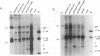Abstract
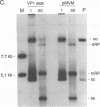
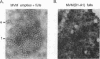
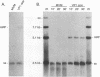
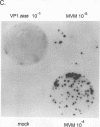
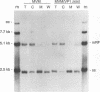
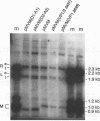
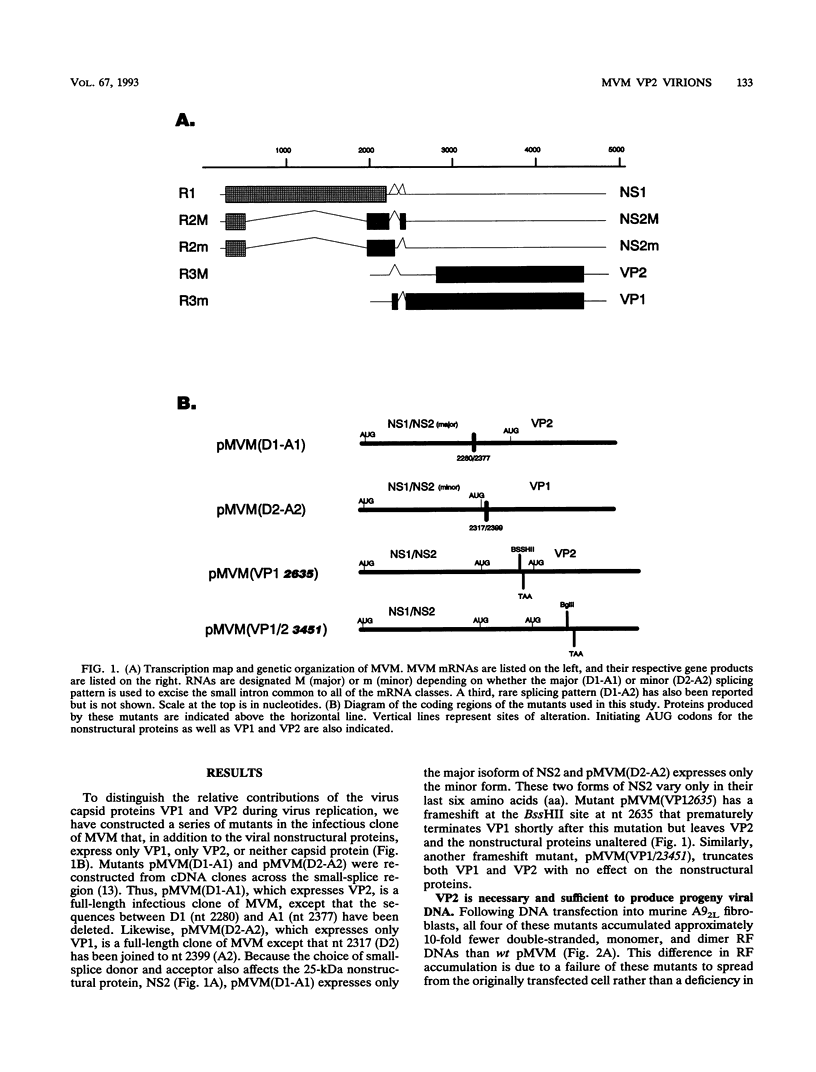
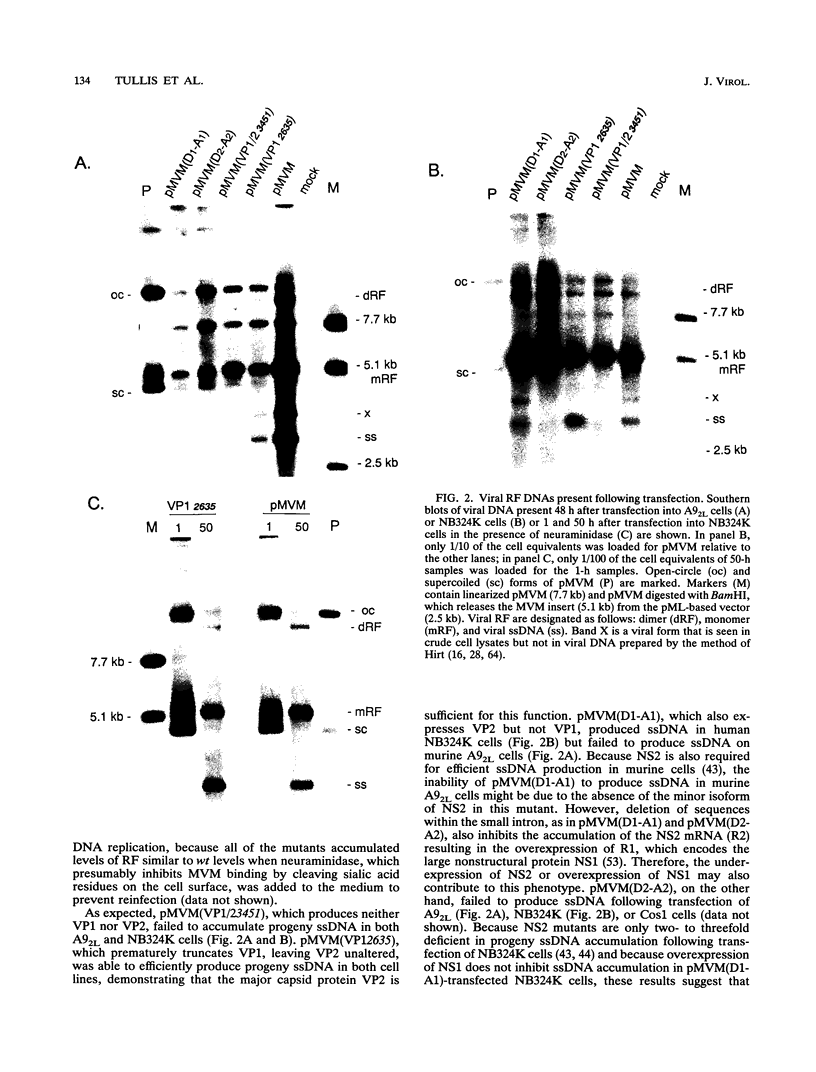
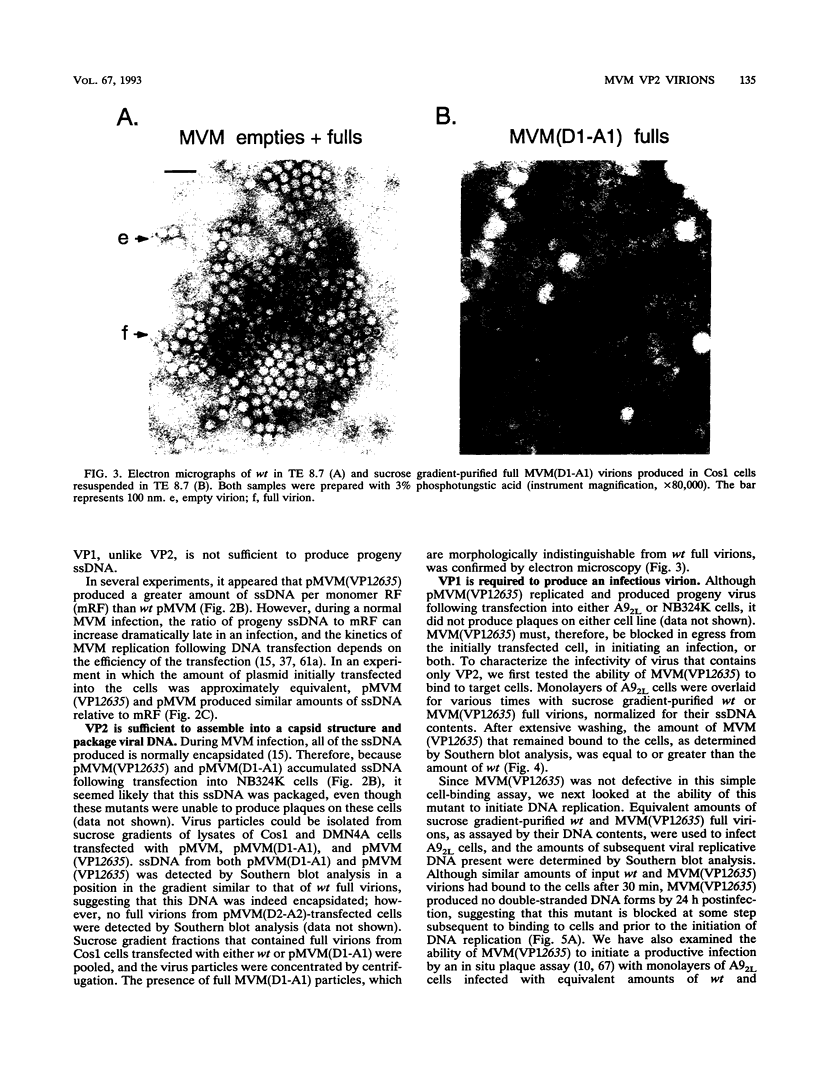
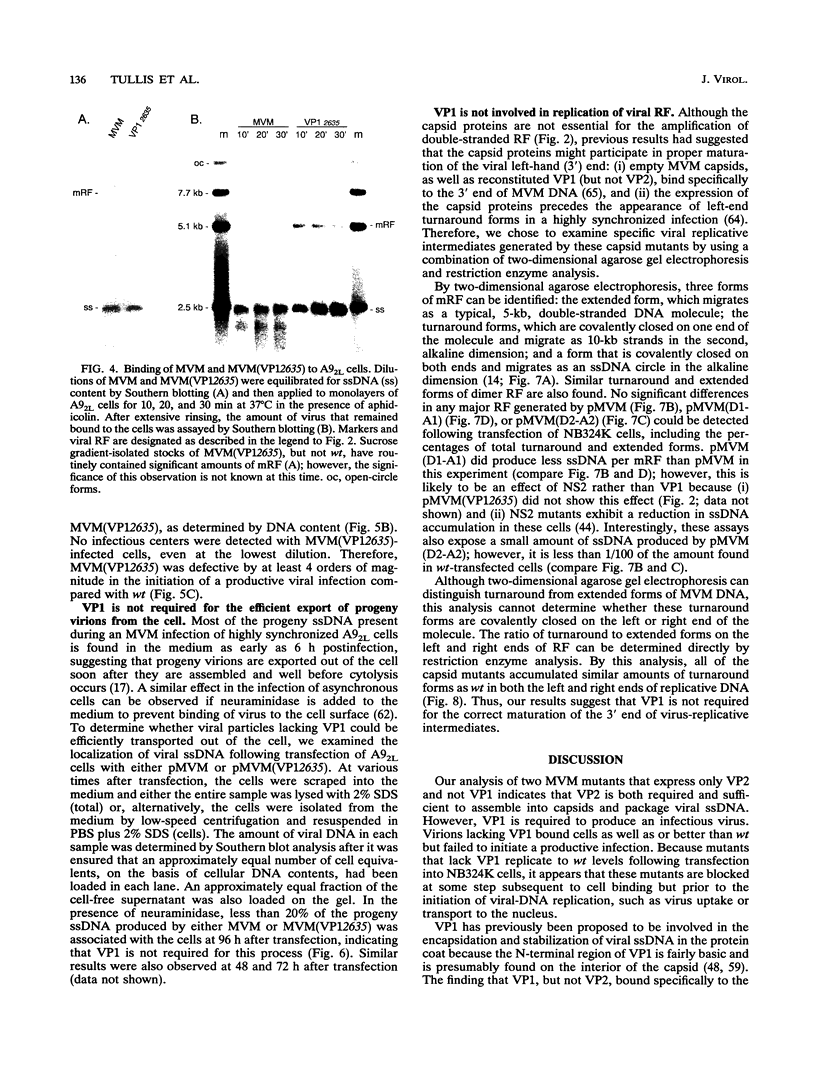
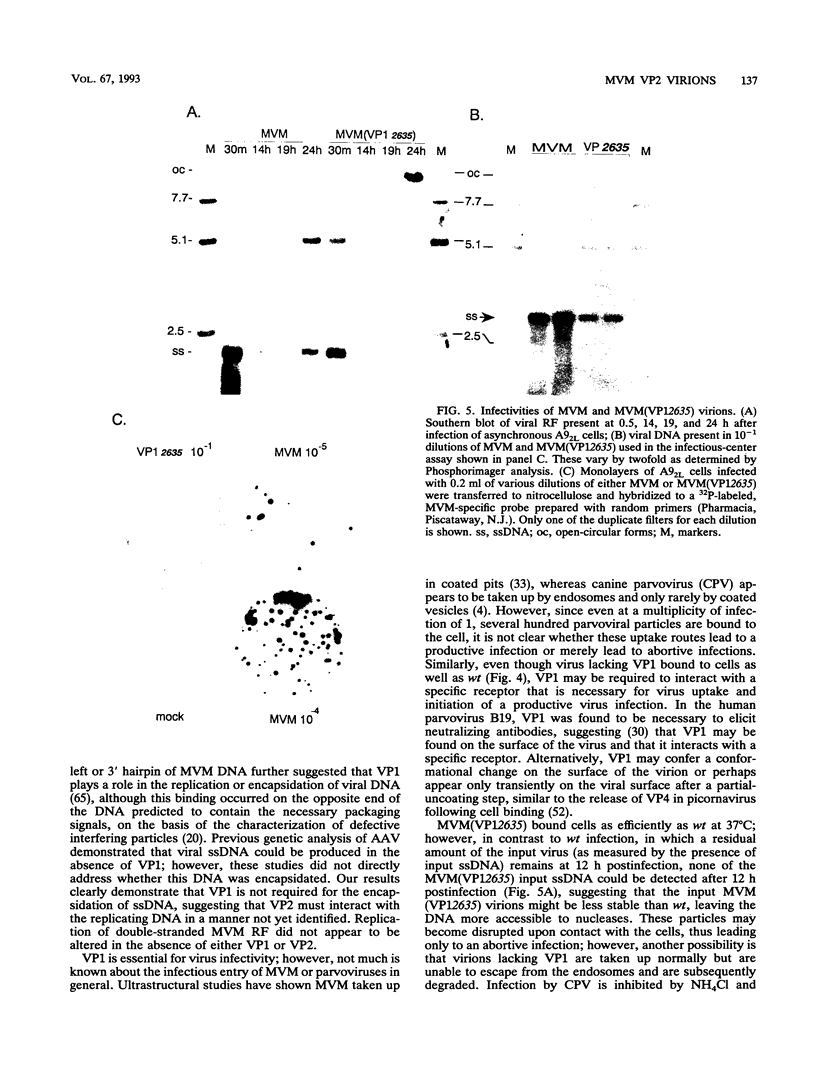
The two capsid proteins of minute virus of mice, VP1 and VP2, are generated from a single large open reading frame by alternate splicing of the capsid gene mRNA. Examination of the replication of a series of mutants that express only VP1, only VP2, or neither capsid protein demonstrates that VP2 is necessary for the accumulation and encapsidation of virus progeny single-stranded DNA. VP1 is dispensable for these functions but is required to produce an infectious virion. Virus that lacks VP1 binds to cells as efficiently as wild-type minute virus of mice but fails to initiate a productive infection. Because neither capsid protein is required for viral-DNA replication, these results suggest that virus lacking VP1 is blocked at a step during virus entry, subsequent to cell binding and prior to the initiation of DNA replication.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexandersen S., Bloom M. E., Perryman S. Detailed transcription map of Aleutian mink disease parvovirus. J Virol. 1988 Oct;62(10):3684–3694. doi: 10.1128/jvi.62.10.3684-3694.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astell C. R., Thomson M., Merchlinsky M., Ward D. C. The complete DNA sequence of minute virus of mice, an autonomous parvovirus. Nucleic Acids Res. 1983 Feb 25;11(4):999–1018. doi: 10.1093/nar/11.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball-Goodrich L. J., Tattersall P. Two amino acid substitutions within the capsid are coordinately required for acquisition of fibrotropism by the lymphotropic strain of minute virus of mice. J Virol. 1992 Jun;66(6):3415–3423. doi: 10.1128/jvi.66.6.3415-3423.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basak S., Turner H. Infectious entry pathway for canine parvovirus. Virology. 1992 Feb;186(2):368–376. doi: 10.1016/0042-6822(92)90002-7. [DOI] [PubMed] [Google Scholar]
- Becerra S. P., Rose J. A., Hardy M., Baroudy B. M., Anderson C. W. Direct mapping of adeno-associated virus capsid proteins B and C: a possible ACG initiation codon. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7919–7923. doi: 10.1073/pnas.82.23.7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom M. E., Alexandersen S., Perryman S., Lechner D., Wolfinbarger J. B. Nucleotide sequence and genomic organization of Aleutian mink disease parvovirus (ADV): sequence comparisons between a nonpathogenic and a pathogenic strain of ADV. J Virol. 1988 Aug;62(8):2903–2915. doi: 10.1128/jvi.62.8.2903-2915.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissy R., Astell C. R. An Escherichia coli recBCsbcBrecF host permits the deletion-resistant propagation of plasmid clones containing the 5'-terminal palindrome of minute virus of mice. Gene. 1985;35(1-2):179–185. doi: 10.1016/0378-1119(85)90170-2. [DOI] [PubMed] [Google Scholar]
- Bouck N., di Mayorca G. Chemical carcinogens transform BHK cells by inducing a recessive mutation. Mol Cell Biol. 1982 Feb;2(2):97–105. doi: 10.1128/mcb.2.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassinotti P., Weitz M., Tratschin J. D. Organization of the adeno-associated virus (AAV) capsid gene: mapping of a minor spliced mRNA coding for virus capsid protein 1. Virology. 1988 Nov;167(1):176–184. [PubMed] [Google Scholar]
- Cater J. E., Pintel D. J. The small non-structural protein NS2 of the autonomous parvovirus minute virus of mice is required for virus growth in murine cells. J Gen Virol. 1992 Jul;73(Pt 7):1839–1843. doi: 10.1099/0022-1317-73-7-1839. [DOI] [PubMed] [Google Scholar]
- Chen K. C., Shull B. C., Moses E. A., Lederman M., Stout E. R., Bates R. C. Complete nucleotide sequence and genome organization of bovine parvovirus. J Virol. 1986 Dec;60(3):1085–1097. doi: 10.1128/jvi.60.3.1085-1097.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citovsky V., Zupan J., Warnick D., Zambryski P. Nuclear localization of Agrobacterium VirE2 protein in plant cells. Science. 1992 Jun 26;256(5065):1802–1805. doi: 10.1126/science.1615325. [DOI] [PubMed] [Google Scholar]
- Clemens K. E., Cerutis D. R., Burger L. R., Yang C. Q., Pintel D. J. Cloning of minute virus of mice cDNAs and preliminary analysis of individual viral proteins expressed in murine cells. J Virol. 1990 Aug;64(8):3967–3973. doi: 10.1128/jvi.64.8.3967-3973.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotmore S. F., Gunther M., Tattersall P. Evidence for a ligation step in the DNA replication of the autonomous parvovirus minute virus of mice. J Virol. 1989 Feb;63(2):1002–1006. doi: 10.1128/jvi.63.2.1002-1006.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotmore S. F., Tattersall P. A genome-linked copy of the NS-1 polypeptide is located on the outside of infectious parvovirus particles. J Virol. 1989 Sep;63(9):3902–3911. doi: 10.1128/jvi.63.9.3902-3911.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotmore S. F., Tattersall P. The NS-1 polypeptide of minute virus of mice is covalently attached to the 5' termini of duplex replicative-form DNA and progeny single strands. J Virol. 1988 Mar;62(3):851–860. doi: 10.1128/jvi.62.3.851-860.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotmore S. F., Tattersall P. The autonomously replicating parvoviruses of vertebrates. Adv Virus Res. 1987;33:91–174. doi: 10.1016/s0065-3527(08)60317-6. [DOI] [PubMed] [Google Scholar]
- Dingwall C., Laskey R. A. Nuclear targeting sequences--a consensus? Trends Biochem Sci. 1991 Dec;16(12):478–481. doi: 10.1016/0968-0004(91)90184-w. [DOI] [PubMed] [Google Scholar]
- Dworetzky S. I., Feldherr C. M. Translocation of RNA-coated gold particles through the nuclear pores of oocytes. J Cell Biol. 1988 Mar;106(3):575–584. doi: 10.1083/jcb.106.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faust E. A., Ward D. C. Incomplete genomes of the parvovirus minute virus of mice: selective conservation of genome termini, including the origin for DNA replication. J Virol. 1979 Oct;32(1):276–292. doi: 10.1128/jvi.32.1.276-292.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldherr C. M., Kallenbach E., Schultz N. Movement of a karyophilic protein through the nuclear pores of oocytes. J Cell Biol. 1984 Dec;99(6):2216–2222. doi: 10.1083/jcb.99.6.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner E. M., Tattersall P. Evidence that developmentally regulated control of gene expression by a parvoviral allotropic determinant is particle mediated. J Virol. 1988 May;62(5):1713–1722. doi: 10.1128/jvi.62.5.1713-1722.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith G. R., Marriott S. J., Rintoul D. A., Consigli R. A. Early events in polyomavirus infection: fusion of monopinocytotic vesicles containing virions with mouse kidney cell nuclei. Virus Res. 1988 Apr;10(1):41–51. doi: 10.1016/0168-1702(88)90056-1. [DOI] [PubMed] [Google Scholar]
- Hamm J., Darzynkiewicz E., Tahara S. M., Mattaj I. W. The trimethylguanosine cap structure of U1 snRNA is a component of a bipartite nuclear targeting signal. Cell. 1990 Aug 10;62(3):569–577. doi: 10.1016/0092-8674(90)90021-6. [DOI] [PubMed] [Google Scholar]
- Hermonat P. L., Labow M. A., Wright R., Berns K. I., Muzyczka N. Genetics of adeno-associated virus: isolation and preliminary characterization of adeno-associated virus type 2 mutants. J Virol. 1984 Aug;51(2):329–339. doi: 10.1128/jvi.51.2.329-339.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Jongeneel C. V., Sahli R., McMaster G. K., Hirt B. A precise map of splice junctions in the mRNAs of minute virus of mice, an autonomous parvovirus. J Virol. 1986 Sep;59(3):564–573. doi: 10.1128/jvi.59.3.564-573.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajigaya S., Fujii H., Field A., Anderson S., Rosenfeld S., Anderson L. J., Shimada T., Young N. S. Self-assembled B19 parvovirus capsids, produced in a baculovirus system, are antigenically and immunogenically similar to native virions. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4646–4650. doi: 10.1073/pnas.88.11.4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. THREE DEGREES OF GUANYLIC ACID--INOSINIC ACID PYROPHOSPHORYLASE DEFICIENCY IN MOUSE FIBROBLASTS. Nature. 1964 Sep 12;203:1142–1144. doi: 10.1038/2031142a0. [DOI] [PubMed] [Google Scholar]
- Labieniec-Pintel L., Pintel D. The minute virus of mice P39 transcription unit can encode both capsid proteins. J Virol. 1986 Mar;57(3):1163–1167. doi: 10.1128/jvi.57.3.1163-1167.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lederman M., Shull B. C., Stout E. R., Bates R. C. Bovine parvovirus DNA-binding proteins: identification by a combined DNA hybridization and immunodetection assay. J Gen Virol. 1987 Jan;68(Pt 1):147–157. doi: 10.1099/0022-1317-68-1-147. [DOI] [PubMed] [Google Scholar]
- Martin K., Helenius A. Transport of incoming influenza virus nucleocapsids into the nucleus. J Virol. 1991 Jan;65(1):232–244. doi: 10.1128/jvi.65.1.232-244.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchlinsky M. J., Tattersall P. J., Leary J. J., Cotmore S. F., Gardiner E. M., Ward D. C. Construction of an infectious molecular clone of the autonomous parvovirus minute virus of mice. J Virol. 1983 Jul;47(1):227–232. doi: 10.1128/jvi.47.1.227-232.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf J. B., Bates R. C., Lederman M. Interaction of virally coded protein and a cell cycle-regulated cellular protein with the bovine parvovirus left terminus ori. J Virol. 1990 Nov;64(11):5485–5490. doi: 10.1128/jvi.64.11.5485-5490.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaud N., Goldfarb D. S. Multiple pathways in nuclear transport: the import of U2 snRNP occurs by a novel kinetic pathway. J Cell Biol. 1991 Jan;112(2):215–223. doi: 10.1083/jcb.112.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreland R. B., Montross L., Garcea R. L. Characterization of the DNA-binding properties of the polyomavirus capsid protein VP1. J Virol. 1991 Mar;65(3):1168–1176. doi: 10.1128/jvi.65.3.1168-1176.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan W. R., Ward D. C. Three splicing patterns are used to excise the small intron common to all minute virus of mice RNAs. J Virol. 1986 Dec;60(3):1170–1174. doi: 10.1128/jvi.60.3.1170-1174.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeger L. K., Cater J., Pintel D. J. The small nonstructural protein (NS2) of the parvovirus minute virus of mice is required for efficient DNA replication and infectious virus production in a cell-type-specific manner. J Virol. 1990 Dec;64(12):6166–6175. doi: 10.1128/jvi.64.12.6166-6175.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradiso P. R., Williams K. R., Costantino R. L. Mapping of the amino terminus of the H-1 parvovirus major capsid protein. J Virol. 1984 Oct;52(1):77–81. doi: 10.1128/jvi.52.1.77-81.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrish C. R., Aquadro C. F., Carmichael L. E. Canine host range and a specific epitope map along with variant sequences in the capsid protein gene of canine parvovirus and related feline, mink, and raccoon parvoviruses. Virology. 1988 Oct;166(2):293–307. doi: 10.1016/0042-6822(88)90500-4. [DOI] [PubMed] [Google Scholar]
- Ranz A. I., Manclús J. J., Díaz-Aroca E., Casal J. I. Porcine parvovirus: DNA sequence and genome organization. J Gen Virol. 1989 Oct;70(Pt 10):2541–2553. doi: 10.1099/0022-1317-70-10-2541. [DOI] [PubMed] [Google Scholar]
- Rhode S. L., 3rd, Paradiso P. R. Parvovirus genome: nucleotide sequence of H-1 and mapping of its genes by hybrid-arrested translation. J Virol. 1983 Jan;45(1):173–184. doi: 10.1128/jvi.45.1.173-184.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Replication process of the parvovirus H-1 V. Isolation and characterization of temperature-sensitive H-1 mutants. J Virol. 1976 Feb;17(2):659–667. doi: 10.1128/jvi.17.2.659-667.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spalholz B. A., Tattersall P. Interaction of minute virus of mice with differentiated cells: strain-dependent target cell specificity is mediated by intracellular factors. J Virol. 1983 Jun;46(3):937–943. doi: 10.1128/jvi.46.3.937-943.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A., Lusby E. W., Berns K. I. Nucleotide sequence and organization of the adeno-associated virus 2 genome. J Virol. 1983 Feb;45(2):555–564. doi: 10.1128/jvi.45.2.555-564.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersall P., Bratton J. Reciprocal productive and restrictive virus-cell interactions of immunosuppressive and prototype strains of minute virus of mice. J Virol. 1983 Jun;46(3):944–955. doi: 10.1128/jvi.46.3.944-955.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersall P., Cawte P. J., Shatkin A. J., Ward D. C. Three structural polypeptides coded for by minite virus of mice, a parvovirus. J Virol. 1976 Oct;20(1):273–289. doi: 10.1128/jvi.20.1.273-289.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersall P., Shatkin A. J., Ward D. C. Sequence homology between the structural polypeptides of minute virus of mice. J Mol Biol. 1977 Apr 25;111(4):375–394. doi: 10.1016/s0022-2836(77)80060-0. [DOI] [PubMed] [Google Scholar]
- Tratschin J. D., Miller I. L., Carter B. J. Genetic analysis of adeno-associated virus: properties of deletion mutants constructed in vitro and evidence for an adeno-associated virus replication function. J Virol. 1984 Sep;51(3):611–619. doi: 10.1128/jvi.51.3.611-619.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao J., Chapman M. S., Agbandje M., Keller W., Smith K., Wu H., Luo M., Smith T. J., Rossmann M. G., Compans R. W. The three-dimensional structure of canine parvovirus and its functional implications. Science. 1991 Mar 22;251(5000):1456–1464. doi: 10.1126/science.2006420. [DOI] [PubMed] [Google Scholar]
- Tullis G. E., Labieniec-Pintel L., Clemens K. E., Pintel D. Generation and characterization of a temperature-sensitive mutation in the NS-1 gene of the autonomous parvovirus minute virus of mice. J Virol. 1988 Aug;62(8):2736–2744. doi: 10.1128/jvi.62.8.2736-2744.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willwand K., Hirt B. The minute virus of mice capsid specifically recognizes the 3' hairpin structure of the viral replicative-form DNA: mapping of the binding site by hydroxyl radical footprinting. J Virol. 1991 Sep;65(9):4629–4635. doi: 10.1128/jvi.65.9.4629-4635.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willwand K., Kaaden O. R. Proteins of viral and cellular origin bind to the Aleutian disease virus (ADV) DNA 3'-terminal hairpin: presentation of a scheme for encapsidation of ADV DNA. J Virol. 1990 Apr;64(4):1598–1605. doi: 10.1128/jvi.64.4.1598-1605.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakobson B., Hrynko T. A., Peak M. J., Winocour E. Replication of adeno-associated virus in cells irradiated with UV light at 254 nm. J Virol. 1989 Mar;63(3):1023–1030. doi: 10.1128/jvi.63.3.1023-1030.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]