Abstract
The ability of several naturally occurring substances known as osmolytes to induce helix formation in an alanine-based peptide have been investigated. As predicted by the osmophobic effect hypothesis, the osmolytes studies here do induce helix formation. Trimethylamine-N-oxide (TMAO) is the best structure-inducing osmolytes investigated here, but it is not as effective in promoting helix formation as the common cosolvent trifluoroethanol (TFE). We also provide a semiquantitative study of the ability of TMAO to induce helix formation and urea, which acts as a helix (and protein) denaturant. We find that on a molar basis, these agents are exactly counteractive as structure inducing and unfolding agents. Finally, we extend the investigations to the effects of urea and TMAO on the stability of a dimeric coiled-coil peptide and find identical results. Together these results support the tenets of the osmophobic hypothesis and highlight the importance of the polypeptide backbone in protein folding and stability.
Keywords: TMAO, peptide stability, helix-coil transition, leucine zipper
Many organisms and cells accumulate small organic molecules, termed osmolytes, to counteract either external osmotic stresses such as dehydration, freezing, and high salinity or internal stresses such as high concentrations of urea. For example, to offset the potential deleterious effects of having high levels of intracellular urea, many organisms produce and accumulate large amounts of a trimethylamine N-oxide (TMAO), a representative of the general class of compounds known as protective osmolytes (Yancey and Somero 1979; Yancey et al. 1982). Such osmolytes have been shown in vitro to enhance the stability of many proteins without substantial changes in the function of the proteins.
It has been proposed that osmolytes have a property that forces proteins to fold, and this general solvophobic property has been termed the osmophobic effect (Bolen 2001; Bolen and Baskakov 2001). Based on an elegant series of experiments that measured the transfer free energy of amino acids and models for the polypeptide backbone, Bolen and associates have proposed that osmolytes exert their stabilizing effects on proteins chiefly because of an unfavorable interaction of osmolytes with the peptide backbone (Liu and Bolen 1995; Wang and Bolen 1997; Qu et al. 1998). The molecular mechanism underlying the osmophobic effect is grounded in the work of Timasheff and coworkers who showed that osmolytes are preferentially excluded from the immediate contact area around the protein molecule (Lee and Timasheff 1981; Arakawa et al. 1990; Timasheff 1993).
A hypothesis that emerges from this idea is that osmolytes should be able to induce structure in otherwise unfolded polypeptides. For many osmolytes, this has been shown to be true. Reduced and carboxyamidated ribonuclease A, which is unfolded in aqueous solution, becomes more compact when transferred to 1M solutions of a variety of osmolytes as measured by changes in the Stokes radius (Qu et al. 1998). Furthermore, TMAO has been shown to cooperatively refold reduced carboxyamidated ribonuclease T1 with a Cmid of ≈1.3 M TMAO (Baskakov and Bolen 1998) and the protein subunit of ribonuclease P with a Cmid of ≈1 M TMAO at 37°C (Henkels et al. 2001).
Here, we provide another test of the hypothesis that osmolytes act as structure-inducing agents by comparing their ability to induce helical structure in a model alanine-based peptide. This allows us to make a qualitative comparison between several osmolytes, salts, and the known helix-inducing solvent TFE. We also provide a semiquantitative comparison between TMAO as a structure-inducing agent and urea, a common protein, and peptide denaturant. We also extend the studies on urea and TMAO mixtures to a simple coiled-coil peptide system using a model derived from the leucine zipper region of GCN4.
Results and Discussion
Osmolyte effects on helix formation
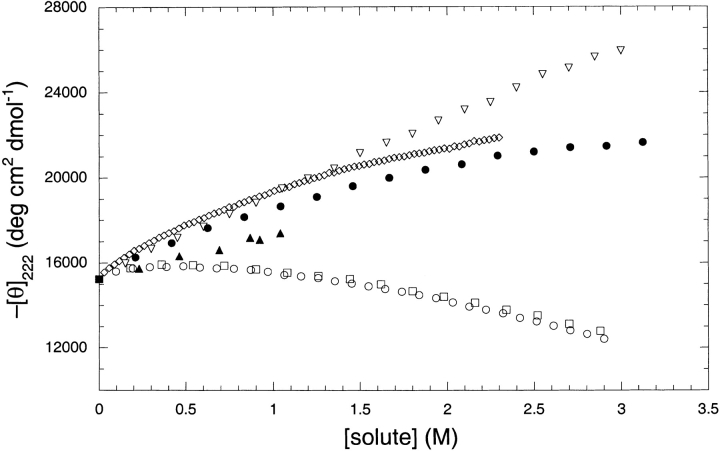
To compare the relative helix stabilizing effects of TFE, TMAO, potassium phosphate, NaCl, KCl, and sucrose, an alanine-based peptide was titrated with each compound at pH 7.0 and helical structure was monitored by circular dichroism at 222 nm, as shown in Figure 1 ▶. This peptide, which has been studied before (Scholtz et al. 1993), shows approximately 40% helical structure at pH 7.0 and 0°C in 10 mM potassium phosphate without any additives (Scholtz et al. 1993). The peptide, Ac-AAQAA-AEQAA-AAQAAY-NH2, was designed to be devoid of any stabilizing side-chain interactions, and the helical structure is due principally to the high helix-forming propensity of alanine (Chakrabartty et al. 1994; Chakrabartty and Baldwin 1995; Scholtz and Baldwin 1995), and thus helix formation is driven by the formation of hydrogen bond between backbone amides.
Fig. 1.
The effects of various solutes on the helix formation in the alanine-based peptide. The solutes are KCl (open circles), K/Pi (open diamonds), NaCl (open squares), sucrose (filled triangles), TFE (open triangles), and TMAO (filled circles). The CD signal at 222 nm was recorded at 0°C in a pH 7 in 10 mM phosphate buffer.
At low concentrations, potassium phosphate induces helix formation to the largest extent for any of the additives studied, but this effect diminishes at higher concentrations. As expected, TFE also proved very effective at inducing helix formation, becoming better than potassium phosphate above a concentration of 1 M. TMAO showed helix induction that is nearly identical to that of potassium phosphate. Sucrose titrations could only be performed to a concentration of 1 M for technical reasons related to sample viscosity. Although sucrose does induce helical structure, it is less effective than the compounds already discussed. Sodium and potassium chloride had virtually identical effects upon helix formation in the peptide, inducing structure to a concentration of 0.5 M followed by a decline and becoming destabilizing above 1 M concentration as noted before for a related peptide (Scholtz et al. 1991).
Because TMAO has a significant buffering capacity at high concentrations in aqueous solution, we determined if different buffer components alter the effects of TMAO as a helix-inducing osmolyte. Titrations were performed on the alanine-based peptide with TMAO solutions adjusted to pH 7.0 using hydrochloric, phosphoric, or sulfuric acids. In the concentration range of 0–2 M TMAO, the helix induction effects of each solution were identical, and thus TMAO, and not any other component of the solution, is causing the peptide to adopt the helical structure (data not shown).
Urea and TMAO effects on helix formation
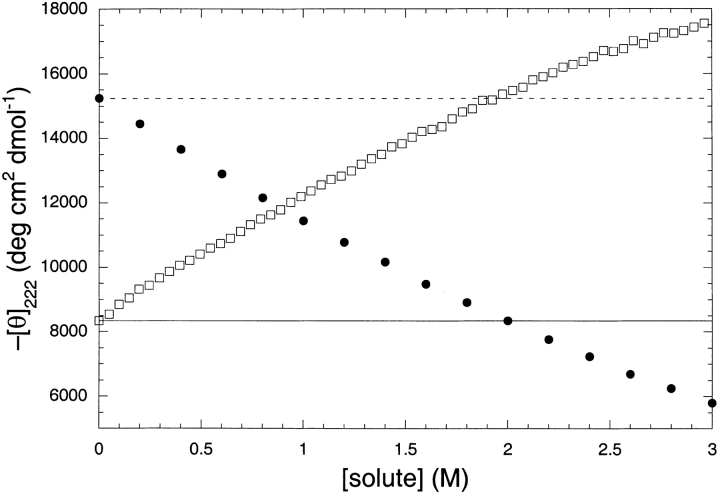
Urea is known to induce helix unfolding in related alanine-based peptides (Scholtz et al. 1995; Smith and Scholtz 1996). In Figure 2 ▶, we show that urea does unfold our peptide as well. The alanine-based peptide was also treated with TMAO at a constant urea concentration of 2 M. Figure 2 ▶ shows this curve compared with a urea titration of the same peptide. The two curves intersect at 0.92 M titrant, and 2 M urea is completely counteracted by 1.9 M TMAO. This suggests the full counteraction ratio of TMAO to urea is approximately 1:1 for the alanine-based peptide, and furthermore, that on a quantitative basis urea and TMAO are identical in their ability to unfold or refold the α-helix, respectively.
Fig. 2.
The additivity of TMAO and urea on helix formation in thealanine-based peptide. Shown in filled circles is the urea-induced helix unfolding curve. The open squares shows how TMAO induces helix formation in a solution that contains 2 M urea. The dotted line shows the value of the CD signal in the absence of any added solute, and the solid line shows the CD signal for the peptide in 2 M urea. The buffers also contained 10 mM K/Pi (pH 7), and the CD signal was recorded at a constant temperature of 0°C. The curves cross near 1 M additive, suggesting that urea and TMAO have equal and opposite efficiencies as helix unfolding and refolding agents.
Previously, we quantitated the ability of urea to unfold helical peptides (Scholtz et al. 1995; Smith and Scholtz 1996). By measuring the urea-dependence to helix formation in a series of alanine-based peptides, and fitting the data with either the Zimm-Bragg (Zimm and Bragg 1959) or Lifson-Roig (Lifson and Roig 1961) models for the helix to random coil transition, we were able to determine the m-value for the urea-induced unfolding of the peptide helix. This m-value was found to be 23 ± 1 cal mol−1 M−1 per residue for helix propagation. Here, we find that TMAO and urea are fully counteractive at very near to a 1:1 ratio, implying that the absolute values for the m-values for TMAO and urea are identical, with urea acting as a denaturant and TMAO as a renaturant of the helical peptide.
TMAO and urea effects on coiled-coil stability
We also investigated the combined effects of urea and TMAO on the stability of a dimeric coiled-coil peptide derived from the leucine zipper domain of GCN4. The peptide, called MIINN (see Materials and Methods for sequence and nomenclature), has been thoroughly characterized in a previous study (Zhu et al. 2000). Shown in Figure 3 ▶ are the results of thermal and solvent denaturation curves for MIINN. The analysis of the urea denaturation studies in the presence and absence of 1 M TMAO show that the Cmid for urea increases by 1 M in the presence of 1 M TMAO, suggesting that 1 M TMAO completely counteracts 1 M urea (Fig. 3A ▶). To further examine the additivity of TMAO and urea on the stability of the coiled-coil peptide, thermal denaturations were performed on MIINN (Fig. 3B ▶). The effects of urea and TMAO on a molar basis were found to be equal, opposite, and completely additive. Urea (1 M) was completely counteracted by 1 M TMAO, yielding a Tm equal to that of the peptide in the simple buffer. Furthermore, the Tm of the peptide in 1 M TMAO + 2 M urea was identical to that found in 1 M urea.
Fig. 3.
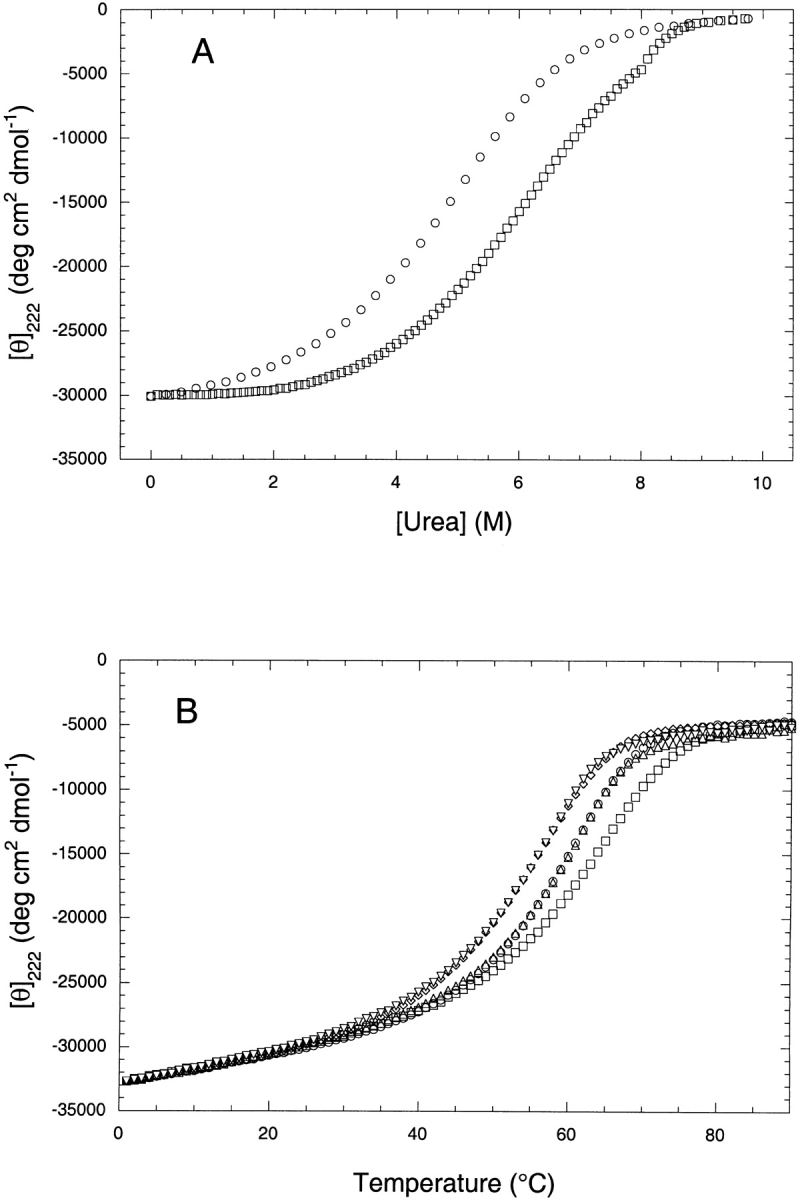
The effects of urea and TMAO on the stability of the coiled-coil peptide MIINN. (A) Urea denaturation curves in 1 M TMAO (open squares) and in buffer only (open circles). The peptide concentration was 10 μM in a 10 mM K/Pi buffer at pH 7, and the temperature was 25°C. TMAO (1 M) increases the Cmid for the urea denaturation curve by 1.0 M. (B) Five thermal unfolding curves for the MIINN peptide at pH 7 in 10 mM K/Pi buffer alone (open circles), in 1 M TMAO (open squares), 1 M urea (open diamonds), 1 M TMAO + 1 M urea (open up triangles), or 1 M TMAO + 2 M urea (open down triangles).
Concluding remarks
We have compared the ability of several different osmolytes to induce helical structure in a model alanine-based peptide. As predicted by the osmophobic effect hypothesis, the osmolytes do, in fact, induce helix formation. TMAO is the best of the osmolytes investigated here, but it is not as effective in inducing helix formation as the common helix-inducing cosolvent TFE. Furthermore, we provide a semiquantitative study of the differences between TAMO to induce structure and urea, a common protein denaturant. For helix formation in this peptide, we find that on a molar basis, TMAO is just as effective as a helix stabilizing agent as urea is as a helix denaturant. When we extended the studies to the dimeric coiled-coil model system, we found identical results. Urea and TMAO completely counteract each other, on a molar basis, and the effects on the stability of the coiled-coil peptide are equal, opposite, and completely additive. These simple experiments support the general hypothesis put forward about the nature of osmophobic effect and specifically address the special role of the polypeptide backbone in protein folding and stability.
Materials and methods
Trimethylamine-N-oxide (TMAO) and sucrose were obtained from Fluka. Sodium chloride, potassium chloride, trifluoroethanol, and all buffers were obtained from Sigma, and the urea was obtained from Nacalai Tesque. All chemicals, with the exception of TMAO, were used without further purification. TMAO was purified by dissolving it in ddH2O and stirring in mixed bed ion exchange resin to remove decomposition products and impurities. The solution was then filtered to remove the resin and the pH was adjusted with concentrated phosphoric acid, HCl or H2SO4. The concentration of TMAO was then determined by refractive index measurements as previously described (Wang and Bolen 1997).
The monomeric peptide used in this study, Ac-AAQAA-AEQAA-AAQAAY-NH2, was synthesized with solid-phase Fmoc chemistry methods on an Applied Biosystems 431A Peptide Synthesizer using rink resin and amino acids from Advanced Chemtech. The peptide was cleaved from the resin in 95% trifluoroacetic acid 5% anisole, both obtained from Aldrich for 30 min, followed by precipitation in tert-butyl methyl ether. The precipitate was dissolved in water, lyophilized, and purified by reverse phase FPLC on a Resource RPC column with acidic water–acetonitrile gradients. The identity of the peptide was confirmed by mass spectroscopy (MALDI-TOF).
The variant of the leucine zipper peptide from GCN4 was prepared as described previously (Zhu et al. 2000). The peptide has the sequence:
STH-MKQLEDK-IEELLSK-IYHLENE-NARLKKL-NGER and is named MIINN for the identity of the five a positions in the heptad repeat of the coiled coil (Zhu et al. 2000).
Concentrated osmolyte stock solutions were prepared daily for all solutes and diluted with buffer to the appropriate concentration for each experiment. Urea was dissolved in the appropriate buffer and the pH adjusted to 7.0, and the urea concentration was determined by refractive index measurements (Pace 1986; Pace and Scholtz 1997). The TMAO concentration was determined by refractive index measurements according to the procedure described by Wang and Bolen (1997). All other osmolyte solutions were prepared using quantitative methods by dissolving a known mass of solute in buffer using a volumetric flask.
All circular dichroism experiments were performed on an Aviv Circular Dichroism Spectrometer Model 62DS and monitored at 222 nm. With the exception of the sucrose experiments, all titrations were performed by a Hamilton Microlab 500 series automatic titrator controlled by IGOR Pro software. The sucrose titration was accomplished by preparing individual samples at the desired concentration of sucrose.
Acknowledgments
We thank Nick Pace and members of the Pace and Scholtz laboratories for helpful discussions. This work was supported by grants from the National Institutes of Health (GM-52483) and the Robert A. Welch Foundation (BE-1281). J.M.S. is an Established Investigator of the American Heart Association.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked "advertisement" in accordance with 18 USC section 1734 solely to indicate this fact.
Abbreviations
TMAO, trimethylamine N-oxide
TFE, 1,1,1-trifluoroethanol
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1110/ps.0211702.
References
- Arakawa, T., Bhat, R., and Timasheff, S.N. 1990. Why preferential hydration does not always stabilize the native structure of globular proteins. Biochemistry 29 1924–1931. [DOI] [PubMed] [Google Scholar]
- Baskakov, I. and Bolen, D.W. 1998. Forcing thermodynamically unfolded proteins to fold. J. Biol. Chem. 273 4831–4834. [DOI] [PubMed] [Google Scholar]
- Bolen, D.W. 2001. Protein stabilization by naturally occurring osmolytes. Methods Mol. Biol. 168 17–36. [DOI] [PubMed] [Google Scholar]
- Bolen, D.W. and Baskakov, I.V. 2001. The osmophobic effect: Natural selection of a thermodynamic force in protein folding. J. Mol. Biol. 310 955–963. [DOI] [PubMed] [Google Scholar]
- Chakrabartty, A. and Baldwin, R.L. 1995. Stability of α-helices. Adv. Protein Chem. 46 141–176. [PubMed] [Google Scholar]
- Chakrabartty, A., Kortemme, T., and Baldwin, R.L. 1994. Helix propensities of the amino acids measured in alanine-based peptides without helix-stabilizing side-chain interactions. Protein Sci. 3 843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkels, C.H., Kurz, J.C., Fierke, C.A., and Oas, T.G. 2001. Linked folding and anion binding of the Bacillus subtilis ribonuclease P protein. Biochemistry 40 2777–2789. [DOI] [PubMed] [Google Scholar]
- Lee, J.C. and Timasheff, S.N. 1981. The stabilization of proteins by sucrose. J. Biol. Chem. 256 7193–7201. [PubMed] [Google Scholar]
- Lifson, S. and Roig, A. 1961. On the theory of helix-coil transitions in biopolymers. J. Chem. Phys. 34 1963–1974. [Google Scholar]
- Liu, Y. and Bolen, D.W. 1995. The peptide backbone plays a dominant role in protein stabilization by naturally occurring osmolytes. Biochemistry 34 12884–12891. [DOI] [PubMed] [Google Scholar]
- Pace, C.N. 1986. Determination and analysis of urea and guanidine hydrochloride denaturation curves. Methods Enzymol. 131 266–280. [DOI] [PubMed] [Google Scholar]
- Pace, C.N. and Scholtz, J.M. 1997. Measuring the conformational stability of a protein. In Protein structure: A practical approach, 2 ed. (ed. T.E. Creighton), pp. 299–321. IRL Press, Oxford.
- Qu, Y., Bolen, C.L., and Bolen, D.W. 1998. Osmolyte-driven contraction of a random coil protein. Proc. Natl. Acad. Sci. 95 9268–9273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholtz, J.M. and Baldwin, R.L. 1995. α-Helix formation by peptides in water. In Peptides: Synthesis, structures, and applications (ed. B. Gutte), pp. 171–192. Academic Press, San Diego.
- Scholtz, J.M., Barrick, D., York, E.J., Stewart, J.M., and Baldwin, R.L. 1995. Urea unfolding of peptide helices as a model for interpreting protein unfolding. Proc. Natl. Acad. Sci. 92 185–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholtz, J.M., Qian, H., Robbins, V.H., and Baldwin, R.L. 1993. The energetics of ion-pair and hydrogen-bonding interactions in a helical peptide. Biochemistry 32 9668–9676. [DOI] [PubMed] [Google Scholar]
- Scholtz, J.M., York, E.J., Stewart, J.M., and Baldwin, R.L. 1991. A neutral, water-soluble α-helical peptide: The effect of ionic strength on the helix-coil equilibrium. J. Am. Chem. Soc. 113 5102–5104. [Google Scholar]
- Smith, J.S. and Scholtz, J.M. 1996. Guanidine hydrochloride unfolding of peptide helices: Separation of denaturant and salt effects. Biochemistry 35 7292–7297. [DOI] [PubMed] [Google Scholar]
- Timasheff, S.N. 1993. The control of protein stability and association by weak interactions with water: How do solvents affect these processes? Annu. Rev. Biophys. Biomol. Struct. 22 67–97. [DOI] [PubMed] [Google Scholar]
- Wang, A. and Bolen, D.W. 1997. A naturally occurring protective system in urea-rich cells: Mechanism of osmolyte protection of proteins against urea denaturation. Biochemistry 36 9101–9108. [DOI] [PubMed] [Google Scholar]
- Yancey, P.H. and Somero, G.N. 1979. Counteraction of urea destabilization of protein structure by methylamine osmoregulatory compounds of elasmobranch fishes. Biochem. J. 183 317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey, P.H., Clark, M.E., Hand, S.C., Bowlus, R.D., and Somero, G.N. 1982. Living with water stress: Evolution of osmolyte systems. Science 217 1214–1222. [DOI] [PubMed] [Google Scholar]
- Zhu, H., Celinski, S., Scholtz, J.M., and Hu, J.C. 2000. The contribution of buried polar groups to the conformational stability of the GCN4 coiled-coil. J. Mol. Biol. 300 1379–1389. [DOI] [PubMed] [Google Scholar]
- Zimm, B.H. and Bragg, J.K. 1959. Theory of the phase transition between helix and random coil in polypeptidechains. J. Chem. Phys. 31 526–535. [Google Scholar]