prophylaxis coverage increases, with a curve passing through the mean values. Using 25% clinical attack rate in the GP, samples of size n=1000 were generated by the LHS technique for the changes in susceptibility to the disease due to prophylaxis (αs : 25–35%) and a range of attack rate among HCWs (c2 : 25–40%).
The effect of prophylaxis of HCWs may be deceptive, as its primary purpose is to maintain the quality of healthcare and reduce the disease burden, not to contain the epidemic. Our results shows that although the averaged control reproduction number is very marginally affected by expanding prophylactic treatment of HCWs, the potential substantial impact of this strategy appears to be limiting the spread of infection and reducing disease-related mortality. Box plots in figure 4a for the variations in the total number of recovered clinical infections show that the mean value is reduced by 72% (with full prophylaxis coverage of HCWs). A similar impact is observed in figure 4b with 73% reduction in the mean value of the total number of deaths in the population. The effectiveness of prophylaxis will be dramatically influenced by attack rates, and simulations reveal that the mean values of recovered clinical infections and deaths are reduced by 45 and 46%, respectively, when attack rates are increased by 30% in the GP and 30–45% among HCWs.
Figure 4.
Sensitivity analysis: box plots for the variations of (a) the total number of recovered clinical infections and (b) the total number of deaths, as prophylaxis coverage increases. Using 25% clinical attack rate in the GP, samples of size n=1000 were generated by the LHS technique for the changes in susceptibility to the disease due to prophylaxis (αS : 25–35%) and a range of attack rate among HCWs (c2 : 25–40%).
5. Discussion
The published literature suggesting that the use of neuraminidase inhibitors reduces susceptibility to influenza and substantially decreases the infectivity if infection occurs (Jefferson et al. 2006) has provided the basis for our study, assessing the effect of treatment and prophylaxis on reducing morbidity and mortality during a pandemic. The results show that maintaining high treatment level of clinical cases is essential for reducing fatality and containing a pandemic, regardless of the prophylaxis coverage of HCWs. In practice, however, the quality of healthcare system, and therefore the treatment level, is influenced by the number of HCWs who contribute to the caring for patients with influenza (Nguyen-Van-Tam et al. 1999; Simeonsson et al. 2004). Considering the nature of interaction between infected individuals and HCWs, the lack or poor administration of preventive measures may leave HCWs highly vulnerable to the infection, which in turn will compromise the effectiveness of the treatment strategy. For population demographics similar to those of Ontario, Canada, the findings suggest that with the full prophylaxis coverage of HCWs, a 45% reduction in population morbidity is achievable.
Given the available resources of public health, none of the previous studies (Meltzer et al. 1999; Balicer et al. 2005; Lee et al. 2006) addresses the fact that HCWs are central to any treatment or prophylaxis strategy. It should be emphasized that keeping HCW force in place is critical not just to respond to the needed care of pandemic influenza patients, but also to prevent deaths from all other causes. Conventional wisdom considers access to inpatient and outpatient care to be central to any pandemic response. If modern healthcare resources were unavailable, ill individuals would not be able to access antivirals, antibiotics, oxygen therapy, intravenous therapy, intensive care and other therapies. The unavailability of these modern options during 1918–1919 undoubtedly contributed to the high mortality rate during that pandemic.
The WHO recommendations on non-pharmaceutical public health interventions call for the use of gowns, gloves and surgical masks to protect HCWs while providing care (WHO Writing Group 2006). While the use of such equipment may decrease the risk of transmission for an individual encounter with an infectious patient, the sheer number of encounters over the course of a working day would probably still result in disease transmission. Even with excellent infection control practices, attack rates of greater than 10% are likely to occur among HCWs in the absence of vaccination (Salgado et al. 2002). There is some evidence that infants, elderly and immunocompromised subjects may shed virus for several weeks (Salgado et al. 2002), which makes transmission of the disease even more difficult to control in the hospital and the community. It is also well recognized that even if protected at work, HCWs are just as probable as the GP to become infected outside of the workplace.
This study has several limitations that merit further discussion. Our model assumes homogeneous mixing, which may underestimate R0 and result in a high sensitivity of the epidemic size to interventions, particularly at the early stages of an outbreak when stochastic effects play a dominant role in spreading the disease. Therefore, when the detailed structure of network contacts and mobility patterns are well identified, further evaluation of a prophylaxis strategy should provide more accurate quantitative predictions. We assumed that antiviral therapy will provide the same protection during a pandemic as it has been shown to provide in seasonal influenza. This assumption is common to other modelling studies (Longini et al. 2004, 2005; Ferguson et al. 2005). We have only considered the impact of prophylaxis of HCWs as it relates to the provision of antiviral treatment to the GP, yet we understand that HCWs provide far more care than this: hospital beds and modern therapies are essentially irrelevant without staffing. This simplification will result in an underestimation of the impact of prophylaxis of HCW on the morbidity and mortality of the GP. We have also considered all HCWs as a uniform group, yet they clearly fill different roles in the healthcare setting. Our model assumed that the supply of antiviral treatment for the entire course of the outbreak is secured. If the scarcity of antiviral drugs appears while the outbreak is peaking, it may leave HCWs susceptible to a high incidence of infection. This is unfavourable to the treatment strategy, which can be concomitant with a large absenteeism of HCWs due to the concern over infection at healthcare settings. This may suggest that susceptibility of HCWs at the early stages of an outbreak would become favourable to the treatment strategy by providing immunity upon recovery from the disease. However, a pandemic strain with high mortality rate, particularly in younger adults, can significantly affect the outcome of the treatment strategy. Finally, we have not considered the emergence of drug-resistant viral strains through the widespread or indiscriminate use of antiviral drugs (Moscona 2005; Rogers & Bonhoeffer 2006). This can substantially limit the impact of antiviral drugs and increase the likelihood of multiple pandemic waves caused by the evolution of resistant viral mutants.
Our findings suggest that the overall healthcare benefit of prophylaxis strategy may be much greater than other protective measures. The cost of providing universal oseltamivir prophylaxis to 135 025 HCWs in the province of Ontario is approximately $CDN 316 000 per day or approximately 17.7 million dollars over an eight-week period. The necessary infrastructure to deliver this therapy would be an additional cost. While this strategy may appear cost prohibitive, its financial burden must be balanced with the inevitably far greater cost savings obtained through preventing substantial influenza-related morbidity and mortality in the GP. With the knowledge that prophylaxis of HCWs alone may not prevent the occurrence of an outbreak if expected, it may indeed be necessary to decelerate the spread of infection in the population and afford the time for vaccine manufacturing. While efforts are being made to shorten the delay in vaccine availability, retarding the progression of the first pandemic wave is crucial for preventing excessive deaths and diminishing socio-economic disruption.
Acknowledgments
This research was partially supported by the Natural Sciences and Engineering Research Council of Canada (NSERC), Canada Research Chairs Program (CRCP), Mathematics of Information Technology and Complex Systems (MITACS) and Ontario Ministry of Health and Long-Term Care (OMHLC). The authors also would like to thank Fred Brauer, Ping Yan, John Edmunds, Beni Sahai and Allison McGeer for their critical comments on the preliminarily version of this paper that was presented during ‘Pandemic Influenza Modelling Meeting’ organized by the Public Health Agency of Canada in Toronto, on 27–28 March 2006.
Supplementary Material
Model development, analysis, and parameter estimation
References
- Arino J, Brauer F, van den Driessche P, Watmough J, Wu J. Simple models for containment of a pandemic. J. R. Soc. Interface. 2006;3:453–457. doi: 10.1098/rsif.2006.0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balicer R.D, Huerta M, Davidovitch N, Grotto I. Cost-benefit of stockpiling drugs for influenza pandemic. Emerg. Infect. Dis. 2005;11:1280–1282. doi: 10.3201/eid1108.041156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges C.B, Kuehnert M.J, Hall C.B. Transmission of influenza: implications for control in health care settings. Clin. Infect. Dis. 2003;37:1094–1101. doi: 10.1086/378292. [DOI] [PubMed] [Google Scholar]
- Cinti S, Chenoweth C, Monto A.S. Preparing for pandemic influenza: should hospitals stockpile oseltamivir? Infect. Cont. Hosp. Epidemiol. 2005;26:852–854. doi: 10.1086/502507. [DOI] [PubMed] [Google Scholar]
- Cox N.J, Tamblyn S.E, Tam T. Influenza pandemic planning. Vaccine. 2003;21:1801–1803. doi: 10.1016/S0264-410X(03)00076-8. [DOI] [PubMed] [Google Scholar]
- Diekmann O, Heesterbeek J.A.P. Wiley; Chichester, UK: 2000. Mathematical epidemiology of infectious diseases. [Google Scholar]
- Elder A.G, O'Donnell B, McCruden E.A, Symington I.S, Carman W.F. Incidence and recall of influenza in a cohort of Glasgow healthcare workers during the 1993–4 epidemic: results of serum testing and questionnaire. Br. Med. J. 1996;313:1241–1242. doi: 10.1136/bmj.313.7067.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson N.M, Mallett S, Jackson H, Roberts N, Ward P. A population-dynamic model for evaluating the potential spread of drug-resistant influenza virus infections during community-based use of antivirals. J. Antimicrob. Chemother. 2003;51:977–990. doi: 10.1093/jac/dkg136. [DOI] [PubMed] [Google Scholar]
- Ferguson N.M, Cummings D.A.T, Cauchemez S, Fraser C, Riley S, Meeyai A, Iamsirithaworn S, Burke D.S. Strategies for containing an emerging influenza pandemic in Southeast Asia. Nature. 2005;437:209–214. doi: 10.1038/nature04017. [DOI] [PubMed] [Google Scholar]
- Ferguson N.M, Cummings D.A, Fraser C, Cajka J.C, Cooley P.C, Burke D.S. Strategies for mitigating an influenza pandemic. Nature. 2006;442:448–452. doi: 10.1038/nature04795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gani R, Hughes H, Fleming D, Griffin T, Medlock J, Leach S. Potential impact of antiviral drug use during influenza pandemic. Emerg. Infect. Dis. 2005;9:1355–1362. doi: 10.3201/eid1109.041344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoft D.F, Belshe R.B. The genetic archaeology of influenza. New Engl. J. Med. 2004;351:2550–2551. doi: 10.1056/NEJMcibr043708. [DOI] [PubMed] [Google Scholar]
- Horcajada J.P, et al. A nosocomial outbreak of influenza during a period without influenza epidemic activity. Eur. Respir. J. 2003;21:303–307. doi: 10.1183/09031936.03.00040503. [DOI] [PubMed] [Google Scholar]
- Jefferson T, Demicheli V, Rivetti D, Jones M, Di Pietrantonj C, Rivetti A. Antivirals for influenza in healthy adults: systematic review. Lancet. 2006;367:303–313. doi: 10.1016/S0140-6736(06)67970-1. [DOI] [PubMed] [Google Scholar]
- Lee V.J, Phua K.H, Chen M.I, Chow A, Ma S, Goh K.T, Leo Y.S. Economics of neuraminidase inhibitor stockpiling for pandemic influenza, Singapore. Emerg. Infect. Dis. 2006;12:95–102. doi: 10.3201/eid1201.050556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longini I.M, Jr, Halloran M.E, Nizam A, Yang Y. Containing pandemic influenza with antiviral agents. Am. J. Epidemiol. 2004;159:623–633. doi: 10.1093/aje/kwh092. [DOI] [PubMed] [Google Scholar]
- Longini I.M, Jr, Nizam A, Xu S, Ungchusak K, Hanshaoworakul W, Cummings D.A.T, Halloran M.E. Containing pandemic influenza at the source. Science. 2005;309:1083–1087. doi: 10.1126/science.1115717. [DOI] [PubMed] [Google Scholar]
- Low J.G.H, Wilder-Smith A. Infectious respiratory illnesses and their impact on healthcare workers: a review. Ann. Acad. Med. 2005;34:105–110. [PubMed] [Google Scholar]
- Moscona A. Oseltamivir resistance—disabling our influenza defenses. New Engl. J. Med. 2005;353:2633–2636. doi: 10.1056/NEJMp058291. [DOI] [PubMed] [Google Scholar]
- McKay M.D, Conover W.J, Beckman R.J. A comparison of three methods for selecting values of input variables in the analysis of output from a computer code. Technometrics. 1979;21:239–245. doi: 10.2307/1268522. [DOI] [Google Scholar]
- Meltzer M.I, Cox N.J, Fukuda K. The economic impact of pandemic influenza in the United States: priorities for intervention. Emerg. Infect. Dis. 1999;5:659–671. doi: 10.3201/eid0505.990507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen-Van-Tam J, Granfield R, Pearson J, Fleming D, Keating N. Do influenza epidemics affect patterns of sickness absence among British hospital staff? Infect. Control Hosp. Epidemiol. 1999;20:691–694. doi: 10.1086/501568. [DOI] [PubMed] [Google Scholar]
- Nicholson K.G, Wood J.M, Zambon M. Influenza. Lancet. 2003;362:1733–1745. doi: 10.1016/S0140-6736(03)14854-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers R.R, Bonhoeffer S. Emergence of drug-resistance influenza virus: population dynamical considerations. Science. 2006;312:389–391. doi: 10.1126/science.1122947. [DOI] [PubMed] [Google Scholar]
- Salgado C.D, Farr B.M, Hall K.K, Hayden F.G. Influenza in the acute hospital setting. Lancet Infect. Dis. 2002;2:145–155. doi: 10.1016/S1473-3099(02)00221-9. [DOI] [PubMed] [Google Scholar]
- Simeonsson K, Summers-Bean C, Connolly A. Influenza vaccination of healthcare workers: institutional strategies for improving rates. NC Med. J. 2004;65:323–329. [PubMed] [Google Scholar]
- Taubenberger J.K, Morens D.M. 1918 influenza: the mother of all pandemics. Emerg. Infect. Dis. 2006;12:15–22. doi: 10.3201/eid1201.050979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Driessche P, Watmough J. Reproduction numbers and subthreshold endemic equilibria for compartmental models of disease transmission. Math. Biosci. 2002;180:29–48. doi: 10.1016/S0025-5564(02)00108-6. [DOI] [PubMed] [Google Scholar]
- Weingarten S, Riedinger M, Bolton L.B, Miles P, Ault M. Barriers to influenza vaccine acceptance. A survey of physicians and nurses. Am. J. Infect. Control. 1989;17:202–207. doi: 10.1016/0196-6553(89)90129-6. [DOI] [PubMed] [Google Scholar]
- WHO report on global surveillance of epidemic-prone infectious diseases, 2000, WHO/CDS/CSR/ISR/2000.1.
- World Health Organization Writing Group. Nonpharmaceutical interventions for pandemic influenza, international measures. Emerg. Infect. Dis. 2006;12:81–87. doi: 10.3201/eid1201.051370. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Model development, analysis, and parameter estimation