Abstract
The gene encoding hemolysin II (HlyII) was amplified from Bacillus cereus genomic DNA and a truncated mutant, HlyII(ΔCT), was constructed lacking the 94 amino acid extension at the C terminus. The proteins were produced in an E. coli cell-free in vitro transcription and translation system, and were shown to assemble into SDS-stable oligomers on rabbit erythrocyte membranes and liposomes. The hemolytic activity of HlyII was measured with rabbit erythrocytes yielding an HC50 value of 1.64 ng mL−1, which is over 15 times more potent than staphylococcal α-hemolysin. HlyII(ΔCT) was about eight times less potent than HlyII in this assay. Limited proteolysis of the oligomers formed by HlyII and HlyII(ΔCT) on red cell membranes showed that the C-terminal extension is sensitive to digestion, while HlyII(ΔCT) is protease resistant and migrates with an electrophoretic mobility similar to that of digested HlyII. HlyII forms moderately anion selective, rectifying pores (I+80/I−80 = 0.57, 1 M KCl, pH 7.4) in planar lipid bilayers of diphytanoylphosphatidylcholine with a unitary conductance of 637 pS (1 M KCl, 5 mM HEPES, pH 7.4) and exhibits no gating over a wide range of applied potentials (−160 to +160 mV). In addition, it was demonstrated that HlyII forms a homoheptameric pore by using gel shift electrophoresis aided by a genetically encoded oligoaspartate tag. Although they share limited primary sequence identity (30%), these data confirm that HlyII is a structural and functional homolog of staphylococcal α-hemolysin.
Keywords: β-Barrel, hemolysin, membrane protein, pore-forming toxin, staphylococcal α-hemolysin, subunit stoichiometry
In this work, we examine the properties of hemolysin II (HlyII), a β-barrel pore forming toxin (β-PFT) from Bacillus cereus. The β-PFTs consist of several subfamilies of bacterial exotoxins that are related by sequence (Gouaux et al. 1997; Menestrina et al. 2001) and structure (Song et al. 1996; Gouaux et al. 1997; Olson et al. 1999; Pédelacq et al. 1999). These polypeptides are secreted as water-soluble molecules that bind to the surfaces of susceptible cells and assemble into oligomeric transmembrane pores leading to cell permeation and lysis (Bhakdi et al. 2000; Menestrina et al. 2001). The crystal structure of a heptameric staphylococcal α-hemolysin (αHL) pore has been determined in detergent at 1.9-Å resolution (Song et al. 1996). αHL has also been shown to form heptamers on red cell membranes (Gouaux et al. 1994), in planar lipid bilayers (Krasilnikov et al. 2000), supported bilayers (Fang et al. 1997), and after spontaneous assembly in solution (Cheley et al. 1997). In a working scheme for the assembly of αHL, the 293-residue polypeptide first binds to the membrane as a monomer, associates to form a heptameric prepore, and finally inserts into the bilayer to form the transmembrane pore. This scheme is supported by numerous biophysical and biochemical experiments (Cheley et al. 1997), and has been refined to accommodate recent structural data (Olson et al. 1999; Pédelacq et al. 1999).
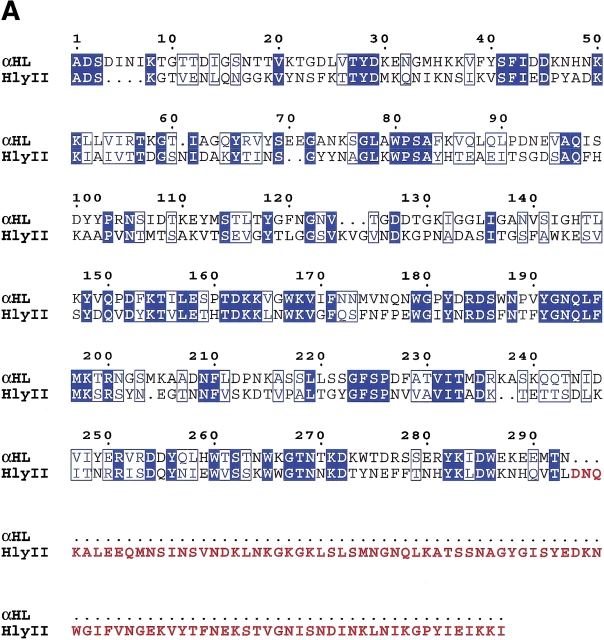
Bacillus cereus is an opportunistic pathogen. It is associated with a wide range of clinical symptoms, but encountered primarily in cases of severe food poisoning (Drobeniwski 1993; Lund et al. 2000). Over 20 exotoxins are produced and secreted by B. cereus, including several nonhemolytic enterotoxins (Alouf and Freer 1999). Two distinct hemolytic proteins produced by this bacterium, hemolysin II (HlyII) (Baida et al. 1999) and cytotoxin K (CytK) (Lund et al. 2000), have recently been cloned and shown to be homologous with the β-PFTs. HlyII has the longest polypeptide chain in the β-PFT family, with 412 residues, and contains a C-terminal 94 amino acid extension that has no homology with any other known β-PFT. The remainder of HlyII shares 30% sequence identity with αHL, and the level of identity with other members of the β-PFT family does not exceed this value. Although regions of similarity are dispersed throughout the aligned sequences (Fig. 1A ▶), the majority are concentrated in the strands that comprise the “cap” domain (Song et al. 1996), and are probably necessary to preserve the fold of the cap (Fig. 1B ▶).
Fig. 1.
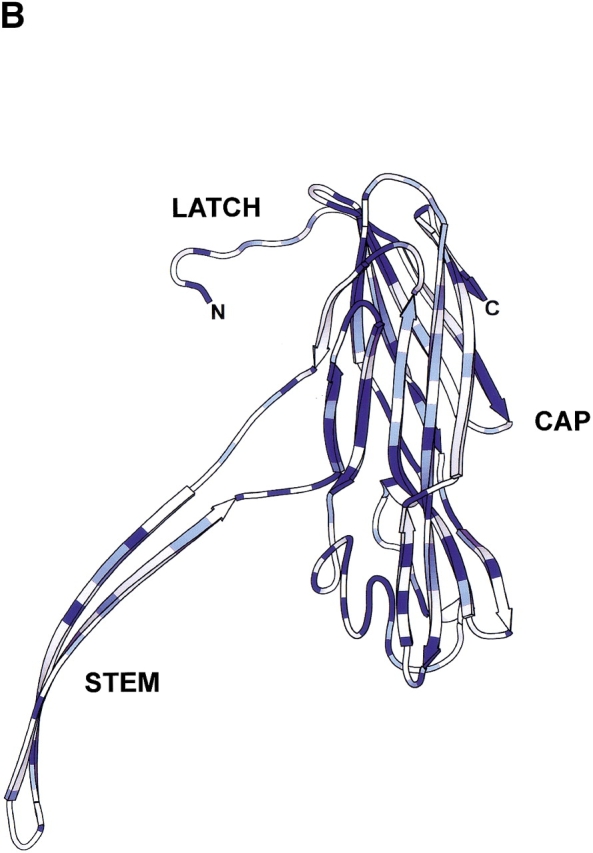
Comparison of Bacillus hemolysin II and staphylococcal α-hemolysin. (A) Primary sequence alignment of Bacillus cereus hemolysin II (HlyII) and staphylococcal α-hemolysin (αHL). Residues highlighted with a blue background are identical, while similar residues (Blosum62 similarity scoring matrix; Henikoff and Henikoff 1992) are shown as blue characters. The 94 residue C-terminal extension of HlyII is shown in maroon characters; it is the portion that was deleted to form the truncation mutant HlyII(ΔCT). The figure was generated using ClustalW 1.81 (Thompson et al. 1994) and rendered using ESPript 2.0 (Gouet et al. 1999). (B) Structure of one protomer taken from the crystal structure of the αHL heptamer (7aHL.pdb). Areas in dark blue and light blue correspond to identical and similar residues, respectively, as shown in (A). The image was created with SPOCK 6.3 (Christopher 1998) and rendered with Molscript (Kraulis 1991).
Members of the β-PFT family other than αHL have not been thoroughly investigated. In particular, limited data exist on the molecular architecture of the pores formed by them. The bicomponent leukocidin pore has a relatively large unitary conductance (2.5 nS in 1 M KCl) compared to other members of the β-PFT family (Miles et al. 2001), and it has been recently demonstrated to form an octameric transmembrane pore (Miles et al. 2002). The clostridial β-toxin has been shown to oligomerize into an SDS-stable multimer of unknown composition on human endothelial cells (Steinthorsdottir et al. 2000), and forms cation-selective channels with two conductance states of 60 and 110 pS (100 mM NaCl, 10 mM HEPES, pH 7.4, +60 mV) (Shatursky et al. 2000). Recently, it was shown that CytK purified from B. cereus supernatants forms pores in planar lipid bilayers, and is cytotoxic towards intestinal epithelial cells (Hardy et al. 2001). The subunit stoichiometries of the Bacillus toxins, HlyII and CytK, have not been determined.
In this study, we demonstrate that HlyII, produced by in vitro transcription and translation, forms a heptameric transmembrane pore in red cell membranes, which is resistant to SDS. In planar lipid bilayers, the pores are rectifying and lack voltage-induced gating. HlyII with the C-terminal extension removed, HlyII(ΔCT), has similar properties. Knowledge of the subunit stoichiometry and channel properties of this relative of αHL adds to the understanding of the β-PFTs family, and will be helpful in protein engineering aimed at the construction of pore-forming proteins with new properties (Bayley 1999; Bayley and Cremer 2001).
Results and Discussion
HlyII and HlyII(ΔCT) form SDS-resistant oligomers on red cell membranes and liposomes
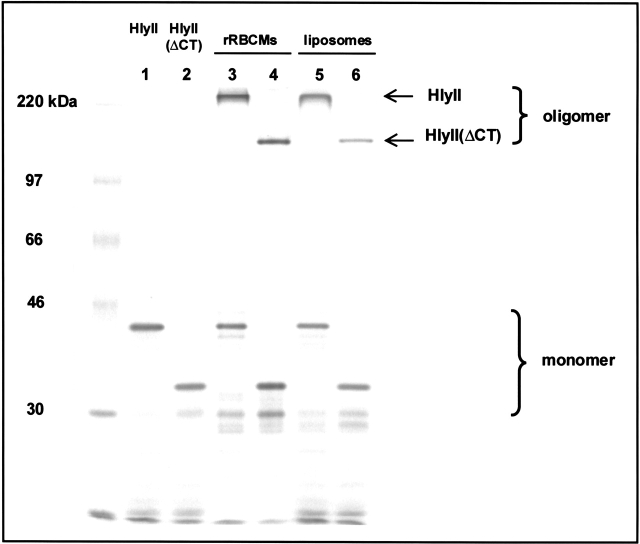
HlyII includes a 94-amino acid C-terminal extension, which is not homologous with any known β-PFT. The two most similar candidates from a BLAST search (Altshul et al. 1997) were a 46-amino acid segment of the pX01–124 gene product from Bacillus anthracis (39% identity, 67% similarity, one gap) and a 78-amino acid segment of orf16 from Streptococcus phage Cp-1 (34% identity, 49% similarity, three gaps). The genes for these proteins are both associated with genetic material linked with virulence, but the roles of the proteins have not been fully defined (Welkos 1991; Martín et al. 1996). We constructed a truncation mutant, HlyII(ΔCT), which lacks the extension (residues 289–382) (Fig. 1A ▶). 35S-labeled HlyII and HlyII(ΔCT) were produced in an E. coli S30 transcription and translation system. When HlyII was translated in the presence of rabbit red cell membranes (rRBCM) or incubated with small unilamellar vesicles (SUVs) composed of egg yolk phosphatidylcholine, cholesterol, and phosphatidic acid at a molar ratio of 55:25:20, a single high-molecular mass band above the 220-kD marker appeared upon SDS-polyacrylamide gel electrophoresis of unheated samples (Fig. 2 ▶). The extent of oligomerization on rRBCM is comparable to that of αHL, with 74% of the total membrane-bound protein in the oligomeric form (compared to 83% seen with αHL). In the case of HlyII(ΔCT), the extent of oligomerization is reduced to 49%. Oligomers formed by HlyII and HlyII(ΔCT) are stable in 2.3% SDS (1× Laemmli sample buffer) at room temperature and dissociate at 82°C and 78°C, respectively. αHL dissociates at 70°C in the sample buffer (data not shown).
Fig. 2.
35S-Labeled HlyII and HlyII(ΔCT) synthesized by coupled in vitro transcription and translation (IVTT) with an S30 extract from E. coli. An autoradiogram of a 10% SDS-polyacrylamide gel is shown. Lane 1, HlyII translated in the absence of rRBCM; lane 2, HlyII(ΔCT) translated in the absence of rRBCM; lane 3, HlyII translated in the presence of rRBCM; lane 4, HlyII(ΔCT) translated in the presence of rRBCM; lane 5, HlyII incubated in the presence of freshly prepared liposomes for 1 h at room temperature; lane 6, HlyII(ΔCT) incubated in the presence of liposomes. In the samples used in lanes 3 and 4, the rRBCM were washed before electrophoresis of the bound protein. In the samples used in lanes 5 and 6, the entire sample was loaded onto the gel. The high molecular weight bands (arrows) represent oligomerized HlyII and HlyII(ΔCT).
A deletion variant lacking the extension (and two additional amino acids) was previously shown to retain some hemolytic activity towards human red blood cells (Baida et al. 1999). The removal of the two additional amino acids is expected to lead to a reduction in activity. We found earlier that the removal of three amino acids from the C terminus of αHL causes a dramatic reduction in hemolytic activity that is associated with an almost complete loss of ability to form SDS-stable oligomers (Walker et al. 1992a).
Examination of the HlyII oligomer by limited proteolysis suggests structural similarity to αHL
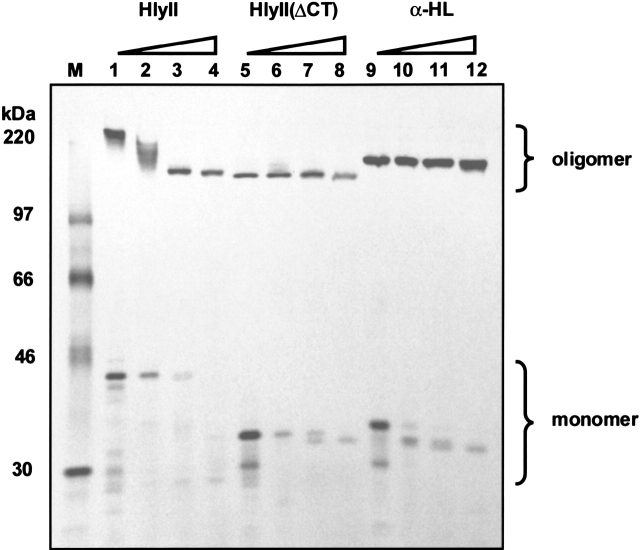
The susceptibility of HlyII and HlyII(ΔCT) to cleavage by proteinase K was compared with that of αHL (Fig. 3 ▶). The HlyII oligomer shows marked susceptibility to proteinase K, yielding multiple proteolytic fragments at low protease concentrations (5 μg mL−1; Fig. 3 ▶, lane 2). In contrast, the oligomeric form of HlyII(ΔCT), like the αHL heptamer, was protease resistant. At the highest concentration of proteinase K (500 μg mL−1), a protease-resistant, SDS-stable form of oligomeric HlyII was formed, which migrated in SDS gels with a similar mobility to oligomers formed from the genetically truncated HlyII(ΔCT) (Fig. 3 ▶). These results suggest that the C-terminal extension either has a structure with pronounced sensitivity to proteolytic cleavage, or that the extension is connected to the rest of the protein through a readily accessible linker. The former is favored because the C terminus (TL, see below) expressed as a separate domain is protease sensitive (data not shown). In any case, the stability of the proteolyzed pore implies that it is unlikely that the extension contributes significantly to the stability of the cap domain, the formation of which initiates heptamerization at the prepore stage (Cheley et al. 1997). In addition, when the sequences of HlyII from several different B. cereus and B. thuringiensis strains, BGSC 4A1, 4A4, 4A7, 6A3, and 6E2, were compared to the HlyII sequence from Bacillus cereus strain 6A5, the nonredundant sequence differences were concentrated in the C-terminal extension (14 differences in the 94 residues, compared with 9 differences in the 288 residues of the remainder of the polypeptide). This relative lack of conservation again suggests that the extension does not play a major role in oligomerization.
Fig. 3.
Conformational states of HlyII, HlyII(ΔCT) and αHL examined by limited proteolysis. HlyII, HlyII(ΔCT) and αHL were translated in the presence of rRBCM. The membranes were washed, treated with proteinase K, solubilized and subjected to electrophoresis in a 10% SDS-polyacrylamide gel prior to autoradiography. Lanes 1–4, HlyII; lanes 5–8, HlyII(ΔCT); lanes 9–12, αHL. The final proteinase K concentrations were: lanes 1, 5, and 9, 0 μg mL−1; lanes 2, 6, and 10, 5 μg mL−1; lanes 3, 7, and 11, 50 μg mL−1; lanes 4, 8, and 12, 500 μg mL−1.
Hemolysin II is a potent hemolytic toxin
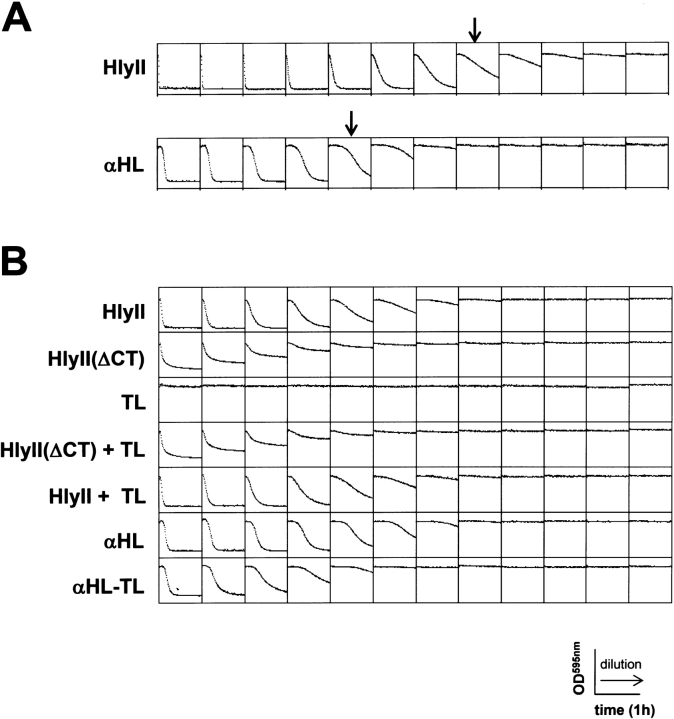
The hemolytic activities of HlyII and HlyII(ΔCT), produced by IVTT, were compared with αHL in a quantitative assay. The concentration of HlyII at which 50% of the rabbit erythrocytes were lysed in 1 h at 20°C (HC50) was 1.64 ng mL−1 (Fig. 4A ▶). By contrast, αHL gives an HC50 value of 25 ng mL−1 at 37°C (Walker et al. 1992b) and a similar value at 20°C (unpublished results). The HC50 value for CytK is similar to that of αHL (Lund et al. 2000). Therefore, the specific activity of HlyII is more than 15 times greater than that of αHL or CytK. The HC50 value amounts to about 1000 monomers per cell. HlyII(ΔCT) had a lower specific activity of 4.8 ng mL−1 (Fig. 4B ▶), in keeping with the somewhat less efficient oligomerization seen with this mutant (Fig. 3 ▶). The C-terminal extension by itself (TL) showed no hemolytic activity (Fig. 4B ▶), and did not affect the activity of HlyII(ΔCT) or HlyII. We have found that other β-PFTs can accommodate large extensions at their C termini. For example, the mutant αHL-TL was constructed in which the Bacillus tail was spliced genetically onto the C terminus of αHL. The HC50 of αHL-TL was 64 ng mL−1, i.e. αHL-TL was 2.5 times less active than wild type αHL. In the case of the F and S subunits of leukocidin, the hemolytic activities were unchanged when TL was fused to the C termini (Miles et al. 2002). The cytotoxic effects of HlyII against other cell lines has not been examined. It has been shown recently that CytK has potent activity against human intestinal epithelia, and is believed to have caused necrotic enteritis in a food poisoning outbreak resulting in several deaths (Lund et al. 2000; Hardy et al. 2001).
Fig. 4.
Quantitative hemolysis assays with rabbit erythrocytes. (A) One-hour activity assays on HlyII and αHL synthesized by IVTT. The first well in each panel contained IVTT mix diluted to a final volume of 100 μL in MBSA. The concentration of αHL in an IVTT mix was determined as previously described (Walker et al. 1992b; Miles et al. 2001) and used to calculate the concentration of HlyII produced by IVTT (see Materials and Methods). On this basis, the final concentrations of HlyII or αHL were made equal in the first well (0.21 μg/mL). Twofold serial dilutions from left to right were then made. The final concentration of rRBC in all wells was 0.5%. The arrows indicate ∼50% lysis. (B) One-hour activity assays on various constructs synthesized by IVTT. IVTT mixes containing HlyII, HlyII(ΔCT), αHL, and αHL-TL were diluted 10-fold with MBSA before addition (1 μL) to the first well. TL, the C-terminal extension of HlyII was translated as a separate polypeptide, and added where indicated in 32-molar excess as determined by phosphorimager quantitation (5 μL of undiluted IVTT mix in the first well). Twofold serial dilutions were made from left to right.
By comparison with αHL, there is a shorter initial lag period associated with HlyII hemolysis (Fig. 4 ▶): HlyII, 5 min to 5% lysis at HC50; αHL, 18 min to 5% lysis at HC50. The short lag period for HlyII suggests that the overall rate of pore formation (membrane binding, oligomerization, and membrane penetration) is greater than that of αHL. By contrast, the rates of hemolysis after the lag period were similar at comparable effective concentrations. Further investigation will be necessary to better define the origin of the difference in lag time.
HlyII forms ionic channels in planar lipid bilayers
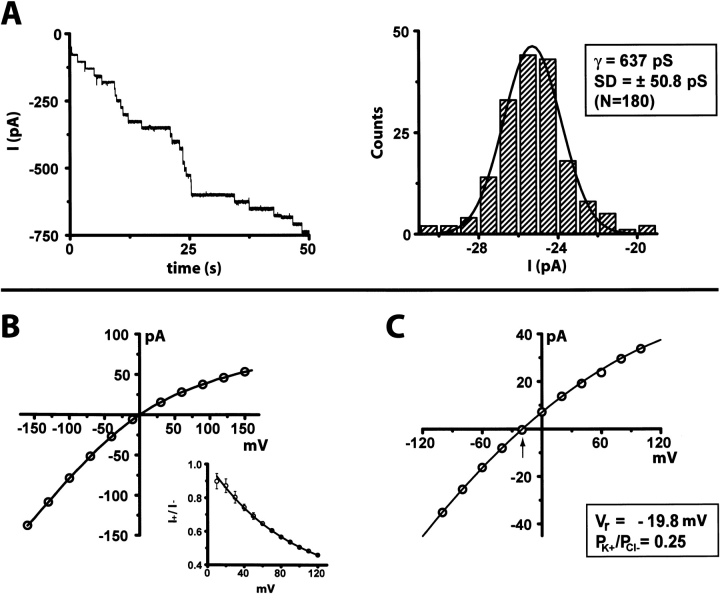
Upon insertion into planar lipid bilayers, the gel-purified oligomeric form of HlyII obtained by IVTT (Fig. 2 ▶) produced discrete conductance steps of 25.5 pA in 1 M KCl at pH 7.4 at a transmembrane potential of −40 mV (Fig. 5A ▶). If each step is presumed to correspond to a single ionic channel, the unitary conductance of the HlyII pore is 637 ± 51 pS (n = 180), under the prevailing conditions (Fig. 5A ▶). A similar result was obtained with gel-purified HlyII(ΔCT) oligomers, 636 ± 73 pS (Table 1). By comparison, the conductance of the αHL pore was 775 ± 38 pS, under the same conditions (Miles et al. 2001) and that of CytK 627 ± 14 pS (1 M NaCl, 5 mM HEPES, pH 7.2) (Hardy et al. 2001). Therefore, the functional diameters of the HlyII, CytK and αHL pores are similar.
Fig. 5.
Single-channel recordings from HlyII pores. (A) Individual insertions of HlyII pores into planar lipid bilayers. HlyII oligomer was prepared by IVTT in the presence of rRBCM, purified by SDS-polyacrylamide gel electrophoresis, and added to the cis chamber of a bilayer apparatus. Both chambers contained 1 M KCl, 5 mM HEPES, pH 7.4, and the applied potential was −40 mV. Stepwise changes in current are shown as a function of time. A histogram of the current steps is displayed and represents a compilation from 15 independent recordings. The histogram was fitted to a Gaussian function to obtain the single-channel conductance. (B) Single-channel current–voltage (I-V) relationship. A representative plot is shown. (Inset) Plot of I+/I− versus voltage representing data (± SD) from four independent recordings. The conditions were as described in (A). (C) HlyII is anion selective at neutral pH. The reversal potential (Vr), the applied voltage that gave zero current (arrow), was determined from single channel I-V plots with asymmetrical KCl solutions (1000 mM KCl cis, 200 mM KCl trans, both in 5 mM HEPES, pH 7.4). A representative plot is shown. The permeability ratio (PK+/PCl−) was derived from the GHK equation.
Table 1.
Single-channel properties of wild-type and engineered HlyII pores
| Protein | g (pS)a | n | I+80/I−80b | Vr(mV); PK+/PCl−c |
| HlyII(WT) | 637 ± 51 | 180 | 0.566 ± 0.004 | −18.0 ± 1.3; 0.29 (n = 4) |
| HlyII(ΔCT) | 636 ± 73 | 142 | 0.583 ± 0.001 | n.d. |
| HlyII(ΔCT)-D8 | 610 ± 54 | 273 | 0.591 ± 0.032 | −19.3 ± 1.2; 0.26 (n = 3) |
| [HlyII(ΔCT)]6/ [HlyII(ΔCT)-D8]1 | 642 ± 62 | 144 | 0.691 ± 0.077 | n.d. |
a Recordings were made at −40 mV in 1 M KCl, 5 mM HEPES, pH 7.4. Single-channel conductances were determined by fitting the peaks in amplitude histograms to Gaussian functions (e.g., Figs. 5, 8 ▶ ▶).
b Rectification ratios were determined from three or more experiments.
c The reversal potentials (Vr) and permeability ratios (PK+/PCl−) are mean values ± SD determined with the following buffers: cis, 1 M KCl, 5 mM HEPES, pH 7.4; trans, 0.2 M KCl, 5 mM HEPES, pH 7.4. n.d., not determined.
The HlyII pores show rectification when examined at applied potentials from −160 to +160 mV (I+80/I−80 = 0.566, 1 M KCl, pH 7.4) (Fig. 5B ▶, Table 1), which is the opposite of that observed with the clostridial β-toxin (Shatursky et al. 2000) (note that these authors use the opposite polarity sign convention in their bilayer recordings). The charge selectivity of the HlyII pore was determined by measuring the reversal potential of single-channel currents in asymmetrical KCl solutions. HlyII forms anion selective pores, with a permeability ratio (PK+/PCl−) of 0.29 ± 0.03 (1000 mM KCl (cis), 200 mM KCl (trans), pH 7.5) (Fig. 5C ▶). The HlyII pore is significantly more anion selective than the αHL pore (PK+/PCl− = 0.79, under the same conditions) (Gu et al. 2000), whereas the leukocidin pore is cation selective (PK+/PCl− = 1.64) (Miles et al. 2001). Of the known β-PFTs, HlyII and CytK share the greatest sequence identity within the putative transmembrane domain (48%). In HlyII, each subunit contributes seven charged residues that are predicted on the basis of their hydrophilicity and a sequence alignment with αHL to face the lumen of the transmembrane barrel (K106, E110, K120, D125, K126, D131, K141). However, only two charged side chains (K128, E139) are predicted to project into the lumen of the CytK pore, which is almost nonselective (Hardy et al. 2001). The overall positive charge in the HlyII pore is likely to contribute to the observed anion selectivity.
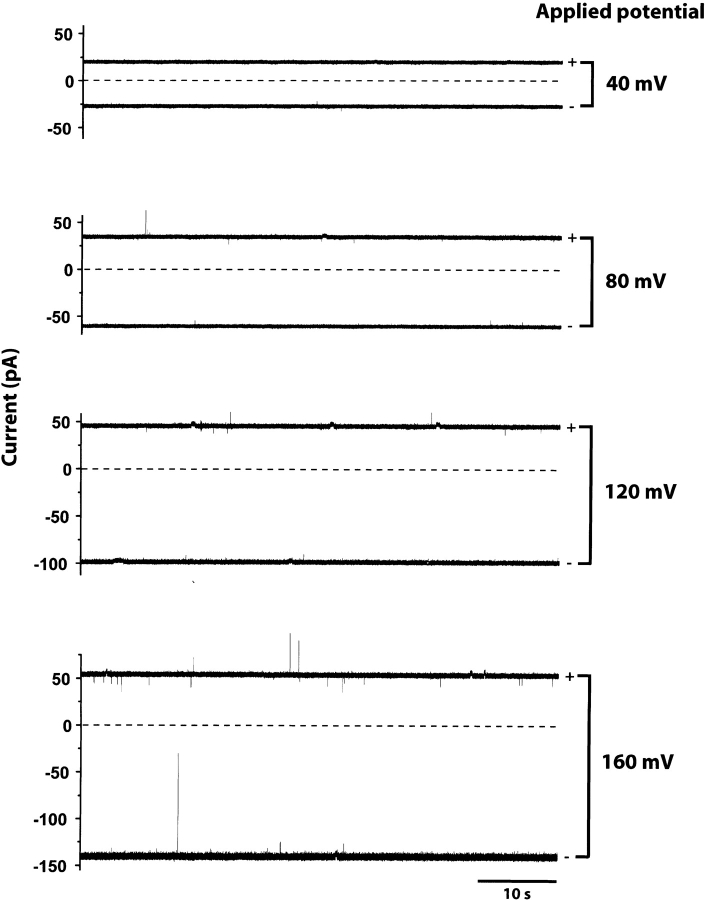
In addition, the HlyII pore remains predominantly open at the applied potentials tested (−160 to +160 mV) (Fig. 6 ▶). This is similar to what is observed with αHL (Korchev et al. 1995), but in contrast with the properties of most porins and many other β-PFTs, which exhibit voltage-induced gating. For example, the leukocidin pore gates at positive potentials above +60 mV, but remains fully open at negative potentials up to −160 mV (Miles et al. 2001). Two rings of charged residues facing the lumen of the HlyII pore form the boundaries of the putative transmembrane region, E110/K141 and K120/D131, based on the alignment of residues in the stem domain (unpublished results) with those in the αHL β-barrel (Song et al. 1996). We speculate that these side chains form salt bridges that strengthen the β-barrel, holding it in a rigid conformation even under high applied potentials. This argument is reinforced by the ruggedness of the HlyII oligomer, which dissociates in SDS at 82°C, as it is known that it is barrel formation that stabilizes the αHL heptamer to heat denaturation in SDS (Valeva et al. 1996; Cheley et al. 1999).
Fig. 6.
The HlyII pore exhibits minimal gating over a wide range of applied potentials. Each trace is representative of a single channel from HlyII oligomer prepared by IVTT in the presence of rRBCM and purified by SDS-polyacrylamide gel electrophoresis. The fully open state of the channel is shown at both polarities of the indicated voltage.
Hemolysin II assembles as a heptamer on membranes
Finally, we determined the subunit composition of the HlyII pore by gel shift electrophoresis. Whereas the original gel-shift method used to evaluate the stoichiometry of the αHL pore was based on a mobility change brought about by site-specific chemical modification (Gouaux et al. 1994; Braha et al. 1997), gel-shift experiments based on genetically engineered truncations or extensions have proved successful in other cases (Heginbotham et al. 1997; Zitzer et al. 1999; Miyata et al. 2001; Miles et al. 2002).
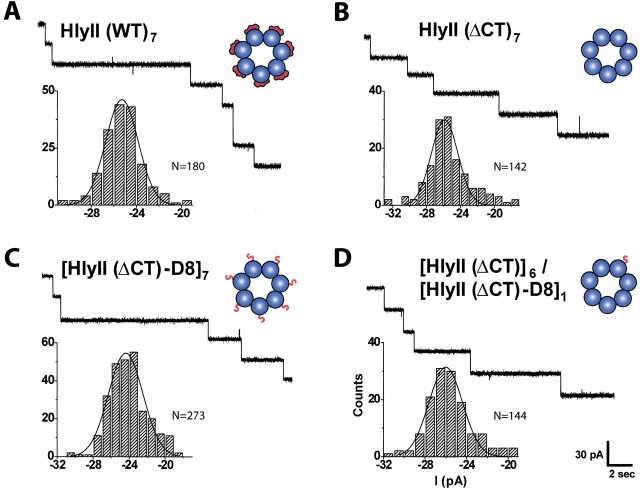
In a similar approach to the one we used here, transthyretin heterotetramers were separated by using an N-terminal charged extension (Flag tag) (Schneider et al. 2001). Similarly, hybrid trimers of the catalytic subunits of aspartate transcarbamoylase were resolved with a six-residue aspartate tail (Sakash and Kantrowitz 2000). The mutant HlyII-D8 was constructed in which an extension encoding eight aspartate residues was incorporated at the C terminus of HlyII (Fig. 7A ▶). Oligomers containing various ratios of HlyII to HlyII-D8 were produced by cotranslation in the presence of rRBCM. Analysis of the oligomers by SDS-polyacrylamide gel electrophoresis and autoradiography lacked sufficient resolution to enable a count of individual bands (Fig. 7B ▶). It is unclear why the D8 tail on full-length HlyII fails to yield sharp bands; it is possible that the various permutations of each combination of subunits (Braha et al. 1997) are spread out on the gel. By contrast, eight distinct bands were obtained by using various mixtures of αHL and αHL-D8 (Fig. 7C ▶). Each downward shift in electrophoretic mobility corresponds to the incorporation of one αHL-D8 subunit into the αHL heptamer. Therefore, we engineered the D8 tail onto HlyII(ΔCT). When assembled with HlyII(ΔCT), eight species were resolved by gel electrophoresis (Fig. 7D ▶). This result indicates that the HlyII(ΔCT) oligomer contains seven subunits.
Fig. 7.
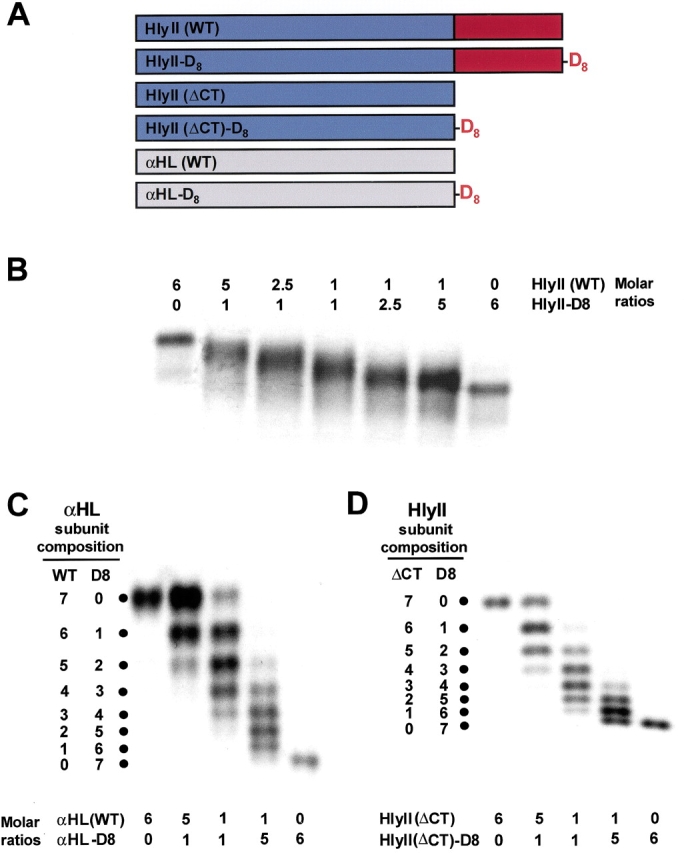
Subunit stoichiometry of the HlyII pore. (A) Constructs used in the study. The maroon block represents the 94-residue C-terminal extension. The oligoaspartate (D8) tail is shown in red. (B) SDS-polyacrylamide gel electrophoresis of heteromers generated from WT HlyII and/or HlyII-D8. (C) Heteromers generated from αHL and/or αHL-D8. (D) Heteromers generated from HlyII(ΔCT) and/or HlyII(ΔCT)-D8. The DNA constructs were mixed at various molar ratios, as indicated in (B–D), and cotranslated in the presence of rRBCM. The membranes were washed, solubilized in sample buffer without heating, and subjected to electrophoresis in a 5% gel. Autoradiograms of dried gels are shown. The results in B are from a long gel (36 cm), which was run for 72 h at 50 V.
Because the properties of the pores formed by HlyII and HlyII(ΔCT) are similar, HlyII is also likely to form a heptamer. HlyII and HlyII(ΔCT) produced pores with similar conductance values (Fig. 8A,B ▶; Table 1). Together with the proteolysis experiments and measurements of hemolytic activity, these results support the idea that the 94-amino acid C-terminal extension is neither essential for pore formation nor for determining functional properties. Oligomers composed entirely of HlyII(ΔCT)-D8 subunits and a heteromer containing one D8 subunit [HlyII(ΔCT)]6/[HlyII(ΔCT)-D8]1 also exhibited similar conductance values, rectification properties and charge selectivities when compared with the pores formed by HlyII and HlyII(ΔCT) (Fig. 8A,B ▶;Table 1). Therefore, despite the additional 56 negative charges in [HlyII(ΔCT)-D8]7, the conductive pathway remains unaffected, in keeping with the location of the C terminus distant from the channel entrance.
Fig. 8.
Representative channel insertion events and single channel conductance histograms for [HlyII]7, [HlyII(ΔCT)]7, [HlyII(ΔCT)-D8]7, and the heteroheptamer [HlyII(ΔCT)]6[HlyII(ΔCT)-D8]1. (A) HlyII homoheptamer. (B) HlyII(ΔCT) homoheptamer. (B) HlyII(ΔCT)-D8 homoheptamer. (C) [HlyII(ΔCT)]6[HlyII(ΔCT)-D8]1 heteroheptamer. After a preparative IVTT reaction in the presence of rRBCM and SDS-polyacrylamide gel electrophoresis, the desired band was excised from the gel and processed as described in Materials and Methods. Current traces were recorded in 1 M KCl, 5 mM HEPES (pH 7.4). The scale for the current trace in (D), applies to the entire figure. The histograms of the current steps represent compilations from at least 15 independent recordings. Conductance values, rectification properties, and ion selectivities are summarized in Table 1.
Several different N- and C-terminal oligoaspartate extensions have been evaluated in our laboratory for their ability to produce electrophoretic shifts in β-PFTs. For instance, a D8 tail provides about 1.5 times the separation between oligomeric species in SDS gels compared with a D4 extension (G. Miles and S. Cheley, unpubl.). Besides its utility in evaluating the subunit composition of proteins, this approach provides a convenient way to separate functional heteromers (Sakash and Kantrowitz 2000; Howorka et al. 2001a, 2001b).
General implications and future prospects
Here, we have shown that the properties of the pore-forming protein HlyII from Bacillus cereus conforms, in general, with those of other members of the class of β-PFTs. HlyII has the most potent hemolytic activity yet found in the β-PFTs; 50% lysis of rabbit red cells occurs at a ratio of ∼1000 HlyII monomers per cell. HlyII possesses additional properties that might be improved for applications in biotechnology (Eroglu et al. 2000; Bayley and Cremer 2001). For example, the heptameric pore is more stable than that formed by αHL, dissociating in SDS at 82°C. Remarkably, the pore formed by clostridial β toxin is yet more stable, resisting “boiling” in SDS (Steinthorsdottir et al. 2000). The pore formed by HlyII remains open at high transmembrane potentials, which is a useful property for applications in sensor technology (Bayley et al. 2000; Bayley and Cremer 2001). The results provided here also demonstrate that the tail carried by HlyII has little influence on the properties of the pore. This suggests that the tail might be replaced by polypeptide sequences that provide additional functionality such as the ability to form lattices or bind to surfaces, or a linked catalytic activity. Finally, the use of an oligoaspartate extension provides a convenient means to purify heterooligomeric pores. Combined with new findings about the subunit–subunit interface, revealed by the ability of the related binary pore-forming toxin, leukocidin, to form octamers (Miles et al. 2002), the present work suggests that it should be possible to extend the range of heteromeric pores that can be formed and purified.
Materials and methods
Isolation of genomic DNA from Bacillus cereus
Bacillus cereus strain 6A5 (Bacillus Genetic Stock Center, Ohio State University) (equivalent to ATCC #14579) was grown to saturation from log-phase inocula and stored frozen at −80°C in 50% glycerol. To isolate genomic DNA, a portion of a culture (5 mL) grown overnight at 30°C was centrifuged at 3000g. The pellet was resuspended in buffer B1 (Qiagen #19060; 1 mL, 50 mM Tris.HCl, pH 8.0, 0.5% Tween-20, 0.5% Triton X-100), supplemented with 125 U of mutanolysin (Sigma. #M4782; 25 μL of a stock made at 5000 U mL−1 in deionized water and stored in frozen aliquots), 0.4 mg proteinase K (GibcoBRL; 20 μL of a stock made at 20 mg mL−1 in 1× TE [10 mM Tris.HCl, 1 mM EDTA, pH 8.0, supplemented with 10 mM NaCl]) and 0.2 mg RNase A (Qiagen; 2 μL of a 100 mg mL−1 stock), and incubated at 37°C overnight. Genomic DNA was purified on an anion-exchange resin using a Qiagen Genomic tip 20/G (#10223) and redissolved in 1× TE. The DNA was then sheared by two passes through an 18-gauge needle.
Amplification of Bacillus hemolysin-II genes
The coding sequences for HlyII were amplified from the genomic DNA by using the following primers: HlyII (sense) 5`-ACATATGGCAGATTCTAAAGGAACTGTAGAAAATC-3`; HlyII (antisense) 5`-CAAGCTTATCAGATTTTTTTAATCTCA ATATAAGG-3`. The sense primer generated an NdeI site (CATATG), containing a new initiation codon, immediately before the first codon of the mature polypeptide, predicted by sequence aligment (Fig. 1A ▶). The antisense primer encodes two stop codons and a HindIII site immediately following the last codon of the gene. PCR was carried out in a 50-μL mixture containing sheared genomic DNA (50 ng), primers (50 pmole of each), 200 μM dNTPs (Stratagene), and 1.5 U of Taq/Pwo DNA polymerase mixture in PCR buffer 1 (Expand Long Template PCR System) with the following program: 95°C for 2 min, 25 cycles of 94°C (60 sec), 45 to 56°C gradient (60 sec), 72°C (80 sec), followed by a final extension at 72°C for 7 min. The PCR product was then cloned into the TOPO-TA plasmid (#K4500–01, Invitrogen) to yield pHlyII-TA.
pHlyII-TA was digested with NdeI and HindIII, and the liberated DNA insert was ligated into the pT7 expression vector pT7-SC1 (Miles et al. 2001). Upon sequencing, two independently amplified clones from Bacillus cereus strain 6A5; the following differences were noted from the published sequence (B. cereus VKM-B771) (Baida et al. 1999): G66S (GGG→AGC), S236T (TCT→ACT), N276H (AAC→CAC), P294L (CCT→CTT), I299N (ATT→AAT), G300S (GGT→AGT), N303S (AAC→AGC), N306D (AAC→GAT), Q307K (CAG→AAA), F317L (TTT→CTT), T358S (ACA→TCA). With the exception of three residues (in bold), the variations noted occurred in the C-terminal extension. In addition, there were 35 silent changes throughout the sequence. The DNA sequence from strain 6A5 has been deposited in GenBank with the accession number AF448485.
Hemolysin-II C-terminal truncation
Unless otherwise noted, the constructs used in this study were created by PCR and cloned into the TOPO-TA plasmid prior to being subcloned into pT7-SC1 by ligation. Each construct was verified by DNA sequencing.
HlyII(ΔCT), a mutant of HlyII truncated at the C terminus, was constructed by removing the sequence encoding the last 94 amino acids of HlyII (residues 289–382). PCR was carried out on linearized pT7-HlyII(6A5) using the forward primer SC001 5`-CAC TATAGGGAGACCACAACGG-3` and the reverse primer BAC6 A5TRN1 5`-TAAGCTTCATTAAAGAGTAACTTGATG-3`. The latter encodes two stop codons and a HindIII site immediately after the Leu-288 codon in the HlyII gene.
The C-terminal extension (TL) was cloned separately into the pT7 vector by using PCR. The sense primer BAC6A5TAILBEGIN 5`-ACATATGGATAACCAAAAAGCCCTT-3` generated an NdeI site (CATATG), containing a new initiation codon, immediately before the first codon of the C-terminal extension (Asp–289). The antisense primer was SC011 5`-CCCCTCAAGACCCGTTTAG AGGC-3`, which hybridized at a site in the vector downstream of the stop codons.
Construction of mutants with C-terminal (oligo)–aspartic acid extensions
We sought to develop a genetic alternative to the original gel shift method for counting subunits, which was based on a mobility change brought about by site-specific chemical modification (Gouaux et al. 1994). The mutants HlyII-D8, HlyII(ΔCT)-D8, and αHL-D8 were constructed with an extension encoding eight C-terminal aspartate residues (Fig. 7A ▶). The “D8 tail” was expected to change the electrophoretic mobility of the assembled pore based on charge. PCR was carried out with SC001 as the sense primer, using each of the following reverse primers: HlyII-D8, 5`-AAGCT TATCAATCGTCATCGTCATCGTCATCGTCGATTTTTTTAA TCTCAA-3`; HlyII(ΔCT)-D8, 5`-AAGCTTATCAATCGTCATC GTCATCGTCGTCGTCAAGAGT AACAAGATGGTT-3`; αHLD8, 5`-AAGCTTATCAATCATCGTCGTCATCATCGTCATCATTTGTCATTTCTTCTTTTTCCC-3`. These electrophoretically purified antisense oligonucleotides incorporated two stop codons (bold) after the D8 tail codons (underscored) followed by a HindIII site (italics).
Construction of the fusion protein, αHL-TL
In vivo recombination (Howorka and Bayley 1998) was used to fuse a 3` extension directly to the last codon of the αHL gene (Jones 1995). The extension encoded the 94 amino acids of the Bacillus hemolysin II C-terminal tail (residues 289–382). The fused gene (αHL-TL) was generated in pT7-SC1 by cotransforming Escherichia coli XL-10 Gold cells with two PCR products encompassing the Bacillus tail and the αHL gene. The primer sets for the two amplification reactions were: (1) nonmutagenic primer (F-NM) (sense), 5`-GTATTCAACATTTCCGTGTCGCCCTTAT TC-3`; αHL-TAIL-αHL, (antisense), 5`-AAGGGCTTTAAGGTTATCATTTGTCATTTCTTCTTT-3`; and (2) αHL-TAIL-TL (sense), 5`-AAAGAAGAAATGACAAATGATAACCAAAAAGCCCTT-3`; nonmutagenic primer (R-NM) (antisense) 5`-GAATAAGGG CGACACGGAAATGTTGAATAC-3`. The underlined 18-nt sequences form the overlap for recombination between the two PCR products.
In vitro transcription and translation (IVTT)
Polypeptides were synthesized in a cell-free in vitro transcription and translation (IVTT) system by using an S30 extract from E. coli (T7 S30 No. L114A, Promega) supplemented with rifampicin (20 μg mL−1) (Walker et al. 1992b). Radiolabeled polypeptides were synthesized by IVTT with the complete amino acid mix supplemented with [35S]methionine (ICN Biomedicals, Inc., 1175 Ci mmole−1, 10 μCi per 25 μL translation). The concentrations of the translated polypeptides were determined by phosphorimager analysis, by comparison with αHL standards radiolabeled in parallel IVTT reactions (Miles et al. 2001). The specific radioactivity of the αHL, in phosphorimager units, was determined after deducing the concentration of the protein from its hemolytic activity, assuming a specific hemolytic activity of 25 ng mL−1 (Walker et al. 1992b). The specific radioactivity was normalized to the number of Met residues in the polypeptide chain (assuming that the N-terminal Met is intact). The concentration of HlyII, and its variants, in a translation mix could then be determined from the strengths of the phosphorimager signals and the number of Met residues in the polypeptide chains.
Quantitative hemolysis assay
HlyII proteins, synthesized by IVTT with the complete amino acid mix, were diluted into MBSA (10 mM 3-[N-morpholino]propane sulfonic acid; MOPS, Cat. No. AB1270, American Bioanalytical, 150 mM NaCl, pH 7.4, containing 1 mg mL−1 bovine serum albumin, Cat. No. 4503, Sigma) and subjected to 12 twofold serial dilutions in the same buffer in microtiter wells (final volume 50 μL). An equal volume of 1% washed rabbit erythrocytes (rRBC) in MBSA was quickly added to each well, beginning with the most diluted sample. Hemolysis was recorded for 1 h at 20°C by monitoring the decrease in light scattering at 595 nm with a Bio-Rad microplate reader (Model 3550-UV) and using the Microplate Manager 4.0 software.
HlyII oligomer formation on rabbit erythrocyte membranes
Radiolabeled HlyII oligomers were prepared by IVTT in the presence of [35S]methionine and rRBCM (5 μL, 3.0 mg protein mL−1) (Cheley et al. 1999) in a total reaction volume of 25 μL. After 1 h at 30°C, the mixture was centrifuged and the supernatant discarded. The membrane pellet was washed and resuspended in MBSA (80 μL) prior to solubilization by the addition of 5× Laemmli sample buffer (20 μL; Laemmli 1970). A portion (20 μL) was subjected to electrophoresis in a 10% SDS-polyacrylamide gel. The gel was fixed for 1 h prior to drying and autoradiography.
Oligomerization of HlyII on liposomes
Egg yolk phosphatidylcholine, cholesterol, and phosphatidic acid in chloroform were mixed in the desired molar ratio of 55:25:20. After drying under vacuum, the lipid film was resuspended in buffer (10 mM Tris, pH 8.0, 150 mM NaCl, 1 mM EDTA) to a total lipid concentration of 5 mg mL−1. Liposomes were prepared by ultrasonication for 30 min on ice using a probe sonicator (Dynatech Sonic Dismembrator Model 150) (relative output set to 50% power), followed by a brief centrifugation (30 sec, 16,000g) to remove titanium particles. [35S]Methionine-labeled HlyII or HlyII(ΔCT) (2 μL of an IVTT reaction mix) was incubated with freshly prepared liposomes (8 μL) for 1 h at room temperature. After solubilization in Laemmli sample buffer, the mixture was subjected to SDS polyacrylamide gel electrophoresis.
Proteinase K treatment of HlyII, HlyII(ΔCT) and αHL polypeptides on membranes
Proteinase K (Sigma, #P-0390) solutions (5.0, 0.5, and 0.05 mg mL−1 in water) were prepared by dilution of an enzyme stock (10 mg mL−1 in water) and used immediately. Limited proteolysis was performed on HlyII, HlyII(ΔCT) and αHL bound to rRBCM. The membranes were resuspended in MBSA at 0.19 mg membrane protein mL−1 and divided into four tubes (18 μL in each). Proteinase K or water (2 μL) was added to each tube. After 5 min at room temperature, the reactions were stopped by treatment with PMSF (9 mM final, added in 2 μL of isopropanol, 5 min, room temperature), followed by the addition of 2× Laemmli loading buffer. The samples were subjected to electrophoresis in 10% SDS-polyacrylamide gels (unheated samples) or 12% gels (heated samples: 95°C, 5 min).
Gel purification of HlyII oligomers
HlyII, HlyII(ΔCT) and HlyII(ΔCT)-D8, and were prepared by translation in the presence of rRBCM as described above, but in preparative amounts (75 μL IVTT reaction). To obtain, HlyII(ΔCT)6(ΔCT)-D81, HlyII(ΔCT) and HlyII(ΔCT)-D8 were translated using the corresponding plasmids at a ratio of 5:1. The oligomers were purified by SDS-polyacrylamide gel electrophoresis in an 8% gel in the presence of 0.1 mM sodium thioglycolate (Movileanu et al. 2001; Miles et al. 2001), stored at −80°C in 10 mM Tris.HCl, pH 7.5, and used for bilayer recordings without further treatment.
Hetero-oligomer formation for the determination of stoichiometry
Hetero-oligomers of hemolysin II subunits containing HlyII and/or HlyII-D8, and HlyII(ΔCT) and/or HlyII(ΔCT)-D8 were prepared by mixing the corresponding plasmids in the desired molar ratios (see Fig. 7 ▶ legend) prior to IVTT in the presence of rRBCM. To obtain αHL heteromers, αHL and/or αHL-D8 were used. The washed membrane pellets were solubilized with Laemmli sample buffer and subjected, without heating, to electrophoresis in 5% SDS-polyacrylamide gels. Autoradiographs were made of the dried gels.
Planar lipid bilayer recordings
All measurements were performed at 25°C. Numerical values are given as the mean ± SD (σn−1). Planar lipid bilayer membranes were formed with 1,2-diphytanoyl-sn-glycero-3-phosphocholine (Avanti Polar Lipids) on a 150–160 μm-diameter aperture in a 25 μm-thick Teflon film (Goodfellow Corporation) separating the cis and trans compartments (2 mL each) of a bilayer apparatus (Montal and Mueller 1972). Prior to forming the lipid bilayer, the orifice was pretreated with 10% (v/v) hexadecane (#29,631-7; Aldrich) in n-pentane (Burdick & Jackson) and allowed to dry thoroughly. For most measurements, the cis and trans chambers contained 1 M KCl, 5 mM HEPES, pH 7.4. Various concentrations of KCl in 5 mM HEPES, pH 7.4 were used for ion selectivity measurements. Protein samples were added to the cis chamber, which was at ground.
Currents were recorded by using a Dagan 3900A patch clamp amplifier (Dagan Corporation) with a 3910 expander and a built-in low-pass four-pole Bessel filter set at 5 kHz. Data were stored on digital audio tape with a DAS-75 data recorder (Dagan Corporation). Prior to analysis, the signal was low-pass filtered at 1 kHz with an eight-pole Bessel filter (Model 902, Frequency Devices) and acquired with a Digidata 1200A A/D board with a sampling time interval of 200 μsec. Data were acquired and analyzed with pClamp 8.0 software (Axon Instruments). Single-channel conductance values were determined by fitting the peaks in amplitude histograms to Gaussian functions. Current–voltage (I-V) relationships for single channels were determined by recording the currents obtained after stepwise changes in applied potential. The permeability ratios (PK+/PCl−) were calculated from experimentally determined reversal potentials (Vr) by using the Goldman-Hodgkin-Katz (GHK) equation (Hille 2001) and the appropriate activity coefficients for KCl solutions (Zemaitis et al. 1986). In these measurements, the cis compartment contained 1000 mM KCl, while the other chamber contained 200 mM KCl. Any electrode DC offset was balanced prior to the addition of protein to the cis chamber. The applied voltage that gave zero current was noted. In addition, the reversal potential was more accurately determined by polynomial fits to current–voltage (I-V) data. Symmetrical solutions were then reestablished to evaluate whether or not any DC offset had built up during the course of the experiment. It all cases, the offset was less than 1 mV.
Acknowledgments
This work was supported by grants from the DOE and NIH. G.M. holds an MD-PhD training fellowship at The Texas A&M University System Health Science Center, College of Medicine, and was the recipient of an ASSERT (ARO) award. The authors thank Daniel Zeigler, BGSC Director, Ohio State University, for graciously supplying Bacillus strains; Orit Braha and Li-Qun Gu for their advice on ion selectivity; Brian Lauman for technical help; Michael Palmer for advice on liposomes; and Sean Conlan for guidance on using Spock.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked "advertisement" in accordance with 18 USC section 1734 solely to indicate this fact.
Abbreviations
αHL, α-hemolysin of Staphylococcus aureus
αHL-D8, α-hemolysin with a C-terminal extension of eight aspartate residues
αHL-TL, α-hemolysin fusion protein with a C-terminal extension comprising the C-terminal 94 residues of HlyII
β-PFT, β-barrel pore forming toxin
CytK, cytotoxin K of Bacillus cereus
HEPES, N-[2-hydroxyethyl]piperazine-N`-[2-ethanesulfonic acid]
MBSA, 10 mM Na MOPS, 150 mM NaCl, pH 7.4, containing 1 mg mL−1 bovine serum albumin
MOPS, 3-[N-morpholino]propanesulfonic acid
IVTT, in vitro transcription and translation
PMSF, phenylmethylsulfonylfluoride
rRBC, rabbit erythrocyte
rRBCM, rabbit erythrocyte membranes
SDS, sodium dodecyl sulfate
HlyII, hemolysin II of Bacillus cereus
HlyII-D8, hemolysin II with a C-terminal extension of eight aspartate residues
HlyII(ΔCT), a truncation mutant of HlyII lacking 94 amino acid residues at the C terminus
HlyII(ΔCT)-D8, HlyII(ΔCT) with a C-terminal extension of eight aspartate residues
TL, a polypeptide comprising the C-terminal 94 amino acids of HlyII
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1110/ps.0204002.
References
- Alouf, J.E. and Freer, J.H. 1999. The comprehensive sourcebook of bacterial protein toxins. Academic Press, New York.
- Altshul, S.F., Madden, T.L., Schaffer, A.A., Zhang, J., Zhang, Z., Miller, W., and Lipman, D.J. 1997. Gapped BLAST and PSI-BLAST; A new generation of protein database search programs. Nucleic Acids Res. 25 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baida, G., Budarina, Z.I., Kuzmin, N.P., and Solonin, A.S. 1999. Complete nucleotide sequence and molecular characterization of hemolysin II gene from Bacillus cereus. FEMS Microbiol. Lett. 180 7–14. [DOI] [PubMed] [Google Scholar]
- Bayley, H. 1999. Designed membrane channels and pores. Curr. Opin. Biotechnol. 10 94–103. [DOI] [PubMed] [Google Scholar]
- Bayley, H. and Cremer, P.S. 2001. Stochastic sensors inspired by biology. Nature 413 226–230. [DOI] [PubMed] [Google Scholar]
- Bayley, H., Braha, O., and Gu, L.-Q. 2000. Stochastic sensing with protein pores. Adv. Mater. 12 139–142. [Google Scholar]
- Bhakdi, S., Walev, I., Palmer, M., and Valeva, A. 2000. Staphylococcal α toxin. In Bacterial protein toxins (eds. K. Aktories and I. Just), pp. 509–527. Springer, Berlin.
- Braha, O., Walker, B., Cheley, S., Kasianowicz, J.J., Song, L., Gouaux, J.E., and Bayley, H. 1997. Designed protein pores as components for biosensors. Chem. Biol. 4 497–505. [DOI] [PubMed] [Google Scholar]
- Cheley, S., Braha, O., Lu, X., Conlan, S., and Bayley, H. 1999. A functional protein pore with a "retro" transmembrane domain. Protein Sci. 8 1257–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheley, S., Malghani, M.S., Song, L., Hobaugh, M., Gouaux, J.E., Yang, J., and Bayley, H. 1997. Spontaneous oligomerization of a staphylococcal α-hemolysin conformationally constrained by removal of residues that form the transmembrane β barrel. Protein Eng. 10 1433–1443. [DOI] [PubMed] [Google Scholar]
- Christopher, J.A. 1998. SPOCK: The structural properties observation and calculation kit (program manual). Center for Macromolecular Design, Texas A&M University, College Station, TX.
- Drobeniwski, F.A. 1993. Bacillus cereus and related species. Clin. Microbiol. Rev. 6 324–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eroglu, A., Russo, M.J., Bieganski, R., Fowler, A., Cheley, S., Bayley, H., and Toner, M. 2000. Intracellular trehalose improves the survival of cryopreserved mammalian cells. Nat Biotechnol 18 163–167. [DOI] [PubMed] [Google Scholar]
- Fang, Y., Cheley, S., Bayley, H., and Yang, J. 1997. The heptameric prepore of a staphylococcal α-hemolysin mutant in lipid bilayers imaged by atomic force microscopy. Biochemistry 36 9518–9522. [DOI] [PubMed] [Google Scholar]
- Gouaux, J.E., Braha, O., Hobaugh, M.R., Song, L., Cheley, S., Shustak, C., and Bayley, H. 1994. Subunit stoichiometry of staphylococcal α-hemolysin in crystals and on membranes: A heptameric transmembrane pore. Proc. Natl. Acad. Sci. 91 12828–12831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouaux, E., Hobaugh, M., and Song, L. 1997. α-Hemolysin, γ-hemolysin and leukocidin from Staphylococcus aureus: Distant in sequence but similar in structure. Protein Sci. 6 2631–2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouet, P., Courcelle, E., Stuart, D.I., and Metoz, F. 1999. ESPript: Analysis of multiple sequence alignments in PostScript. Bioinformatics 15 305–308. [DOI] [PubMed] [Google Scholar]
- Gu, L.-Q., Dalla Serra, M., Vincent, J.B., Vigh, G., Cheley, S., Braha, O., and Bayley, H. 2000. Reversal of charge selectivity in transmembrane protein pores by using non-covalent molecular adapters. Proc. Natl. Acad. Sci. 97 3959–3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy, S.P., Lund, T., and Granum, P.E. 2001. CytK toxin of Bacillus cereus forms pores in planar lipid bilayers and is cytotoxic to intestinal epithelia. FEMS Microbiol. Lett. 197 47–51. [DOI] [PubMed] [Google Scholar]
- Heginbotham, L., Odessey, E., and Miller, C. 1997. Tetrameric structure of a prokaryotic K+ channel. Biochemistry 36 10335–10342. [DOI] [PubMed] [Google Scholar]
- Henikoff, S. and Henikoff, J.G. 1992. Amino acid substitution matrices from protein blocks. Proc. Natl. Acad. Sci. 89 10915–10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille, B. 2001. Ion channels of excitable membranes. Sinauer, Sunderland, MA.
- Howorka, S. and Bayley, H. 1998. Improved protocol for high-throughput cysteine scanning mutagenesis. Biotechniques 25 766–772. [DOI] [PubMed] [Google Scholar]
- Howorka, S., Cheley, S., and Bayley, H. 2001a. Sequence-specific detection of individual DNA strands using engineered nanopores. Nat Biotechnol 19 636–639. [DOI] [PubMed] [Google Scholar]
- Howorka, S., Movileanu, L., Braha, O., and Bayley, H. 2001b. Kinetics of duplex formation for individual DNA strands within a single protein nanopore. Proc. Natl. Acad. Sci. 98 12996–13001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, D.H. 1995. PCR mutagenesis and recombination in vivo. In PCR primer: A laboratory manual (eds. C.W. Dieffenbach and G.S. Dveksler), pp. 591–601. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Korchev, Y.E., Alder, G.M., Bakhramov, A., Bashford, C.L., Joomun, B.S., Sviderskaya, E.V., Usherwood, P.N.R., and Pasternak, C.A. 1995. Staphylococcus aureus alpha-toxin-induced pores: Channel-like behavior in lipid bilayers and patch clamped cells. J. Membr. Biol. 143 143–151. [DOI] [PubMed] [Google Scholar]
- Krasilnikov, O.V., Merzlyak, P.G., Yuldasheva, L.N., Rodrigues, C.G., Bhakdi, S., and Valeva, A. 2000. Electrophysiological evidence for heptameric stoichiometry of ion channels formed by Staphylococcus aureus alpha-toxin in planar lipid bilayers. Mol. Microbiol. 37 1372–1378. [DOI] [PubMed] [Google Scholar]
- Kraulis, P.J. 1991. MOLSCRIPT: A program to produce both detailed and schematic plots of protein structure. J. Appl. Crystallogr. 24 946–949. [Google Scholar]
- Laemmli, U.K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227 680–685. [DOI] [PubMed] [Google Scholar]
- Lund, T., De Buyser, M.L., and Granum, P.E. 2000. A new cytotoxin from Bacillus cereus that may cause necrotic enteritis. Mol. Microbiol. 38 254–261. [DOI] [PubMed] [Google Scholar]
- Martín, A.C., López, R., and García, P. 1996. Analysis of the complete nucleotide sequence and functional organization of the genome of the Streptococcus pneumoniae bacteriophage Cp-1. J. Virol. 70 3678–3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menestrina, G., Dalla Serra, M., and Prévost, G. 2001. Mode of action of β barrel pore-forming toxins of the staphylococcal α-hemolysin family. Toxicon 39 1661–1672. [DOI] [PubMed] [Google Scholar]
- Miles, G., Cheley, S., Braha, O., and Bayley, H. 2001. The staphylococcal leukocidin bicomponent toxin forms large ionic channels. Biochemistry 40 8514–8522. [DOI] [PubMed] [Google Scholar]
- Miles, G., Movileanu, L., and Bayley, H. 2002. Subunit composition of a bicomponent toxin: Staphylococcal leukocidin forms an octameric transmembrane pore. Protein Sci. 11 894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata, S., Matsushita, O., Minami, J., Katayama, S., Shimamoto, S., and Okabe, A. 2001. Cleavage of a C-terminal peptide is essential for heptamerization of Clostridium perfringens ɛ-toxin in the synaptosomal membrane. J. Biol. Chem. 276 13778–13783. [DOI] [PubMed] [Google Scholar]
- Montal, M. and Mueller, P. 1972. Formation of bimolecular membranes from lipid monolayers and study of their electrical properties. Proc. Natl. Acad. Sci. 69 3561–3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movileanu, L., Cheley, S., Howorka, S., Braha, O., and Bayley, H. 2001. Location of a constriction in the lumen of a transmembrane pore by targeted covalent attachment of polymer molecules. J. Gen. Physiol. 117 239–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson, R., Nariya, H., Yokota, K., Kamio, Y., and Gouaux, E. 1999. Crystal structure of staphylococcal LukF delineates conformational changes accompanying formation of a transmembrane channel. Nat. Struct. Biol. 6 134–140. [DOI] [PubMed] [Google Scholar]
- Pédelacq, J.-D., Maveyraud, L., Prévost, G., Baba-Moussa, L., González, A., Courcelle, E., Shepard, W., Monteil, H., Samama, J.-P., and Mourey, L. 1999. The structure of Staphylococcus aureus leukocidin component (LukF-PV) reveals the fold of the water-soluble species of a family of transmembrane pore-forming toxins. Structure 7 277–288. [DOI] [PubMed] [Google Scholar]
- Sakash, J.B. and Kantrowitz, E.R. 2000. The contribution of individual interchain interactions to the stabilization of the T and R states of Escherichia coli aspartate transcarbamoylase. J. Biol. Chem. 275 28701–28707. [DOI] [PubMed] [Google Scholar]
- Schneider, F., Hammarström, P., and Kelly, J.W. 2001. Transthyretin slowly exchanges subunits under physiological conditions: A convenient chromatographic method to study subunit exchange in oligomeric proteins. Protein Sci. 10 1606–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatursky, O., Bayles, R., Rogers, M., Jost, B.H., Songer, J.G., and Tweten, R.K. 2000. Clostridium perfringens beta-toxin forms potential-dependent, cation-selective channels in lipid bilayers. Infect. Immun. 68 5546–5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, L., Hobaugh, M.R., Shustak, C., Cheley, S., Bayley, H., and Gouaux, J.E. 1996. Structure of staphylococcal α-hemolysin, a heptameric transmembrane pore. Science 274 1859–1865. [DOI] [PubMed] [Google Scholar]
- Steinthorsdottir, V., Halldorsson, H., and Andresson, O.S. 2000. Clostridium perfringens beta-toxin forms multimeric transmembrane pores in human endothelial cells. Microb. Pathog. 28 45–50. [DOI] [PubMed] [Google Scholar]
- Thompson, J.D., Higgins, D.G., and Gibson, T.J. 1994. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valeva, A., Weisser, A., Walker, B., Kehoe, M., Bayley, H., Bhakdi, S., and Palmer, M. 1996. Molecular architecture of a toxin pore: A 15-residue sequence lines the transmembrane channel of staphylococcal alpha-toxin. EMBO J. 15 1857–1864. [PMC free article] [PubMed] [Google Scholar]
- Walker, B.J., Krishnasastry, M., Zorn, L., and Bayley, H. 1992a. Assembly of the oligomeric membrane pore formed by staphylococcal α-hemolysin examined by truncation mutagenesis. J. Biol. Chem. 267 21782–21786. [PubMed] [Google Scholar]
- Walker, B.J., Krishnasastry, M., Zorn, L., Kasianowicz, J.J., and Bayley, H. 1992b. Functional expression of the α-hemolysin of Staphylococcus aureus in intact Escherichia coli and in cell lysates. J. Biol. Chem. 267 10902–10909. [PubMed] [Google Scholar]
- Welkos, S.L. 1991. Plasmid-associated virulence factors of non-toxigenic (pX01–) Bacillus anthracis. Microb. Pathog. 10 183–198. [DOI] [PubMed] [Google Scholar]
- Zemaitis, J.F., D.M. Clark, M. Rafal, and N. Scriver. 1986. Handbook of aqueous electrolyte thermodynamics: Theory and application. American Institute of Chemical Engineers, New York.
- Zitzer, A., Zitzer, O., Bhakdi, S., and Palmer, M. 1999. Oligomerization of Vibrio cholera cytolysin yields a pentameric pore and has a dual specificity for cholesterol and sphingolipids in the target membrane. J. Biol. Chem. 274 1375–1380. [DOI] [PubMed] [Google Scholar]