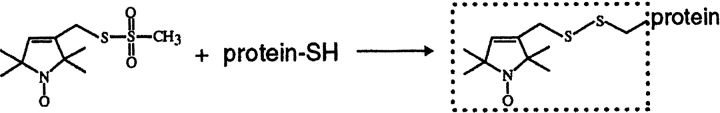Abstract
We used site-directed spin labeling and electron paramagnetic resonance spectroscopy to investigate dynamics and helical packing in the four-helix transmembrane domain of the homodimeric bacterial chemoreceptor Trg. We focused on the first transmembrane helix, TM1, particularly on the nine-residue sequence nearest the periplasm, because patterns of disulfide formation between introduced cysteines had identified that segment as the region of closest approach among neighboring transmembrane helices. Along this sequence, mobility and accessibility of the introduced spin label were characteristic of loosely packed or solvent-exposed side chains. This was also the case for eight additional positions around the circumference and along the length of TM1. For the continuous nine-residue sequence near the periplasm, mobility and accessibility varied only modestly as a function of position. We conclude that side chains of TM1 that face the interior of the four-helix domain interact with neighboring helices but dynamic movement results in loose packing. Compared to transmembrane segments of other membrane proteins reconstituted into lipid bilayers and characterized by site-directed spin labeling, TM1 of chemoreceptor Trg is the most dynamic and loosely packed. A dynamic, loosely packed chemoreceptor domain can account for many experimental observations about the transmembrane domains of chemoreceptors.
Keywords: Bacterial chemotaxis, transmembrane receptors, membrane proteins, protein dynamics, electron paramagnetic resonance (EPR) spectroscopy
Bacterial chemotaxis is mediated by chemoreceptors. These proteins recognize attractants and repellents, control the activity of a noncovalently associated histidine kinase, and mediate sensory adaptation by undergoing covalent modification (Hazelbauer 1992; Falke et al. 1997; Falke and Hazelbauer 2001). Chemoreceptors have been identified across a broad spectrum of bacterial and archaeal species (Zhulin 2001). They share common sequence modules and organizational features. Most are transmembrane proteins that span the cytoplasmic membrane. The best characterized are from Escherichia coli and Salmonella typhimurium. Those receptors are homodimers of ∼60 kD subunits that contain two substantial hydrophilic domains, one in the periplasm that binds ligand and the other in the cytoplasm that interacts with the histidine kinase and contains the sites of adaptational modification. A hydrophobic, transmembrane domain, consisting of four transmembrane helices, connects the two hydrophilic domains structurally and functionally. Structural information from X-ray crystallography of water-soluble fragments of chemoreceptor periplasmic (Milburn et al. 1991; Bowie et al. 1995) and cytoplasmic (Kim et al. 1999; Falke and Kim 2000) domains and from disulfide scanning of intact receptors (Pakula and Simon 1992; Lee et al. 1994; Danielson et al. 1997; Hughson et al. 1997; Bass and Falke 1998; Butler and Falke 1998; Bass et al. 1999) combine to create a model of the native chemoreceptor dimer as an elongated structure consisting primarily, if not exclusively, of helical bundles (Fig. 1A ▶).
Fig. 1.
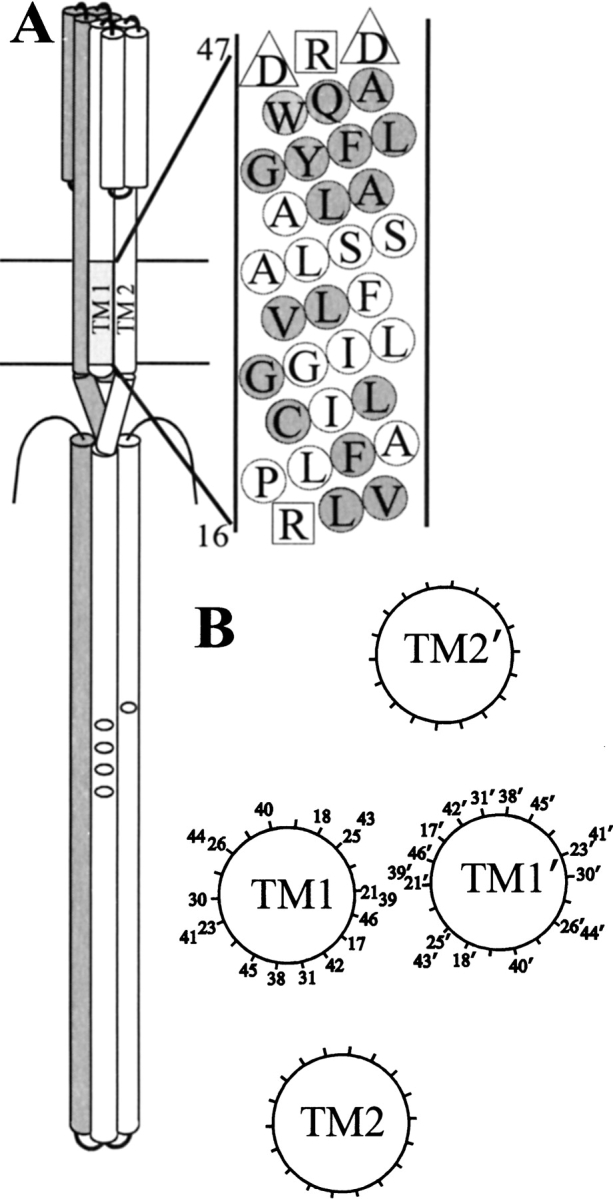
Chemoreceptor organization. (A) Model of a chemoreceptor dimer and details of TM1 of Trg. On the left is a cartoon of a chemoreceptor dimer with one subunit shaded. On one subunit, transmembrane helices 1 (TM1) and 2 (TM2) are labeled and methyl-accepting sites shown as small ovals. On the right, the sequence of TM1 from Trg is shown in a helical net diagram with residue numbers provided for the two bracketing charged residues. Negatively charged residues are indicated by triangles and positively charged residues by squares. Positions characterized by site-directed spin labeling in this study are shaded. (B) Model of the transmembrane domain of Trg showing orientations and relative separations of the four helices determined by cross-linking moments (Lee and Hazelbauer 1995). Positions characterized by site-directed spin labeling in this study are indicated by residue number.
Signaling from ligand-binding site to kinase involves a conformational change that is transmitted through the chemoreceptor transmembrane domain (Falke and Hazelbauer 2001), but information about the structure and dynamics of that domain is incomplete. In the absence of crystallographic information, the organization of this transmembrane domain has been deduced from relative propensities of disulfide formation between pairs of cysteines introduced into neighboring segments (Pakula and Simon 1992; Lee et al. 1994; Hughson et al. 1997). The patterns of these propensities established that the four segments, TM1 and TM2 of one monomer and TM1` and TM2` of the other monomer were helical, and defined their orientation and relative proximity (Fig. 1B ▶). However, the data could not be used to determine whether positions of high cross-linking propensity were sufficiently close to allow side chain packing between neighboring helices nor whether separations between neighboring helices were static or dynamic. Several lines of evidence indicate that the hydrophilic domains of chemoreceptors are flexible and dynamic (Falke and Koshland 1987; Chervitz and Falke 1995; Seeley et al. 1996; Danielson et al. 1997; Bass and Falke 1998; Butler and Falke 1998; Bass et al. 1999; Murphy et al. 2001), but there is little information about the dynamics of the transmembrane segments. To address these issues, we initiated studies of the chemoreceptor transmembrane domain using site-directed spin labeling (Hubbell et al. 1996, 1998, 2000). In this approach a paramagnetic nitroxide side chain is placed at a specific position in a protein through modification of a cysteine residue introduced by site-specific mutagenesis, and the environment of that side chain is assessed by electron paramagnetic resonance (EPR) spectroscopy.
In the present studies, we employed the commonly used methanethiosulfonate reagent that generates a nitroxide side chain, termed R1, at a reactive cysteine (Fig. 2 ▶). The resonance line shape of the EPR spectrum of R1, attached at a specific position in a protein, provides information about the mobility of the modified side chain and the backbone to which it is attached (Hubbell et al. 1996, 1998, 2000; Columbus et al. 2001). In addition, collision rates with small paramagnetic reagents can be used to assess solvent accessibility (Altenbach et al. 1994). The mobility and accessibility of the spin label can characterize the side chain position as buried, involved in a tertiary contact, on a helical surface or exposed on a loop (McHaourab et al. 1996). An "R1-scan" of sequential positions can reveal periodic variation in mobility and accessibility characteristic of regular secondary structure (Hubbell et al. 1998). For transmembrane segments, determination of collision frequencies with hydrophilic and hydrophobic reagents can identify membrane-embedded positions and membrane boundaries (Altenbach et al. 1994). In this report, we describe our application of these strategies to the characterization of a transmembrane segment in chemoreceptor Trg.
Fig. 2.
Reaction of the methanethiosulfonate spin label with a cysteine-containing protein to produce the nitroxide side chain designated R1.
Results
Testing the candidate for the most extensive helical packing
We used our collection of cysteine-substituted forms of chemoreceptor Trg (Lee et al. 1994) to introduce the R1 spin label at positions in a transmembrane segment of a chemoreceptor. We focused first on the periplasmic end of TM1 because patterns of disulfide formation between introduced cysteines in two different chemoreceptors, Trg and Tar, had identified this segment as the one in closest proximity to its neighboring helices in the four-helix transmembrane domain (Pakula and Simon 1992; Lee et al. 1994; Chervitz and Falke 1995; Lee et al. 1995b; Chervitz and Falke 1996; Hughson et al. 1997). We chose nine consecutive positions (46 through 38), beginning adjacent to the bracketing charged residue asp47 and extending approximately three helical turns into the membrane (Fig. 1 ▶). Detergent-solubilized, purified receptors were reacted with methanethiosulfonate spin label (Fig. 2 ▶) and reconstituted into phosphatidylcholine vesicles. The EPR spectra collected are shown in Figure 3 ▶.
Fig. 3.
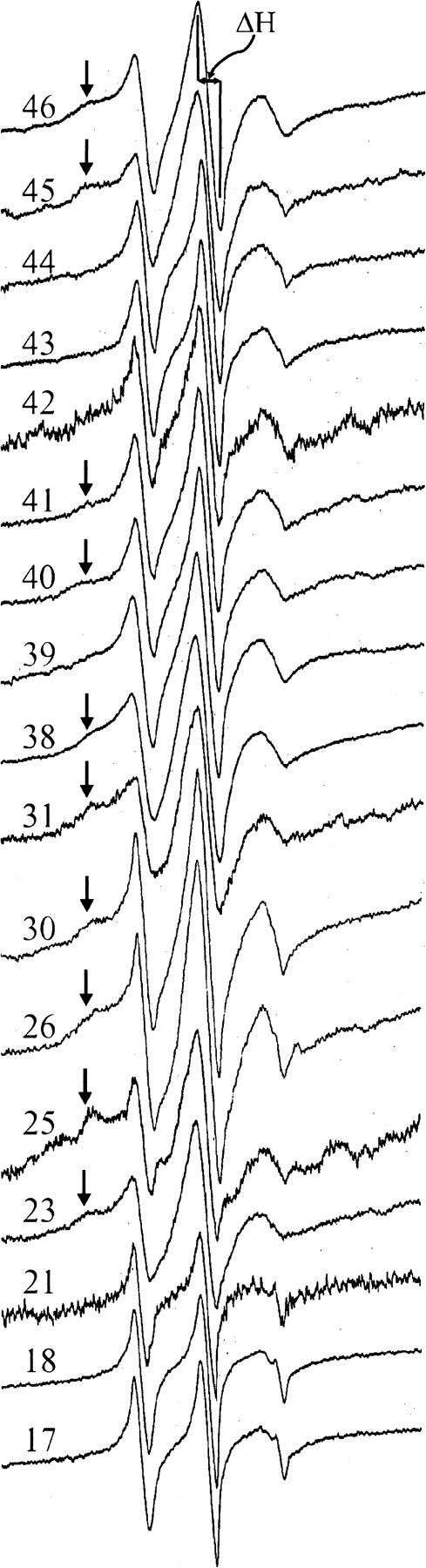
First-derivative EPR spectra of forms of Trg containing a nitroxide side chain R1 at the indicated positions. The spectra are scaled vertically for convenience of presentation. The magnetic field scan width was 98 G. The line width of the central resonance (ΔH) is indicated on the top spectrum. For spectra that appear to have contributions from two or more components, the less-mobile components are marked by arrows. ΔH−1 values for the spectra shown were: 46, 0.294; 45, 0.284; 44, 0.400; 43, 0.380; 42, 0.345; 41, 0.345; 40, 0.351; 39, 0.286; 38, 0.250; 31, 0.286; 30, 0.394; 26, 0.353; 25, 0.333; 23, 0.312; 21, 0.426; 18, 0.455; 17, 0.455.
More than one spectral component
Many of the spectra have two dominant components reflecting different dynamic modes of the R1 side chain, a common situation for both soluble and membrane-inserted proteins (McHaourab et al. 1996; Voss et al. 1996, 1997). In Figure 3 ▶, the component corresponding to the less mobile population is marked with an arrow in the relevant spectra. Multiple dynamic modes of R1 could arise from the existence of two or more rotameric states of the side chain, each having different interactions with the environment. This has in fact been observed in crystal structures of T4 lysozyme bearing R1 at a helix surface site (Langen et al. 2000). Alternatively, Trg could have more than one conformation, resulting in different R1 environments, as observed for the β2 adrenergic receptor (Peleg et al. 2001). This issue will be discussed further below. For spectra with contributions from more than one dynamic population, the more mobile component is weighed more heavily in the approximate measure of side chain mobility employed in this study (ΔH−1) as well in the measure of side chain accessibility (|gP) (see below).
Mobility
Rotational motions of a nitroxide in the nanosecond range (0.5–30 nsec) are reflected in the shape of the EPR spectrum. In general, a lower mobility corresponds to broader spectral features, including the width of the central resonance line, ΔH (see Fig. 3 ▶), and the overall spectral breadth. For the R1 side chain in a protein, motions with nanosecond correlation times arise from both torsional oscillations about the bonds in the side chain (internal modes) and dynamic modes of the backbone (Columbus et al. 2001).
Studies of a number of polytopic membrane proteins in which the R1 side chain was placed sequentially along a segment of a transmembrane helix have identified a periodic dependence of EPR spectral line shape, and hence mobility, on position due to regular alternation of solvent-exposed and internally packed side chains along a helical sequence (Hubbell and Altenbach 1994; Altenbach et al. 1996, 1999; Hubbell et al. 1996, 1998; Voss et al. 1996, 1997; Langen et al. 1998; Salwinski and Hubbell 1999). Surprisingly, the spectra for R1 at positions 38 through 46 of TM1 in Trg reflected only modest modulation of R1 mobility, and none had the broad spectral line shape corresponding to a strongly immobilized state expected for a buried residue. Thus, none of the side chains can be tightly packed against a neighboring helix in a static helix bundle structure.
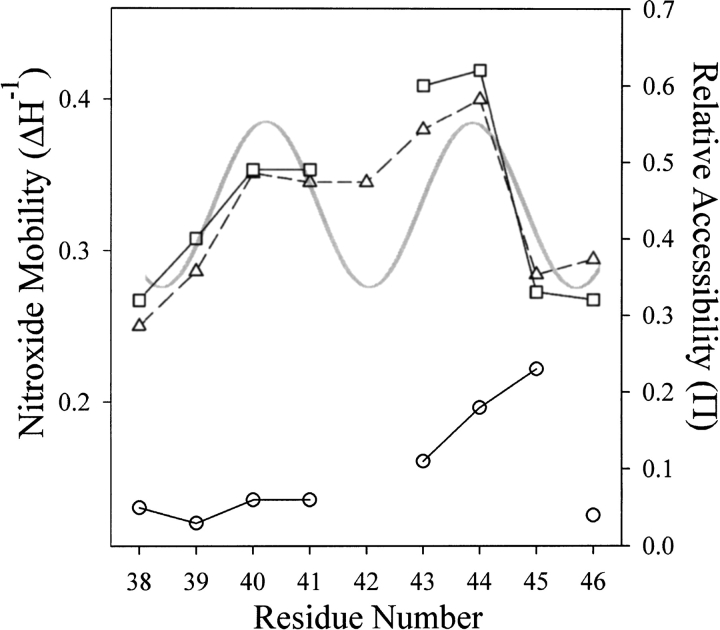
For comparative purposes, R1 side chain mobility can be measured by the inverse of the width of the central resonance line, ΔH−1, a quantity that increases with increased mobility (McHaourab et al. 1996). Figure 4 ▶ shows a plot of ΔH−1 for R1 as a function of position through the sequence 38–46. For reference, we have included in Figure 4 ▶ a plot for the helical periodicity of TM1 derived from cysteine scanning and patterns of disulfide cross-linking between introduced cysteines (Lee et al. 1994; Hughson et al. 1997). The minima in this plot correspond to the face of TM1 closest to its nearest neighbors TM1` and TM2, and maxima correspond to the solvent-exposed face (Fig. 1B ▶). Although the variations in ΔH−1 along the sequence are not dramatic, positions near or at the minima of the helical pattern had either the lowest values of ΔH−1 and thus mobility (38, 39, 45, and 46) or were at local minima in ΔH−1 (42). Similarly, positions near or at the maxima in the helical pattern correspond to residues at local maxima in ΔH−1 (positions 40, 41, 43, and 44; Fig. 1B ▶). Thus, the sequence dependence of R1 residue mobility is consistent with the helical structure defined by cysteine-scanning mutagenesis and patterns of disulfide cross-linking.
Fig. 4.
Mobility and accessibility of the R1 spin label along a nine-residue sequence of TM1. The mobility parameter ΔH−1 (▵) and the accessibility parameters |gP(O2) (□) and |gP(NiEDDA) (○) are plotted for positions 38–46 of chemoreceptor Trg. The gray, continuous curve is a plot of the helical periodicity of TM1 derived from cysteine-scanning (Lee et al. 1995a) and patterns of disulfide cross-linking between introduced cysteines (Lee et al. 1994; Hughson et al. 1997), with minima corresponding to the face of TM1 closest to its nearest neighbors TM1` and TM2 and maxima corresponding to the solvent-exposed face (see Fig. 1B ▶).
Accessibility
An alternative way to probe helical packing in a transmembrane segment is to determine the accessibility of the R1 side chain to a hydrophobic paramagnetic reagent (Altenbach et al. 1994). For this purpose, the hydrophobic molecule O2 was employed as the paramagnetic reagent, and the collision frequency with R1 was expressed in terms of the dimensionless accessibility parameter, |gP, to which it is proportional (Farahbakhsh et al. 1992). In Figure 4 ▶, |gP(O2) values are plotted as a function of sequence position for residues 38 through 46. There is a gap at position 42 where a low signal-to-noise ratio prohibited useful collision measurements. As for the measurements of ΔH−1, there were only modest differences in |gP(O2) values along the TM1 sequence, yet positions of greatest accessibility corresponded to exterior-facing residues in the model of the transmembrane domain based on cross-linking (Fig 1B ▶), and positions of lower accessibility corresponded to interior-facing residues. It is notable that the independent parameters &10 Delta;H−1 and |gP(O2) covaried as a function of position.
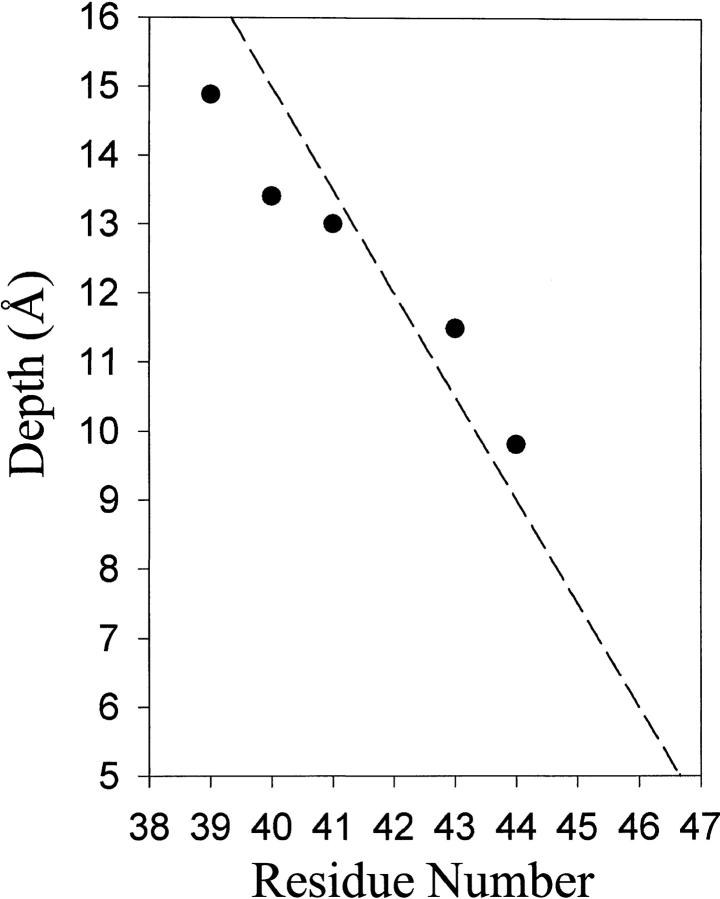
We also determined accessibility of R1 to collision with the hydrophilic, paramagnetic complex Ni(II)ethylenediamine-N-N`-diacetate (NiEDDA) and plotted |gP(NiEDDA) values as a function of residue position (Fig. 4 ▶). For positions 38 through 46 of Trg, the spin label was less accessible to the hydrophilic reagent NiEDDA than to hydrophobic O2 (Fig. 4 ▶), implying that the entire segment was embedded in the hydrocarbon environment of the membrane. The ratio of accessibility parameters |gP(O2)/ |gP(NiEDDA) can be used to calculate the depth of immersion of R1 in the membrane if the side chain is sufficiently accessible that collisions with the larger nickel complex and the smaller oxygen molecule are not influenced by their difference in size (Altenbach et al. 1994; Salwinski and Hubbell 1999; Oh et al. 2000). Using this approach, we computed values for the depth of immersion of R1 from the phosphate head groups of the lipid bilayer from the data in Figure 4 ▶ and plotted them as a function of residue position for appropriate TM1 positions in the nine-residue sequence (Fig. 5 ▶). The immersion depth decreased with increasing residue number in a relationship that approximated the 1.5 Å/residue slope of a canonical helix oriented normal to the plane of the membrane (Fig. 5 ▶).
Fig. 5.
Immersion depths for solvent-exposed positions in TM1 of chemoreceptor Trg. Values of depth from the phosphate groups of the lipid bilayer were calculated as described (Gross et al. 1999). The dotted line has a slope of 1.5 Å/residue. The hydrophobic/hydrophilic boundary of the bilayer is at ∼5 Å.
Extending the analysis
Analysis of the nine-residue segment of TM1 that was the best candidate for extensive helical interaction in the chemoreceptor transmembrane domain indicated only modest packing. To determine whether side chain interactions were more extensive in other parts of TM1, we selected additional positions around the entire circumference and along the length of that helix (Fig. 1B ▶). Five positions (17, 18, 21, 25, and 31) were interior-facing in the model derived from cysteine and disulfide scanning, and three (23, 26, and 30) were modeled as exterior-facing. The spectra for these eight positions are displayed in Figure 3 ▶, and the corresponding ΔH−1 values are provided in the legend.
The spectra for R1 at 17, 18, and 21 resemble the spectrum of R1 at 44, a fully solvent-exposed site, but they have an even higher mobility as measured by ΔH−1. Chemoreceptor residues between the amino terminus and the beginning of TM1 are not necessary for receptor function (Chen and Koshland 1995) and appear to splay away from other segments of the receptor (Chervitz et al. 1995; Peach et al. 2002). Thus the strikingly high mobility of side chains near that end of TM1 could reflect helix fraying (Almeida and Opella 1997; Papavoine et al. 1997).
The spectrum of R1 at 25 is unique, and reflects strong spin-spin interaction between two R1 side chains as evidenced by the overall breadth of the spectrum that exceeds 100 G. Although the spectrum has not been quantitatively analyzed, the breadth of the spectrum requires that the interspin distance be ≤10 Å (Altenbach et al. 2001). This result is consistent with expectations based on the model in Figure 1B ▶, where the two 25 side chains at positions 25 and 25` on TM1 and TM1` of the dimer in the TM1/TM1` contact face. Due to the spin-spin interaction, no conclusions regarding mobility based on line width can be made. The spectra for 23, 26, 30, and 31 are multicomponent, but as for R1 residues in the 38–46 sequence, none have a line shape reflecting the immobilized state expected for a truly buried site in a static structure.
Discussion
Site-directed spin labeling and EPR spectroscopy have been used to investigate dynamics and helical packing in the transmembrane domain of the chemoreceptor Trg. Efforts were concentrated on the first transmembrane helix, TM1, particularly on the segment nearest the periplasm, because patterns of disulfide formation between introduced cysteines had identified that segment as the region of closest approach among helical neighbors in the domain (Pakula and Simon 1992; Lee et al. 1994; Chervitz and Falke 1995; Lee et al. 1995b; Chervitz and Falke 1996; Hughson et al. 1997). Measurements of nitroxide mobility and accessibility indicated that TM1 was notably dynamic and loosely packed. Since the two TM1 helices constitute 50% of the transmembrane domain and the two TM2s are near their TM1 partners but distant from each other (Pakula and Simon 1992; Lee et al. 1994; Hughson et al. 1997), dynamic, loosely packed TM1s imply that the entire chemoreceptor transmembrane domain is a dynamic, loosely packed structure.
Dynamics and packing
Mobility and accessibility of site-specific spin labels varied only modestly along the nine-residue segment of TM1 near the periplasm (Fig. 4 ▶). The most important result is that none of the sites had a low mobility and accessibility characteristic of a truly buried site on the interior surface of a helix. This was also the case for eight additional positions around the circumference and along the length of TM1. However, mobility and accessibility were covariant and the variation was consistent with the model of helical disposition (Fig. 1B ▶) determined from patterns of oxidative cross-linking (Lee et al. 1994; Lee and Hazelbauer 1995b; Hughson et al. 1997) and cysteine scanning (Lee et al. 1995a). We conclude that TM1 side chains facing the interior of the four-helix domain interact with neighboring helices but that dynamic movement alternatively allows and breaks those interactions, resulting in an average state of loose packing. The EPR spectra of R1 at sites predicted to face the interior of the bundle are consistent with this conclusion. For example, the spectrum of 38R1 has two components, one of which corresponds to a strongly immobilized state and the other to a more dynamic state similar to that of 44R1, an exterior site. As mentioned above, one model to account for this result is that TM1 exists in two states. In one state TM1 is tightly packed with neighboring helices, and this state gives rise to the immobilized state of 38R1. In the other state, TM1 is displaced relative to neighboring helices, thus reducing tertiary contact interactions and allowing motion of R1 similar to that at exterior helix sites such as 44. The separation in magnetic field of the two components in 38R1 is ∼10 G. In the context of the two-state model described above, this implies that the exchange rate between the states would be ≤10 MHz, compatible with conformational fluctuation rates observed in other proteins (Wand 2001).
To compare mobility and accessibility values for the continuous segment of TM1 with those for other transmembrane helices investigated by site-specific spin labeling, average values of ΔH−1 (Fig 6A ▶) and |gP(O2) (Fig. 6B ▶) for R1 side chains on interior-facing (buried) surfaces of transmembrane helices were computed from published data for several polytopic membrane proteins reconstituted into phospholipid bilayers. Figure 6C ▶ shows the spectrum of Trg 38R1 and a comparison with representative spectra for R1 at buried sites in Annexin XII (AXII), bacteriorhodopsin (bR) and rhodopsin (Rho). The much narrower spectral features for R1 at Trg38 and AXII145 compared to those in the tightly packed helical bundles of bR and Rho are evident.
Fig. 6.
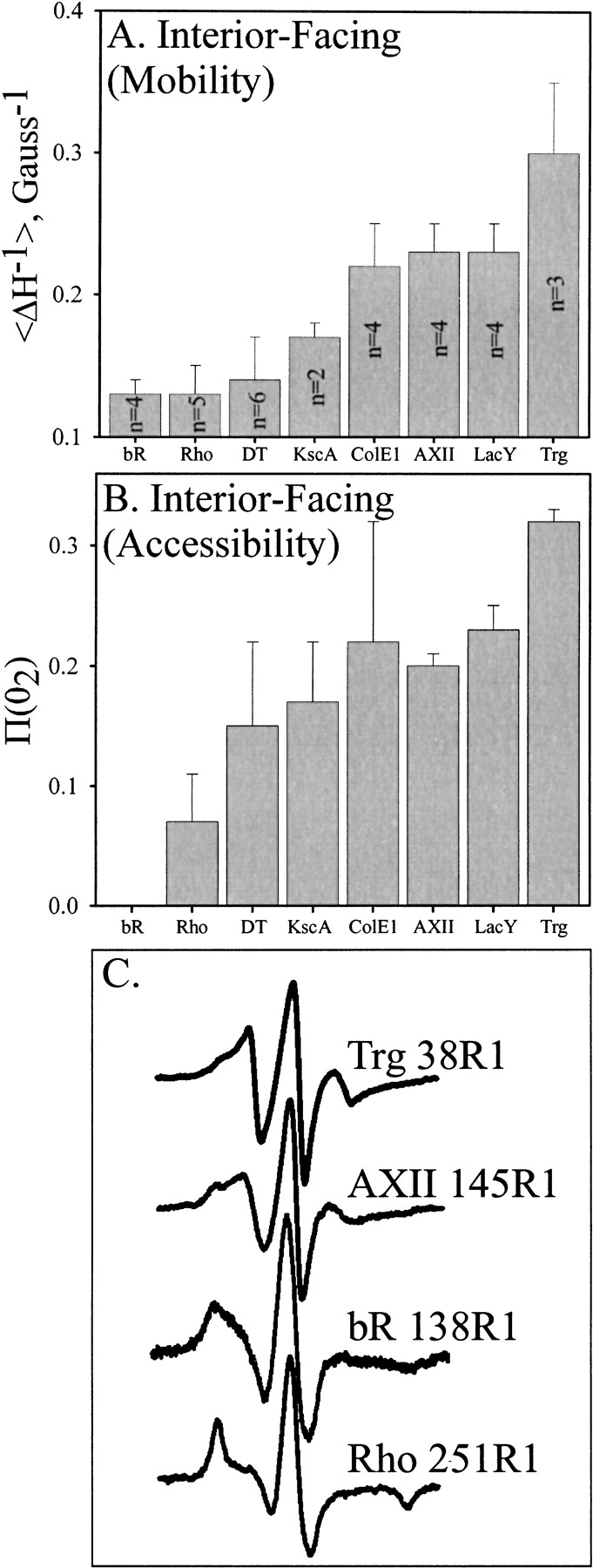
Mobility and accessibility of interior-facing R1 residues on transmembrane α-helices characterized by site-directed spin labeling. Average values for the mobility parameter Δ H−1 (A) or the accessibility parameter |gP(O2) (B) are shown for interior-facing helical positions of bacteriorhodopsin (bR) (Altenbach et al. 1990, 1994), rhodopsin (Rho) (Altenbach et al. 1996), diphtheria toxin T domain (DT) inserted into bilayers at low pH (Oh et al. 1996), Streptomyces lividans potassium channel (KscA) (Gross et al. 1999), colicin E1 (ColE1) inserted into bilayers at low pH (Salwinski and Hubbell 1999), annexin XII (AXII) inserted into membranes at low pH (Langen et al. 1998), lactose permease (LacY) (Voss et al. 1996), and TM1 of Trg (this study). For each protein, all the sites were along a single helix. The number of values averaged is indicated within the bar and was the same for panels A and B. Error bars represent the standard deviation from the mean. Representative spectra from positions used in the calculations for panel A are shown in panel C.
As can be seen in Figure 6 ▶, the helical segments can be roughly divided into two groups based on average side chain mobility of interior-facing positions. In addition, this division is in large part consistent with the corresponding accessibility values shown in Figure 6B ▶, although the dispersion (indicated by the error bars) is relatively high. Helices from diphtheria toxin T domain (DT), bacteriorhodopsin, rhodopsin, potassium channel KcsA, and T4 lysozyme exhibited clearly lower mobilities (average ΔH−1 ∼0.15) compared to those of annexin XII, colicin E1 (ColE1), lactose permease (LacY), and Trg. Thus the former group has features suggesting more tightly packed helices than the latter. The significance of these groups is reinforced by studies of 1H/2H exchange in membrane proteins, in which a wide range of rates have been observed (Downer et al. 1986; Alvarez et al. 1987; Earnest et al. 1990; Dempsey and Butler 1992; Le Coutre et al. 1997), with bacteriorhodopsin exhibiting slow exchange (Downer et al. 1986) and lactose permease exhibiting one of the fastest (Le Coutre et al. 1997), consistent with their classification by site-directed spin labeling as tightly and loosely packed, respectively.
Even within the more loosely packed group of transmembrane helices, Trg exhibited the highest average mobility and accessibility. This is particularly notable since the helical segment characterized was the best candidate for the most extensive packing in the chemoreceptor transmembrane domain.
Membrane boundaries
Accessibility of membrane-embedded, spin-labeled side chains to a hydrophilic paramagnetic reagent is primarily determined by the low solubility of the reagent in the hydrocarbon environment of the lipid bilayer (Altenbach et al. 1994; Hubbell and Altenbach 1994; Hubbell et al. 1998, 2000). The accessibility parameter |gP(NiEDDA) was very low for the four positions (38–41) farthest from the bracketing charged residue at position 47 and increased from positions 43 through 45 as the location of the spin label neared the putative membrane boundary (Fig. 4 ▶). The similar values for |gP(O2) and |gP(NiEDDA) at position 45 implied that the hydrophobic/hydrophilic interface is near that position (Hubbell et al. 1996). Accessibility at position 46 appeared anomalously low, perhaps because of features of the local environment involving the charge density at the membrane boundary, which appears to be close to that position, or side chain packing, since the position is on the interior-facing side of TM1 (Fig. 1B ▶). Calculated values of distance from phosphate head groups of the lipid bilayer plotted as a function of residue position approximated a helical slope of 1.5 Å/residue (Fig. 5 ▶). Extrapolation of that theoretical line to the hydrophobic-hydrophilic interface at approximately 5 Å indicates that TM1 emerges from the hydrocarbon environment of the membrane near the charged aspartyl residue at position 47. This is a reasonable since asp47 marks the boundary between a 30-residue sequence of hydrophobic residues and a sequence with a high frequency of hydrophilic residues (Fig. 1A ▶).
A dynamic transmembrane domain
The conclusion that the chemoreceptor transmembrane domain is dynamic and thus loosely packed is consistent with the following: the very low conservation of sequence in the transmembrane segments of even closely related receptors (Le Moual and Koshland 1996), the lack of significant functional consequences of many single mutational substitutions in transmembrane segments (Oosawa and Simon 1986; Pakula and Simon 1992; Lee et al. 1995a; Baumgartner and Hazelbauer 1996), detectable receptor function upon replacement of much of TM2 by several random sequences (Maruyama et al. 1995), detectable signaling between periplasmic and cytoplasmic domains in a chemoreceptor deleted of the transmembrane domain (Ottemann and Koshland 1997), and indications of flexibility and dynamic movement in the two hydrophilic chemoreceptor domains (Falke and Koshland 1987; Chervitz and Falke 1995; Seeley et al. 1996; Danielson et al. 1997; Bass and Falke 1998; Butler and Falke 1998; Bass et al. 1999; Murphy et al. 2001). An analysis of faces of transmembrane helices that were the most hydrophilic or were the most variable in sequence within families of related membrane proteins (Rees et al. 1989) found that these two faces were usually on opposite sides of the helix and corresponded to packed and exposed faces. Among the few exceptions were both TM1 and TM2 of the E. coli chemoreceptors, in which the faces were essentially adjacent, a situation consistent with dynamic movement and minimal packing.
What is the origin of dynamic movement by TM1? Local unwinding of the transmembrane helix seems unlikely because it would require the energetically unfavorable breaking of backbone hydrogen bonds in the hydrophobic interior of a membrane. Instead, the high mobility of both interior-facing and exterior-facing residues implies that the dynamic motion involves the entire helix. The combination of high mobility and accessibility implies that TM1 and its neighbors move toward and away from each other to create substantial accessibility to side chains in the interaction interface. This is most consistent with movement in the plane of the membrane rather than normal to the plane of the membrane. Such a dynamic situation could result in spin-label spectra with contributions from more than one mobility state, a situation noted for many of the positions characterized in TM1 (see above).
How common are dynamic transmembrane domains? An analysis of helical packing in membrane proteins with known crystal structures revealed packing values higher than those of soluble proteins (Eilers et al. 2000). However, for many membrane proteins, including chemoreceptors, attempts to crystallize the intact protein have not been successful. It may well be that many of these proteins, like Trg, contain dynamic, loosely packed transmembrane helices. This dynamic, loose packing could be important for function but an impediment to crystallization.
Materials and methods
Bacterial strains and plasmids
CP553 is a strain of E. coli K12 carrying chromosomal deletions for trg, tsr, tar, tap, cheR, and cheB, and pGB1 is a pBR322-derived plasmid carrying lacIq, bla, and trg fused to a tac promoter (Burrows et al. 1989). We used derivatives of pGB1 in which the trg codon for cysteine-23 was changed to a serine codon and a codon corresponding to a TM1 residue was changed to a cysteine codon (Lee et al. 1994). All cysteine-substituted proteins were functional receptors (Lee et al. 1995a).
Receptor purification
Cysteine-containing forms of Trg were produced from plasmid-borne genes in CP553, a strain lacking the chromosomal genes for the four closely related chemoreceptors of E. coli and the genes for the two enzymes that catalyze receptor modification. The lack of other chemoreceptors simplified purification, and the lack of receptor modification insured that the receptors had the identical, gene-encoded status at their sites of adaptational modification. Cells were grown with agitation at 35°C in 2.9 L Luria broth containing 100 μg/mL ampicillin. At a density of ∼2.5 × 108 cells/mL, isopropyl-thio-β-D-galactoside was added to 1 mM to induce maximal synthesis of Trg. After 3.5 h, cells were centrifuged, washed once in ice-cold 100 mM sodium phosphate, pH 7.5, 10% w/v glycerol, 10 mM ethylenediaminetetraacetic acid, 5 mM dithiothreitol (DTT), 1 mM phenylmethylsulfonyl fluoride (PMSF), 1 mM 1,10-phenanthroline and suspended in the same, ice-cold buffer. All subsequent steps were done on ice or at 4°C. Cells were broken by two passages through French pressure cell (SLM Instruments, Urbana, IL) at 20,000 psi. Membranes were collected by centrifugation for 40 min at 50,000 rpm in a Beckman 60 Ti rotor and suspended in buffer B (20 mM sodium phosphate, pH 7.5, 10% w/v glycerol, 5 mM DTT, 1 mM PMSF, 1 mM 1,10-phenanthroline, 100 mM NaCl). Membranes were solubilized by addition of octyl β-D-glucopyranoside (octylglucoside) to 1.5% and incubation on ice for 10 min. The mixture was centrifuged for 30 min at 50,000 rpm in the Beckman 60 Ti rotor and the supernatant was applied to a Pharmacia Blue Sepharose Fast Flow column (bed volume 15 mL) equilibrated with buffer B containing 1% octylglucoside. The column was developed by a linear gradient of NaCl (0 to 2 M) in 6 bed volumes of buffer B plus 1% octylglucoside followed by 4 bed volumes of 2 M NaCl in the same buffer. Column fractions containing receptor were pooled, then loaded on a Pharmacia Phenyl-Sepharose Fast Flow column (bed volume 15 mL) equilibrated with two bed volumes of buffer B containing 1% octylglucoside and 2 M NaCl. The column was washed with two bed volumes 0.5 M NaCl in the same buffer and the receptor was eluted with 20 mM Tris, pH 7.5, 10% w/v glycerol, 0.5% Triton X-100, 50 mM NaCl, 5 mM DTT. Fractions containing Trg were pooled and loaded on a Q-Sepharose Pharmacia Hi-Trap column equilibrated with 20 mM Tris, pH 7.5, 10% w/v glycerol, 1% octylglucoside, 50 mM NaCl. The column was developed with a 0.05 to 1 M linear gradient of NaCl in 10 bed volumes of the same buffer, eluting 1.5–2 mg of highly homogeneous receptor at ∼270 mM NaCl.
Spin labeling and membrane reconstitution
Immediately after the final purification step, receptor at ∼10 μM was brought to ∼500 μM of the spin label reagent S-(1-oxy-2,2,5,5-tetramethylpyrroline-3-methyl)methanethiosulfonate (a generous gift of Prof. Kalman Hideg, University of Pecs, Hungary) and incubated overnight at 4°C. When tested, modification of receptor with the R1 spin label was close to stoichiometric. Soybean phosphatidylcholine (Avanti) solubilized in octylglucoside was added to a 20:1 w/w ratio of lipid to protein. A neutral lipid was used to avoid complications in accessibility studies that might arise from charged lipids in the bilayer. Removal of detergent by dialysis against 10 mM sodium phosphate, pH 7.5, 10% w/v glycerol, 100 mM KCl resulted in formation of lipid vesicles containing spin-labeled receptor. The vesicles were pelleted by centrifugation and suspended in a small volume of dialysis buffer.
Chemoreceptors maintain their dimeric organization when solubilized by detergent and reconstituted into lipid vesicles (Milligan and Koshland 1988). This was confirmed for Trg purified and solubilized using the procedure described here by demonstrating intersubunit disulfide formation across the TM1–TM1` interface (Burrows 1991). It was not possible to test spin-labeled, reconstituted forms of Trg for function in ligand-induced transmembrane signaling because such experiments are not feasible in vitro. The ligands for Trg are sugar-occupied ribose-or galactose-binding protein, and the dissociation constant for ligand-receptor interaction is ∼0.5 mM (Yaghmai and Hazelbauer 1993). Thus testing signaling in vitro would require prohibitively high concentrations of the protein ligands. However, spin-labeled forms of the related E. coli chemoreceptor Tar, which binds the small molecule aspartate, have been shown to maintain ligand-induced signaling when reconstituted into lipid vesicles by procedures similar to those we used for Trg (Ottemann et al. 1998, 1999). In addition, the consistency of the EPR data for Trg with patterns of cysteine cross-linking of native Trg present in intact cells provides an indication that purified, spin-labeled and reconstituted Trg maintained a native organization of the transmembrane domain.
EPR spectroscopy
Measurements were performed on concentrated suspensions of vesicles providing 100 to 150 μM receptor, using a Varian E-109 spectrometer fitted with a two-loop, one-gap resonator (Hubbell and Hyde 1987). Samples of 5 μL were placed in TPX capillaries. Spectra were measured using 2 mW incident microwave power and 1 G field modulation amplitude at 100 kHz. Power saturation measurements for the spin-labels attached to Trg were carried out using air, 20 mM Ni(II) ethylenediamine-N-N`-diacetate (NiEDDA) or in the absence of a collider as described (Oh et al. 2000). The accessibility parameter, |gP, was computed using diphenyldipicrylhydrazide as a reference (Gross et al. 1999).
Acknowledgments
This work was supported in part by NIH grants GM29963 (G.L.H.) and EY05216 (W.L.H.), and the Jules Stein Professor endowment (W.L.H.). We thank Linda Columbus for help in refining and revising the manuscript and Wing-Cheung Lai for help in preparation of the text and figures.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked "advertisement" in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1110/ps.0202502.
References
- Almeida, F.C. and Opella, S.J. 1997. fd coat protein structure in membrane environments: Structural dynamics of the loop between the hydrophobic trans-membrane helix and the amphipathic in-plane helix. J. Mol. Biol. 270 481–495. [DOI] [PubMed] [Google Scholar]
- Altenbach, C., Cai, K., Khorana, H.G., and Hubbell, W.L. 1999. Structural features and light-dependent changes in the sequence 306–322 extending from helix VII to the palmitoylation sites in rhodopsin: A site-directed spin-labeling study. Biochemistry 38 7931–7937. [DOI] [PubMed] [Google Scholar]
- Altenbach, C., Greenhalgh, D.A., Khorana, H.G., and Hubbell, W.L. 1994. A collision gradient method to determine the immersion depth of nitroxides in lipid bilayers: Application to spin-labeled mutants of bacteriorhodopsin. Proc. Natl. Acad. Sci. 91 1667–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altenbach, C., Marti, T., Khorana, H.G., and Hubbell, W.L. 1990. Transmembrane protein structure: Spin labeling of bacteriorhodopsin mutants. Science 248 1088–1092. [DOI] [PubMed] [Google Scholar]
- Altenbach, C., Oh, K.J., Trabanino, R.J., Hideg, K., and Hubbell, W.L. 2001. Estimation of inter-residue distances in spin labeled proteins at physiological temperatures: Experimental strategies and practical limitations. Biochemistry 40 15471–15482. [DOI] [PubMed] [Google Scholar]
- Altenbach, C., Yang, K., Farrens, D.L., Farahbakhsh, Z.T., Khorana, H.G., and Hubbell, W.L. 1996. Structural features and light-dependent changes in the cytoplasmic interhelical E-F loop region of rhodopsin: A site-directed spin-labeling study. Biochemistry 35 12470–12478. [DOI] [PubMed] [Google Scholar]
- Alvarez, J., Lee, D.C., Baldwin, S.A., and Chapman, D. 1987. Fourier transform infrared spectroscopic study of the structure and conformational changes of the human erythrocyte glucose transporter. J. Biol. Chem. 262 3502–3509. [PubMed] [Google Scholar]
- Bass, R.B., Coleman, M.D., and Falke, J.J. 1999. Signaling domain of the aspartate receptor is a helical hairpin with a localized kinase docking surface: Cysteine and disulfide scanning studies. Biochemistry 38 9317–9327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass, R.B. and Falke, J.J. 1998. Detection of a conserved alpha-helix in the kinase-docking region of the aspartate receptor by cysteine and disulfide scanning. J. Biol. Chem. 273 25006–25014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner, J.W. and Hazelbauer, G.L. 1996. Mutational analysis of a transmembrane segment in a bacterial chemoreceptor. J. Bacteriol. 178 4651–4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie, J.U., Pakula, A.A., and Simon, M.I. 1995. The 3-dimensional structure of the aspartate receptor from Escherichia coli. Acta Cryst. 51 145–154. [DOI] [PubMed] [Google Scholar]
- Burrow, G.G. 1991. "Purification and biochemical analysis of the chemosensory receptor Trg from Escherichia coli." Ph.D. thesis, Washington State University Pullman, WA.
- Burrows, G.G., Newcomer, M.E., and Hazelbauer, G.L. 1989. Purification of receptor protein Trg by exploiting a property common to chemotactic transducers of Escherichia coli. J. Biol. Chem. 264 17309–17315. [PubMed] [Google Scholar]
- Butler, S.L. and Falke, J.J. 1998. Cysteine and disulfide scanning reveals two amphiphilic helices in the linker region of the aspartate chemoreceptor. Biochemistry 37 10746–10756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, X. and Koshland, D.E. 1995. The N-terminal cytoplasmic tail of the aspartate receptor is not essential in signal transduction of bacterial chemotaxis. J. Biol. Chem. 270 24038–24042. [DOI] [PubMed] [Google Scholar]
- Chervitz, S.A. and Falke, J.J. 1995. Lock on/off disulfides identify the transmembrane signaling helix of the aspartate receptor. J. Biol. Chem. 270 24043–24053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chervitz, S.A. and Falke, J.J. 1996. Molecular mechanism of transmembrane signaling by the aspartate receptor: A model. Proc. Natl. Acad. Sci. 93 2545–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chervitz, S.A., Lin, C.M., and Falke, J.J. 1995. Transmembrane signaling by the aspartate receptor: Engineered disulfides reveal static regions of the subunit interface. Biochemistry 34 9722–9733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Columbus, L., Kalai, T., Jeko, J., Hideg, K., and Hubbell, W.L. 2001. Molecular motion of spin labeled side chains in alpha-helices: Analysis by variation of side chain structure. Biochemistry 40 3828–3846. [DOI] [PubMed] [Google Scholar]
- Danielson, M.A., Bass, R.B., and Falke, J.J. 1997. Cysteine and disulfide scanning reveals a regulatory alpha-helix in the cytoplasmic domain of the aspartate receptor. J. Biol. Chem. 272 32878–32888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey, C.E. and Butler, G.S. 1992. Helical structure and orientation of melittin in dispersed phospholipid membranes from amide exchange analysis in situ. Biochemistry 31 11973–11977. [DOI] [PubMed] [Google Scholar]
- Downer, N.W., Bruchman, T.J., and Hazzard, J.H. 1986. Infrared spectroscopic study of photoreceptor membrane and purple membrane. Protein secondary structure and hydrogen deuterium exchange. J. Biol. Chem. 261 3640–3647. [PubMed] [Google Scholar]
- Earnest, T.N., Herzfeld, J., and Rothschild, K.J. 1990. Polarized Fourier transform infrared spectroscopy of bacteriorhodopsin. Transmembrane alpha helices are resistant to hydrogen/deuterium exchange. Biophys. J. 5/8 1539–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eilers, M., Shekar, S.C., Shieh, T., Smith, S.O., and Fleming, P.J. 2000. Internal packing of helical membrane proteins. Proc. Natl. Acad. Sci. 97 5796–5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falke, J.J., Bass, R.B., Butler, S.L., Chervitz, S.A., and Danielson, M.A. 1997. The two-component signaling pathway of bacterial chemotaxis: A molecular view of signal transduction by receptors, kinases, and adaptation enzymes. Annu. Rev. Cell Dev. Biol. 13 457–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falke, J.J. and Hazelbauer, G.L. 2001. Transmembrane signaling in bacterial chemoreceptors. Trends Biochem. Sci. 26 257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falke, J.J. and Kim, S.H. 2000. Structure of a conserved receptor domain that regulates kinase activity: The cytoplasmic domain of bacterial taxis receptors. Curr. Opin. Struct. Biol. 10 462–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falke, J.J. and Koshland, D.E. 1987. Global flexibility in a sensory receptor: A site-directed cross-linking approach. Science 237 1596–1600. [DOI] [PubMed] [Google Scholar]
- Farahbakhsh, Z.T., Altenbach, C., and Hubbell, W.L. 1992. Spin labeled cysteines as sensors for protein-lipid interaction and conformation in rhodopsin. Photochem. Photobiol. 56 1019–1033. [DOI] [PubMed] [Google Scholar]
- Gross, A., Columbus, L., Hideg, K., Altenbach, C., and Hubbell, W.L. 1999. Structure of the KcsA potassium channel from Streptomyces lividans: A site-directed spin labeling study of the second transmembrane segment. Biochemistry 38 10324–10335. [DOI] [PubMed] [Google Scholar]
- Hazelbauer, G.L. 1992. Bacterial chemoreceptors. Curr. Opin. Struct. Biol. 2 505–510. [Google Scholar]
- Hubbell, W.L. and Altenbach, C. 1994. Investigation of structure and dynamics in membrane proteins using site-directed spin labeling. Curr. Opin. Struct. Biol. 4 556–573. [Google Scholar]
- Hubbell, W.L., Cafiso, D.S., and Altenbach, C. 2000. Identifying conformational changes with site-directed spin labeling. Nat. Struct. Biol. 7 735–739. [DOI] [PubMed] [Google Scholar]
- Hubbell, W.L., Gross, A., Langen, R., and Lietzow, M.A. 1998. Recent advances in site-directed spin labeling of proteins. Curr. Opin. Struct. Biol. 8 649–656. [DOI] [PubMed] [Google Scholar]
- Hubbell, W.L. and Hyde, J.S. 1987. Continuous and stopped flow EPR spectrometer based on a loop gap resonator. Rev. Sci. Instrum. 58 1879–1886. [Google Scholar]
- Hubbell, W.L., McHaourab, H.S., Altenbach, C., and Lietzow, M.A. 1996. Watching proteins move using site-directed spin labeling. Structure 4 779–783. [DOI] [PubMed] [Google Scholar]
- Hughson, A.G., Lee, G.F., and Hazelbauer, G.L. 1997. Analysis of protein structure in intact cells: Crosslinking in vivo between introduced cysteines in the transmembrane domain of a bacterial chemoreceptor. Protein Sci. 6 315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, K.K., Yokota, H., and Kim, S.H. 1999. Four-helical-bundle structure of the cytoplasmic domain of a serine chemotaxis receptor. Nature 400 787–792. [DOI] [PubMed] [Google Scholar]
- Langen, R., Isas, J.M., Hubbell, W.L., and Haigler, H.T. 1998. A transmembrane form of annexin XII detected by site-directed spin labeling. Proc. Natl. Acad. Sci. 95 14060–14065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langen, R., Oh, K.J., Cascio, D., and Hubbell, W.L. 2000. Crystal structures of spin labeled T4 lysozyme mutants: Implications for the interpretation of EPR spectra in terms of structure. Biochemistry 39 8396–8405. [DOI] [PubMed] [Google Scholar]
- Le Coutre, J., Narasimhan, L.R., Patel, C.K., and Kaback, H.R. 1997. The lipid bilayer determines helical tilt angle and function in lactose permease of Escherichia coli. Proc. Natl. Acad. Sci 94 10167–10171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Moual, H. and Koshland, D.E. 1996. Molecular evolution of the C-terminal cytoplasmic domain of a superfamily of bacterial receptors involved in taxis. J. Mol. Biol 261 568–585. [DOI] [PubMed] [Google Scholar]
- Lee, G.F., Burrows, G.G., Lebert, M.R., Dutton, D.P., and Hazelbauer, G.L. 1994. Deducing the organization of a transmembrane domain by disulfide cross-linking. The bacterial chemoreceptor Trg. J. Biol. Chem. 269 29920–29927. [PubMed] [Google Scholar]
- Lee, G.F., Dutton, D.P., and Hazelbauer, G.L. 1995a. Identification of functionally important helical faces in transmembrane segments by scanning mutagenesis. Proc. Natl. Acad. Sci. 92 5416–5420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, G.F. and Hazelbauer, G.L. 1995. Quantitative approaches to utilizing mutational analysis and disulfide crosslinking for modeling a transmembrane domain. Protein Sci. 4 1100–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, G.F., Lebert, M.R., Lilly, A.A., and Hazelbauer, G.L. 1995b. Transmembrane signaling characterized in bacterial chemoreceptors by using sulfhydryl cross-linking in vivo. Proc. Natl. Acad. Sci. 92 3391–3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama, I.N., Mikawa, Y.G., and Maruyama, H.I. 1995. A model for transmembrane signalling by the aspartate receptor based on random-cassette mutagenesis and site-directed disulfide cross-linking. J. Mol. Biol. 253 530–546. [DOI] [PubMed] [Google Scholar]
- McHaourab, H.S., Lietzow, M.A., Hideg, K., and Hubbell, W.L. 1996. Motion of spin-labeled side chains in T4 lysozyme. Correlation with protein structure and dynamics. Biochemistry 35 7692–7704. [DOI] [PubMed] [Google Scholar]
- Milburn, M.V., Prive, G.G., Milligan, D.L., Scott, W.G., Yeh, J., Jancarik, J., Koshland, D.E., and Kim, S.H. 1991. Three-dimensional structures of the ligand-binding domain of the bacterial aspartate receptor with and without a ligand. Science 254 1342–1347. [DOI] [PubMed] [Google Scholar]
- Milligan, D.L. and Koshland, D.E. 1988. Site-directed cross-linking. Establishing the dimeric structure of the aspartate receptor of bacterial chemotaxis. J. Biol. Chem. 263 6268–6275. [PubMed] [Google Scholar]
- Murphy, O.J., Kovacs, F.A., Sicard, E.L., and Thompson, L.K. 2001. Site-directed solid-state NMR measurement of a ligand-induced conformational change in the serine bacterial chemoreceptor. Biochemistry 40 1358–1366. [DOI] [PubMed] [Google Scholar]
- Oh, K.J., Altenbach, C., Collier, R.J., and Hubbell, W.L. 2000. Site-directed spin labeling of proteins. Applications to diphtheria toxin. Methods Mol. Biol. 145 147–169. [DOI] [PubMed] [Google Scholar]
- Oh, K.J., Zhan, H., Cui, C., Hideg, K., Collier, R.J., and Hubbell, W.L. 1996. Organization of diphtheria toxin T domain in bilayers: A site-directed spin labeling study. Science 273 810–812. [DOI] [PubMed] [Google Scholar]
- Oosawa, K. and Simon, M. 1986. Analysis of mutations in the transmembrane region of the aspartate chemoreceptor in Escherichia coli. Proc. Natl. Acad. Sci. 83 6930–6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottemann, K.M. and Koshland, D.E. 1997. Converting a transmembrane receptor to a soluble receptor: Recognition domain to effector domain signaling after excision of the transmembrane domain. Proc. Natl. Acad. Sci. 94 11201–11204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottemann, K.M., Thorgeirsson, T.E., Kolodziej, A.F., Shin, Y.K., and Koshland, D.E. 1998. Direct measurement of small ligand-induced conformational changes in the aspartate chemoreceptor using EPR. Biochemistry 37 7062–7069. [DOI] [PubMed] [Google Scholar]
- Ottemann, K.M., Xiao, W., Shin, Y.K., and Koshland, D.E. 1999. A piston model for transmembrane signaling of the aspartate receptor. Science 285 1751–1754. [DOI] [PubMed] [Google Scholar]
- Pakula, A.A. and Simon, M.I. 1992. Determination of transmembrane protein structure by disulfide cross-linking: The Escherichia coli tar receptor. Proc. Natl. Acad. Sci. 89 4144–4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papavoine, C.H., Remerowski, M.L., Horstink, L.M., Konings, R.N., Hilbers, C.W., and van de Ven, F.J. 1997. Backbone dynamics of the major coat protein of bacteriophage M13 in detergent micelles by 15N nuclear magnetic resonance relaxation measurements using the model-free approach and reduced spectral density mapping. Biochemistry 36 4015–4026. [DOI] [PubMed] [Google Scholar]
- Peach, M.L., Hazelbauer, G.L., and Lybrand, T.P. 2002. Modeling the transmembrane domain of bacterial chemoreceptors. Protein Sci. 11 912–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleg, G., Ghanouni, P., Kobilka, B.K., and Zare, R.N. 2001. Single-molecule spectroscopy of the β2 adrenergic receptor: Observation of conformational substates in a membrane protein. Proc. Natl. Acad. Sci. 98 8469–8474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees, D.C., DeAntonio, L., and Eisenberg, D. 1989. Hydrophobic organization of membrane proteins. Science 245 510–513. [DOI] [PubMed] [Google Scholar]
- Salwinski, L. and Hubbell, W.L. 1999. Structure in the channel forming domain of colicin E1 bound to membranes: The 402–424 sequence. Protein Sci. 8 562–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley, S.K., Weis, R.M., and Thompson, L.K. 1996. The cytoplasmic fragment of the aspartate receptor displays globally dynamic behavior. Biochemistry 35 5199–5206. [DOI] [PubMed] [Google Scholar]
- Voss, J., He, M.M., Hubbell, W.L., and Kaback, H.R. 1996. Site-directed spin labeling demonstrates that transmembrane domain XII in the lactose permease of Escherichia coli is an alpha-helix. Biochemistry 35 12915–12918. [DOI] [PubMed] [Google Scholar]
- Voss, J., Hubbell, W.L., Hernandez-Borrell, J., and Kaback, H.R. 1997. Site-directed spin-labeling of transmembrane domain VII and the 4B1 antibody epitope in the lactose permease of Escherichia coli. Biochemistry 36 15055–15061. [DOI] [PubMed] [Google Scholar]
- Wand, A.J. 2001. Dynamic activation of protein fluctuations: A view emerging from NMR spectroscopy. Nat. Struct. Biol. 8 926–931. [DOI] [PubMed] [Google Scholar]
- Yaghmai, R. and Hazelbauer, G.L. 1993. Strategies for differential sensory responses mediated through the same transmembrane receptor. EMBO J 12 1897–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhulin, I.B. 2001. The superfamily of chemotaxis transducers: From physiology to genomics and back. Adv. Microb. Physiol. 45 157–198. [DOI] [PubMed] [Google Scholar]