Abstract
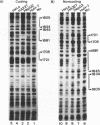
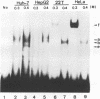
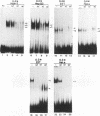
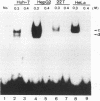
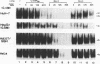
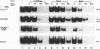
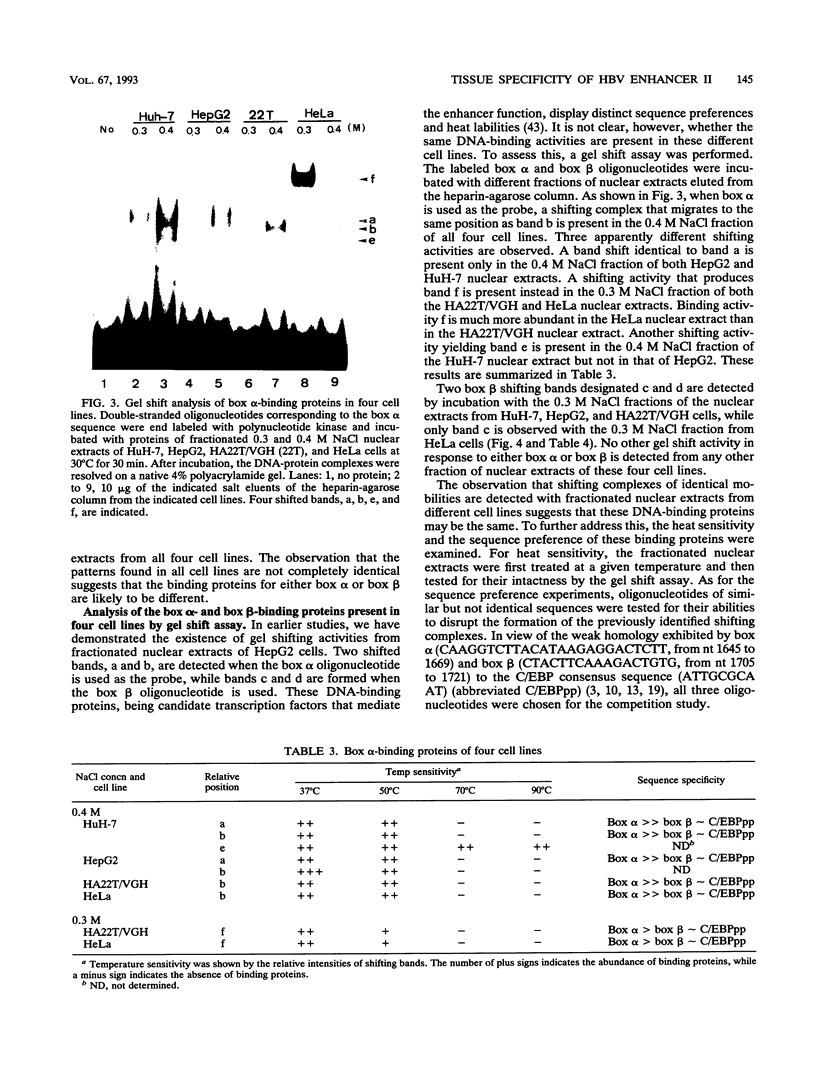
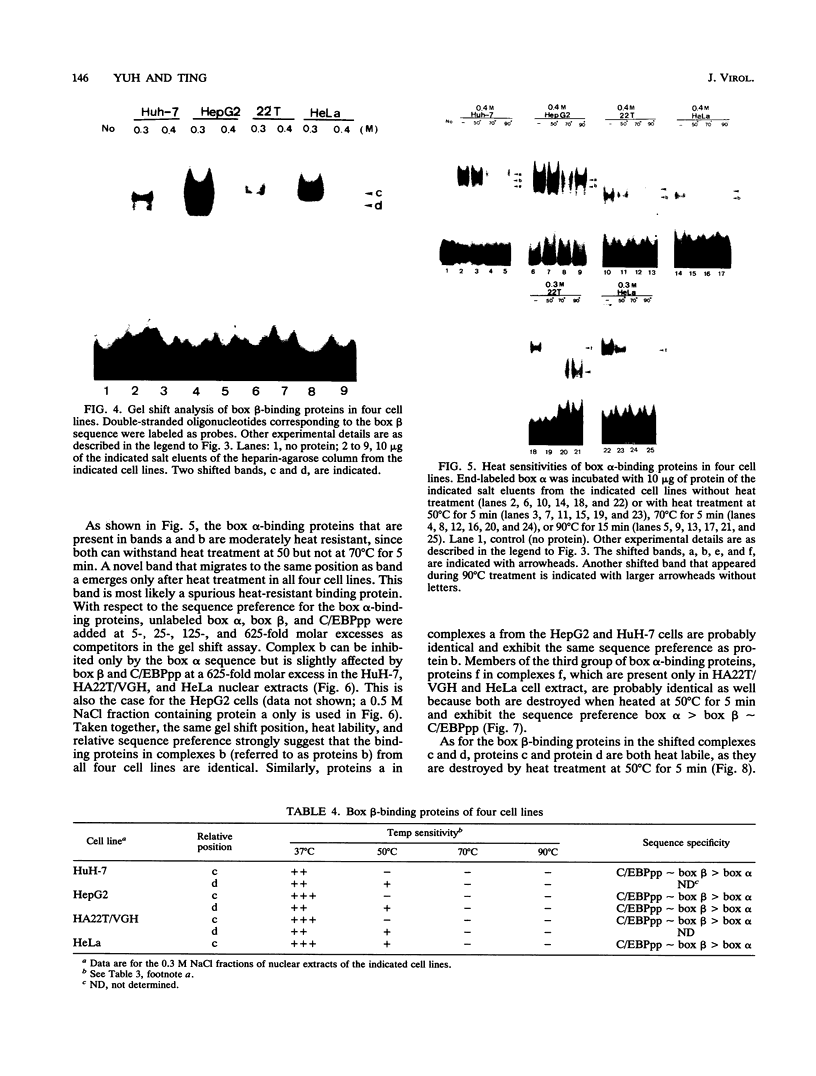
Hepatitis B virus is a hepatotropic virus. Its replication and gene expression are mainly restricted to hepatocytes in the infective process. The viral gene expression thus provides a unique system with which to study the control of tissue-specific gene expression. We have previously reported the identification and characterization of the second enhancer (enhancer II) of hepatitis B virus. In this report, we further demonstrate that the minimal functional constituents of the second enhancer, box alpha and box beta, display liver cell and differentiation state specificity. Moreover, box alpha exhibits the same liver cell and differentiation state specificity when functioning as an upstream regulator for the basal core promoter. Gel shift experiments reveal a unique box alpha-binding protein, protein a, which is present only in differentiated liver cells, where enhancer II is functional. The converse is true for another box alpha-binding protein, protein f, which is present only in poorly differentiated liver cells and nonliver cells. The simplest hypothesis that explains these results is that protein a activates and/or protein f suppresses the enhancer and upstream regulator functions. Although C/EBP is a candidate for a transcription factor that interacts with box alpha or box beta, none of the binding factors identified in the gel shift assays, including protein a and protein f, is likely to be C/EBP because they differ from C/EBP in heat lability and sequence preference.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aden D. P., Fogel A., Plotkin S., Damjanov I., Knowles B. B. Controlled synthesis of HBsAg in a differentiated human liver carcinoma-derived cell line. Nature. 1979 Dec 6;282(5739):615–616. doi: 10.1038/282615a0. [DOI] [PubMed] [Google Scholar]
- Araki K., Miyazaki J., Hino O., Tomita N., Chisaka O., Matsubara K., Yamamura K. Expression and replication of hepatitis B virus genome in transgenic mice. Proc Natl Acad Sci U S A. 1989 Jan;86(1):207–211. doi: 10.1073/pnas.86.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z., Umek R. M., McKnight S. L. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 1991 Sep;5(9):1538–1552. doi: 10.1101/gad.5.9.1538. [DOI] [PubMed] [Google Scholar]
- Cattaneo R., Will H., Hernandez N., Schaller H. Signals regulating hepatitis B surface antigen transcription. Nature. 1983 Sep 22;305(5932):336–338. doi: 10.1038/305336a0. [DOI] [PubMed] [Google Scholar]
- Cattaneo R., Will H., Schaller H. Hepatitis B virus transcription in the infected liver. EMBO J. 1984 Sep;3(9):2191–2196. doi: 10.1002/j.1460-2075.1984.tb02113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. M., Jeng K. S., Hu C. P., Lo S. J., Su T. S., Ting L. P., Chou C. K., Han S. H., Pfaff E., Salfeld J. Production of hepatitis B virus in vitro by transient expression of cloned HBV DNA in a hepatoma cell line. EMBO J. 1987 Mar;6(3):675–680. doi: 10.1002/j.1460-2075.1987.tb04807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C., Lin Y., O-Lee T. W., Chou C. K., Lee T. S., Liu T. J., P'eng F. K., Chen T. Y., Hu C. P. Induction of plasma protein secretion in a newly established human hepatoma cell line. Mol Cell Biol. 1983 Jun;3(6):1133–1137. doi: 10.1128/mcb.3.6.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H. K., Ting L. P. The surface gene promoter of the human hepatitis B virus displays a preference for differentiated hepatocytes. Virology. 1989 May;170(1):176–183. doi: 10.1016/0042-6822(89)90364-4. [DOI] [PubMed] [Google Scholar]
- Chang H. K., Wang B. Y., Yuh C. H., Wei C. L., Ting L. P. A liver-specific nuclear factor interacts with the promoter region of the large surface protein gene of human hepatitis B virus. Mol Cell Biol. 1989 Nov;9(11):5189–5197. doi: 10.1128/mcb.9.11.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christy R. J., Yang V. W., Ntambi J. M., Geiman D. E., Landschulz W. H., Friedman A. D., Nakabeppu Y., Kelly T. J., Lane M. D. Differentiation-induced gene expression in 3T3-L1 preadipocytes: CCAAT/enhancer binding protein interacts with and activates the promoters of two adipocyte-specific genes. Genes Dev. 1989 Sep;3(9):1323–1335. doi: 10.1101/gad.3.9.1323. [DOI] [PubMed] [Google Scholar]
- Elfassi E., Romet-Lemonne J. L., Essex M., Frances-McLane M., Haseltine W. A. Evidence of extrachromosomal forms of hepatitis B viral DNA in a bone marrow culture obtained from a patient recently infected with hepatitis B virus. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3526–3528. doi: 10.1073/pnas.81.11.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farza H., Hadchouel M., Scotto J., Tiollais P., Babinet C., Pourcel C. Replication and gene expression of hepatitis B virus in a transgenic mouse that contains the complete viral genome. J Virol. 1988 Nov;62(11):4144–4152. doi: 10.1128/jvi.62.11.4144-4152.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman A. D., Landschulz W. H., McKnight S. L. CCAAT/enhancer binding protein activates the promoter of the serum albumin gene in cultured hepatoma cells. Genes Dev. 1989 Sep;3(9):1314–1322. doi: 10.1101/gad.3.9.1314. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W. T., Bell K. D., Ou J. H. Characterization of the hepatitis B virus EnhI enhancer and X promoter complex. J Virol. 1991 Dec;65(12):6686–6692. doi: 10.1128/jvi.65.12.6686-6692.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera R., Ro H. S., Robinson G. S., Xanthopoulos K. G., Spiegelman B. M. A direct role for C/EBP and the AP-I-binding site in gene expression linked to adipocyte differentiation. Mol Cell Biol. 1989 Dec;9(12):5331–5339. doi: 10.1128/mcb.9.12.5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honigwachs J., Faktor O., Dikstein R., Shaul Y., Laub O. Liver-specific expression of hepatitis B virus is determined by the combined action of the core gene promoter and the enhancer. J Virol. 1989 Feb;63(2):919–924. doi: 10.1128/jvi.63.2.919-924.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaestner K. H., Christy R. J., Lane M. D. Mouse insulin-responsive glucose transporter gene: characterization of the gene and trans-activation by the CCAAT/enhancer binding protein. Proc Natl Acad Sci U S A. 1990 Jan;87(1):251–255. doi: 10.1073/pnas.87.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., Adashi E. Y., Graves B. J., McKnight S. L. Isolation of a recombinant copy of the gene encoding C/EBP. Genes Dev. 1988 Jul;2(7):786–800. doi: 10.1101/gad.2.7.786. [DOI] [PubMed] [Google Scholar]
- Li X. X., Huang J. H., Rienhoff H. Y., Jr, Liao W. S. Two adjacent C/EBP-binding sequences that participate in the cell-specific expression of the mouse serum amyloid A3 gene. Mol Cell Biol. 1990 Dec;10(12):6624–6631. doi: 10.1128/mcb.10.12.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtsteiner S., Wuarin J., Schibler U. The interplay of DNA-binding proteins on the promoter of the mouse albumin gene. Cell. 1987 Dec 24;51(6):963–973. doi: 10.1016/0092-8674(87)90583-6. [DOI] [PubMed] [Google Scholar]
- López-Cabrera M., Letovsky J., Hu K. Q., Siddiqui A. Multiple liver-specific factors bind to the hepatitis B virus core/pregenomic promoter: trans-activation and repression by CCAAT/enhancer binding protein. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5069–5073. doi: 10.1073/pnas.87.13.5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malpièce Y., Michel M. L., Carloni G., Revel M., Tiollais P., Weissenbach J. The gene S promoter of hepatitis B virus confers constitutive gene expression. Nucleic Acids Res. 1983 Jul 11;11(13):4645–4654. doi: 10.1093/nar/11.13.4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Goodbourn S., Fischer J. A. Regulation of inducible and tissue-specific gene expression. Science. 1987 Jun 5;236(4806):1237–1245. doi: 10.1126/science.3296191. [DOI] [PubMed] [Google Scholar]
- Mitchell P. J., Tjian R. Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins. Science. 1989 Jul 28;245(4916):371–378. doi: 10.1126/science.2667136. [DOI] [PubMed] [Google Scholar]
- Nakabayashi H., Taketa K., Miyano K., Yamane T., Sato J. Growth of human hepatoma cells lines with differentiated functions in chemically defined medium. Cancer Res. 1982 Sep;42(9):3858–3863. [PubMed] [Google Scholar]
- Poli V., Cortese R. Interleukin 6 induces a liver-specific nuclear protein that binds to the promoter of acute-phase genes. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8202–8206. doi: 10.1073/pnas.86.21.8202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall L. B., Standring D. N., Laub O., Rutter W. J. Transcription of hepatitis B virus by RNA polymerase II. Mol Cell Biol. 1983 Oct;3(10):1766–1773. doi: 10.1128/mcb.3.10.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raney A. K., Milich D. R., McLachlan A. Complex regulation of transcription from the hepatitis B virus major surface antigen promoter in human hepatoma cell lines. J Virol. 1991 Sep;65(9):4805–4811. doi: 10.1128/jvi.65.9.4805-4811.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roossinck M. J., Jameel S., Loukin S. H., Siddiqui A. Expression of hepatitis B viral core region in mammalian cells. Mol Cell Biol. 1986 May;6(5):1393–1400. doi: 10.1128/mcb.6.5.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger C., Ganem D., Varmus H. E. Biochemical and genetic evidence for the hepatitis B virus replication strategy. Science. 1986 Apr 25;232(4749):477–484. doi: 10.1126/science.3961490. [DOI] [PubMed] [Google Scholar]
- Shaul Y., Rutter W. J., Laub O. A human hepatitis B viral enhancer element. EMBO J. 1985 Feb;4(2):427–430. doi: 10.1002/j.1460-2075.1985.tb03646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers J., Mason W. S. Replication of the genome of a hepatitis B--like virus by reverse transcription of an RNA intermediate. Cell. 1982 Jun;29(2):403–415. doi: 10.1016/0092-8674(82)90157-x. [DOI] [PubMed] [Google Scholar]
- Sureau C., Romet-Lemonne J. L., Mullins J. I., Essex M. Production of hepatitis B virus by a differentiated human hepatoma cell line after transfection with cloned circular HBV DNA. Cell. 1986 Oct 10;47(1):37–47. doi: 10.1016/0092-8674(86)90364-8. [DOI] [PubMed] [Google Scholar]
- Tiollais P., Pourcel C., Dejean A. The hepatitis B virus. Nature. 1985 Oct 10;317(6037):489–495. doi: 10.1038/317489a0. [DOI] [PubMed] [Google Scholar]
- Tsurimoto T., Fujiyama A., Matsubara K. Stable expression and replication of hepatitis B virus genome in an integrated state in a human hepatoma cell line transfected with the cloned viral DNA. Proc Natl Acad Sci U S A. 1987 Jan;84(2):444–448. doi: 10.1073/pnas.84.2.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams S. C., Cantwell C. A., Johnson P. F. A family of C/EBP-related proteins capable of forming covalently linked leucine zipper dimers in vitro. Genes Dev. 1991 Sep;5(9):1553–1567. doi: 10.1101/gad.5.9.1553. [DOI] [PubMed] [Google Scholar]
- Wilson D. R., Juan T. S., Wilde M. D., Fey G. H., Darlington G. J. A 58-base-pair region of the human C3 gene confers synergistic inducibility by interleukin-1 and interleukin-6. Mol Cell Biol. 1990 Dec;10(12):6181–6191. doi: 10.1128/mcb.10.12.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaginuma K., Shirakata Y., Kobayashi M., Koike K. Hepatitis B virus (HBV) particles are produced in a cell culture system by transient expression of transfected HBV DNA. Proc Natl Acad Sci U S A. 1987 May;84(9):2678–2682. doi: 10.1073/pnas.84.9.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee J. K. A liver-specific enhancer in the core promoter region of human hepatitis B virus. Science. 1989 Nov 3;246(4930):658–661. doi: 10.1126/science.2554495. [DOI] [PubMed] [Google Scholar]
- Yuh C. H., Chang Y. L., Ting L. P. Transcriptional regulation of precore and pregenomic RNAs of hepatitis B virus. J Virol. 1992 Jul;66(7):4073–4084. doi: 10.1128/jvi.66.7.4073-4084.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuh C. H., Ting L. P. C/EBP-like proteins binding to the functional box-alpha and box-beta of the second enhancer of hepatitis B virus. Mol Cell Biol. 1991 Oct;11(10):5044–5052. doi: 10.1128/mcb.11.10.5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuh C. H., Ting L. P. The genome of hepatitis B virus contains a second enhancer: cooperation of two elements within this enhancer is required for its function. J Virol. 1990 Sep;64(9):4281–4287. doi: 10.1128/jvi.64.9.4281-4287.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D. X., Yen T. S. The hepatitis B virus S promoter comprises A CCAAT motif and two initiation regions. J Biol Chem. 1991 Dec 5;266(34):23416–23421. [PubMed] [Google Scholar]