Abstract
The activity of protein tyrosine phosphatases (PTPs) is restricted by their substrate specificities. The analysis of PTP specificity was greatly helped by the discovery that "substrate-trapping" PTP mutants, such as PTP-1B D181A, stably and specifically bind their substrates. We have set up a PTP substrate specificity assay based on the SPOT technique, which involves the microsynthesis of (phospho)peptides on membranes. To validate this approach, substrate trapping PTP-1B was tested on its cognate ligand, the autophosphorylated insulin receptor (IR). On SPOT membranes, IR peptides with phosphotyrosine 1163 were efficiently bound by PTP1B D181A, and dephosphorylated by PTP-1B. Phosphotyrosine 1163 was preferred over the neighboring 1158 and 1162 phosphotyrosines. PTP-1B also recognized IR-like motifs in Trk autophosphorylation domains, and STAT 5 phosphopeptides. Using a gridded 20-by-20 SPOT library, we show that peptides with the YZM motif (Z: phosphotyrosine) are the strongest ligands for PTP-1B D181A, but not the optimal substrates for dephosphorylation by wild-type PTP1B. In addition we show that PTP-1B and PTP-β dephosphorylation efficiency is strongly modulated by the introduction of phospho-serine or phospho-threonine in their cognate phospho-tyrosine substrates. Altogether our data illustrate that the SPOT technique is a highly efficient tool for the study of PTP substrate specificity.
Keywords: PTP, protein-tyrosine phophatase, SPOT, protein-protein interactions
Many cellular responses involve phosphorylation and dephosphorylation steps. The human genome probably encodes around 100 protein tyrosine phosphatases (PTPs) and dual specific phosphatases (DSPs; Lander et al. 2001; Venter et al. 2001). PTPs play important and active roles in the modulation of signaling pathways and have emerged as useful drug targets (Espanel et al. 2001). For example, PTP-1B inactivates insulin signaling by removing docking sites on the insulin receptor (IR) and disrupts STAT 5B dimers (Elchebly et al. 1999; Aoki and Matsuda 2000; Klaman et al. 2000), whereas PTP-α activates Src kinase by dephosphorylation of its autoinhibitory phosphotyrosine (aa 527) involved in the intramolecular recognition by the Src SH2 domain (Zheng et al. 1992).
An understanding of PTP function requires the identification of substrates for these enzymes. "Substrate-trapping" PTP mutants, which retain substrate specificity but remain associated with their substrates, have proven to be useful tools for the study of PTP specificity (Taddei et al. 1994; Garton et al. 1996; Flint et al. 1997; Pasquali et al. 2000; Wälchli et al. 2000). To identify substrates, substrate-trapping mutants have mainly been used in pull-down or overexpression experiments (Fachinger et al. 1999; Zhang et al. 1999). In addition, synthetic phosphopeptide libraries have been used to study PTP substrate specificity (Zhang et al. 1993; Pellegrini et al. 1998; Vetter et al. 2000). An example of the latter is an "alanine reverse scanning" analysis with 153 different peptides harboring the scaffold AAAApYAAAA sequence, where a single alanine per peptide was sequentially replaced by one of the other 19 natural amino acids (Vetter et al. 2000).
In this report, we show that the direct microsynthesis of phosphopeptides on membranes, also called SPOT, is a good alternative for testing PTPs on large peptide libraries. The SPOT technique was first developed for epitope mapping, but it is suitable to assay almost any linear protein-protein interaction, as we and others have shown (Kramer et al. 1993; Martens et al. 1995; Frank and Overwin 1996; Espanel and Sudol 2001).
To validate the SPOT technique for PTP substrate-trapping mutants, we tested PTP-1B D181A on peptides that contain all possible phosphorylation states of the three autophosphorylation sites of the IR (TRDIYETDYYRKGGKGL), a well established substrate for PTP1B (Ebina et al. 1985). We confirmed the binding results by direct dephosphorylation of phosphopeptides on SPOT by wild-type PTP-1B. Using a partially degenerate SPOT library, we identified the optimal PTP-1B recognition sequence, and the most efficient dephosphorylation motif. An analysis of the IR autophosphorylation sequence revealed that similar motifs are present in other proteins such as members of the IR and Trk families, and STAT 5A and 5B. We show here that all of these phosphopeptides can interact with PTP-1B D181A. Lastly, we use SPOT membranes to show that the additional phosphorylation of serine or threonine residues in the vicinity of the phosphotyrosine results in a dramatic change in the PTP’s catalytic efficiency.
Results
On SPOT membranes, PTP-1B preferentially binds and dephosphorylates P-Tyr1163 of the insulin receptor
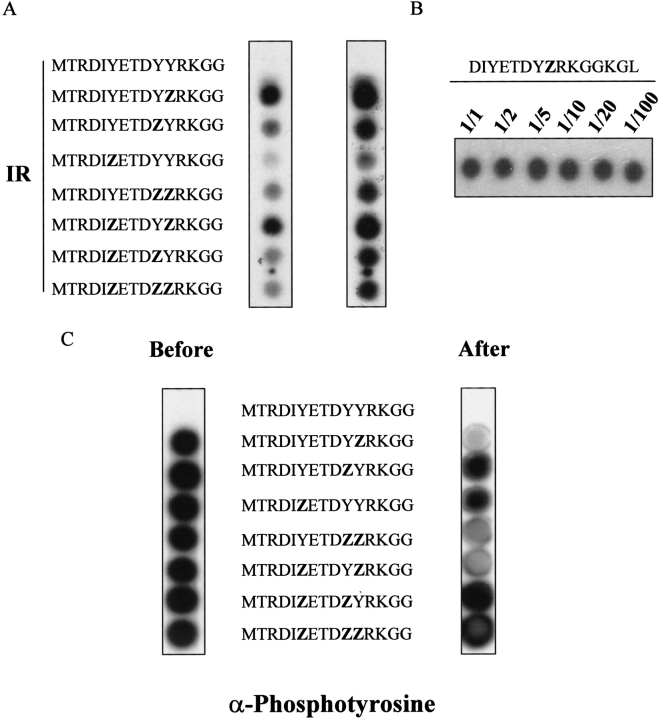
To validate the binding of substrate-trapping PTPs on SPOT membranes, we reproduced the interaction of PTP-1B with the major autophosphorylation site of the insulin receptor. We synthesized 15-mer peptides corresponding to the IR autophosphorylation sequence, with all possible combinations of phosphorylation states for the three tyrosines in this sequence. It is known that all three of these tyrosines are autophosphorylated by the IR kinase (Ebina et al. 1985). As shown in Figure 1A ▶, substrate-trapping GST-PTP-1B D181A bound most strongly to P-Tyr1163 (the C-terminal tyrosine)-containing peptides. We controlled whether the binding intensity differences in Figure 1A ▶ could correlate with variations in peptide synthesis. To do so, the concentration of the Fmoc-phosphotyrosine precursor used for the introduction of P-Tyr1163 was diluted during the synthesis. As shown in Figure 1B ▶, variation in reagent concentration, down to using a 100-fold precursor dilution, did not result in binding intensity modifications, suggesting that the amount of synthesized peptide is limited by the number of membrane anchor sites only.
Fig. 1.
Validation of the SPOT technique for PTP studies. (A) PTP-1B D181A preferentially binds phosphotyrosine 1163. Fifteen-mer peptides of the human IR were directly synthesized on membrane (SPOT technique) with no, one, two, or three phosphotyrosines (Z). The IR membrane was probed with radiolabeled PTP-1B D181A. Left and right panels correspond to short and long exposures, respectively. (B) Variation of SPOT synthesis does not affect binding. The phosphotyrosine 1163 (Z) precursor was diluted up to 100 times during the synthesis, and the membrane was probed, as in Fig. 1A ▶, with radiolabeled PTP-1B D181A. (C) Wild-type PTP-1B most efficiently dephosphorylates phosphopeptides with phosphotyrosine 1163. The two same IR membranes were generated and incubated with (right panel) or without (left panel) PTP-1B wild type. The amount of phosphopeptide was visualized using a mixture of antiphosphotyrosine antibodies (see Materials and Methods).
To test whether peptide dephosphorylation by wild-type PTP-1B would display the same pattern as the binding of substrate-trapping PTP, we incubated the IR SPOT membrane with GST-PTP-1B for 30 min. The residual phosphopeptide was then visualized using an antiphosphotyrosine Western blot analysis (Fig. 1C ▶). Because we found that individual antiphosphotyrosine antisera showed context variability, we used a mixture of commercial antisera (see Materials and Methods). As a control we also probed a second IR SPOT membrane which had not been incubated with wild-type PTP-1B. As expected, dephosphorylation correlated with binding, because peptides containing P-Tyr1163 were the most efficiently dephosphorylated. Interestingly the peptide harboring the double P-Tyr1162 and P-Tyr1163, which was not the best binder (Fig. 1A ▶), was nearly as efficiently dephosphorylated.
Taken together, these findings indicate that the substrate-trapping SPOT technique is suitable for this type of PTP binding/specificity study.
PTP-1B interacts on SPOT membranes with other substrates
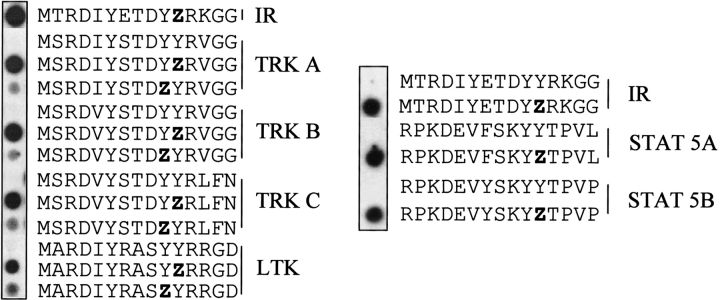
A sequence comparison of the IR autophosphorylation site with other proteins revealed identical or similar motifs in other tyrosine kinases, such as the IR and Trk families (Table 1). This alignment shows an RD(V/I)YxTDYYR consensus motif. Peptides derived from these receptors were tested for binding to substrate-trapping PTP-1B (Fig. 2 ▶). Because of their similarity with this consensus sequence, we included peptides from STAT 5A and STAT 5B, which have also been described as substrates of PTP-1B (Aoki and Matsuda 2000). All of these related peptides were recognized by PTP-1B D181A with almost the same affinity as that of the IR, suggesting that these phosphotyrosines may be dephosphorylated by PTP-1B. Once again, the C-terminal (third) tyrosine in these related motifs is the one highly preferred by PTP-1B D181A (Fig. 2 ▶). While this work was in progress, Tyk2 and Jak2, with EYY(R/K) motifs, were also identified as PTP1B substrates (Myers et al. 2001).
Table 1.
Partial listing of human proteins containing the RDxYxTDYYR motif
| IR | DFGMTRDIYETDYYR ––––––––––––– KGGKGLLPVRWMAPESLKD |
| IGFR–1 | DFGMTRDIYETDYYR ––––––––––––– KGGKGLLPVRWMSPESLKD |
| IRR | DFGMTRDVYETDYYR ––––––––––––– KGGKGLLPVRWMAPESLKD |
| TRK–A | DFGMSRDIYSTDYYR ––––––––––––– VGGRTMLPIRWMPPESILY |
| TRK–B | DFGMSRDVYSTDYYR ––––––––––––– VGGHTMLPIRWMPPESIMY |
| TRK–C | DFGMSRDVYSTDYYRLFNPSGNDFCIWCEVGGHTMLPIRWMPPESIMY |
| LTK | DFGMARDIYRASYYR ––––––––––––– RGDRALLPVKWMPPEAFLE |
| CONSENSUS | DFGMxRDIYxTDYYR ––––––––––––– KGGxxxLPVRWMxPESxxx |
| STAT5A | DRPK–DEVFS–KYYTPV –––––––––––––––– LAKAVDGYVKPQIK |
| STAT5B | DRPK–DEVYS–KYYTPV ––––––––––– PCESATAKAVDGYVKPQIK |
Bold letters correspond to conserved amino acids. Phosphotyrosines of the autophosphorylation sites are underlined.
Fig. 2.
PTP-1B D181A interacted with phosphopeptides from other receptors on SPOT. IR, Trk A, Trk B, Trk C, Ltk, STAT 5A, and STAT 5B peptides were synthesized on SPOT membranes and probed with radiolabeled PTP-1B D181A. Z corresponds to phosphotyrosine residues.
PTP-1B D181A has a high affinity for the (F/Y)ZM motif
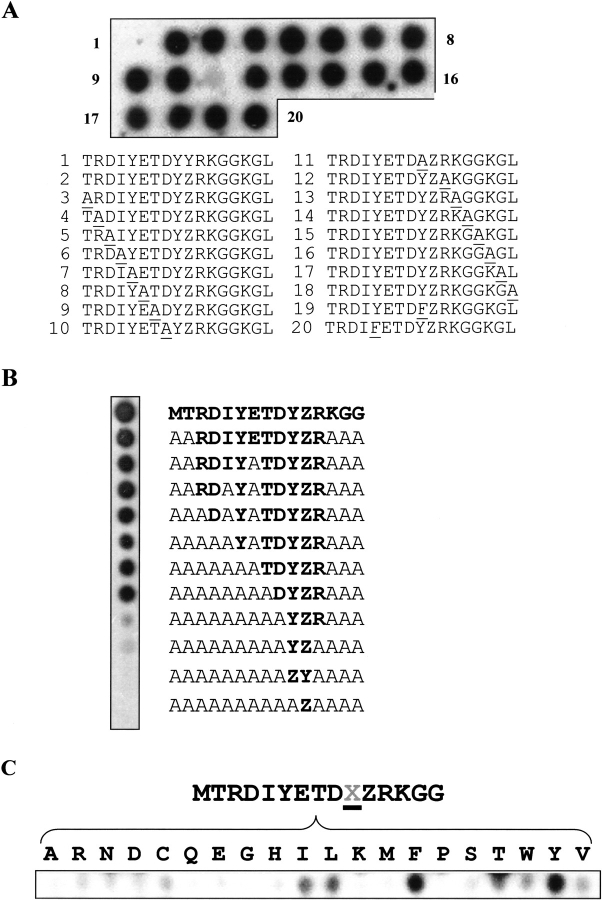
To better understand the interaction between PTP-1B and IR, we performed, by SPOT, an alanine scan on the IR autophosphorylation site. As shown in Figure 3A ▶, the PTP-1B substrate-trapping binding is greatly reduced whenever the essential YZ motif is affected. By making progressive Ala substitutions in the IR peptide we found that the core motif corresponds to the DYZR sequence (Fig. 3B ▶), suggesting that none of the other amino acids in the consensus sequence is essential for an efficient binding of P-Tyr1163. However, a slight decrease in binding affinity was observed between the full IR peptide and peptides in which the DYZR motif was flanked by alanines only. Therefore amino acids outside the core DYZR motif in the RD(V/I)YxTDYZR consensus sequence may contribute to binding and dephosphorylation efficiency.
Fig. 3.
PTP-1B D181A requires the (Y/F)Z motif to strongly interact with IR. SPOT membranes probed with radiolabeled PTP-1B D181A. (A) Alanine scan of the IR autophosphorylation site. (B) Progressive alanine substitution in the IR peptide probed with PTP-1B D181A. (C) The IR peptide was synthesized with the 20 amino acids at the position −1 (X).
To confirm the importance of the YZ dipeptide, we replaced Tyr1162 with the 19 other amino acids within the context of the IR. As shown in Figure 3C ▶, phenylalanine was the only substitution for tyrosine that still allowed strong binding. Weaker binding was seen for the other "bulky" hydrophobic amino acids isoleucine and leucine, but no binding was seen for alanine.
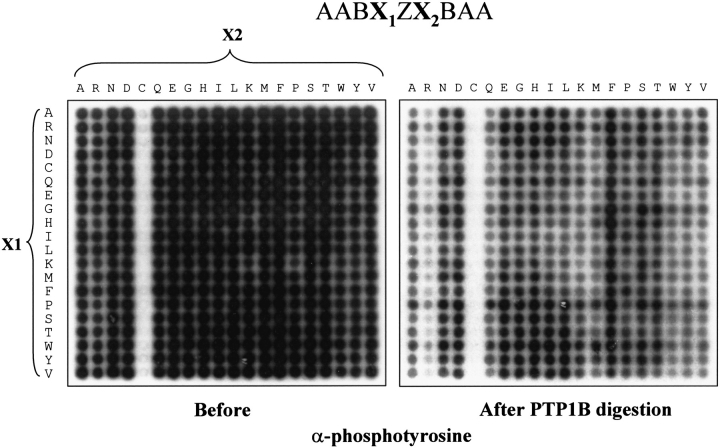
To investigate whether the YZ or FZ motifs are also the best ligands in a context different from the IR, we screened a degenerate SPOT library (Kramer et al. 1993) with PTP-1B D181A. Using a SPOT robot (Intavis), we made the library AABX1ZX2BAAA. B represents a complete mixture of all 20 common amino acids, whereas X1 and X2 both represent each of the 20 amino acids, for a total of 400 different peptides. As shown in Figure 4 ▶, PTP-1B D181A had the best binding affinity for peptides with a Y (tyrosine) or F (phenylalanine) present at −1 with respect to the phosphotyrosine. Interestingly, the YZM motif, with a methionine at +1, was the optimal combination for binding; in this setting, the YZ(R/K) motifs only bound PTP1B weakly.
Fig. 4.
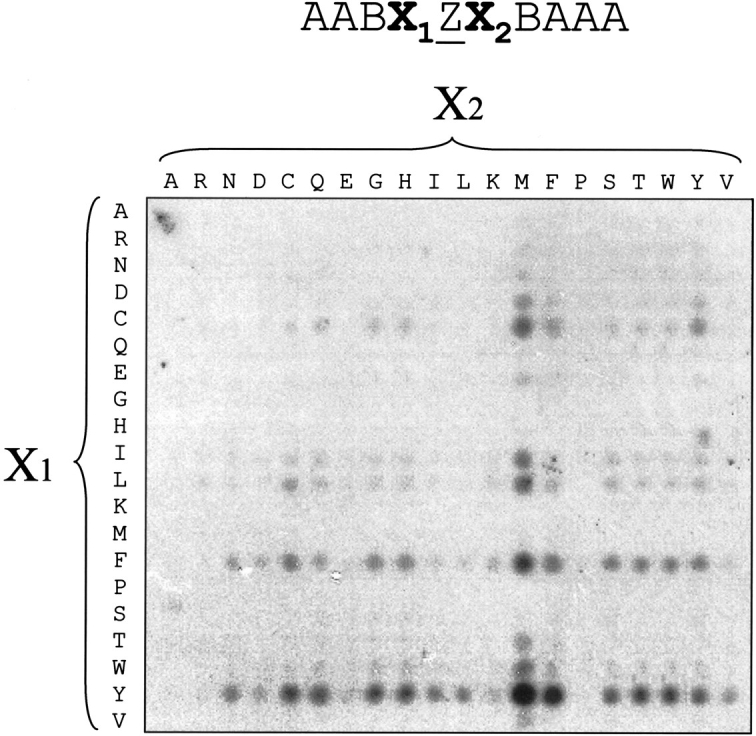
The YZM motif is the best binder for PTP-1B D181A on a SPOT library. An AABX1ZX2BAAA SPOT library was synthesized and probed with radiolabeled PTP-1B D181A. ‘B’ stands for the 20-aa mixture.
Dephosphorylation by PTP-1B of the peptide library
After stripping off the trapping mutant PTP, the SPOT membrane library was first reprobed with a mix of antiphosphotyrosine antibodies to control the quality of the membrane. Surprisingly, a cysteine at position +1 greatly decreased antiphosphotyrosine antibody binding (Fig. 5 ▶, left panel), despite the use of a mixture of antibodies. Since the same lane of peptides in Figure 4 ▶ had bound PTP-1B D181A, we conclude that this empty lane in Figure 5 ▶ is a shortcoming of the antibodies, rather than a lane where peptide synthesis had failed. The same membrane was again stripped, incubated with wild-type PTP-1B enzyme, and reprobed with the antiphosphotyrosine antibody mix. As shown in Figure 5 ▶ (right panel), the best ligand, the YZM peptide, was not the best substrate in this assay, because an arginine at position +1 produced the most effective dephosphorylation.
Fig. 5.
An arginine at position +1 enhanced the PTP-1B dephosphorylation. The membrane shown in Fig. 4 ▶ was probed with an antiphosphotyrosine antibody mixture, as control (left panel). After stripping, the same blot was incubated with PTP-1B wild type. The remaining phosphotyrosine presence was again visualized by ECL.
Potential substrate affinity modification by serine and/or threonine phosphorylations
Because serines and/or threonines are very often present near phosphorylated tyrosines (Table 1), we decided to study the effects of serine/threonine phosphorylation on PTP activity. We addressed this question for the STAT 5A peptide. This peptide has a potential threonine phosphorylation site (YTP) for proline-dependent protein kinases, as predicted by the NetPhos Prediction Server (Blom et al. 1999), that immediately follows the described phosphotyrosine. As shown in Figure 6A ▶, threonine phosphorylation in the STAT 5A peptide enhanced P-Tyr dephosphorylation by PTP-1B (right panel). To determine whether this property was specific for PTP-1B, we also analyzed another phosphatase for which a substrate has been proposed, namely PTP-β and the Tie-2 receptor (Fachinger et al. 1999). Figure 6B ▶ shows that a phosphoserine or a phosphothreonine in the Tie2 autophosphorylation site also enhanced PTP-β-mediated dephosphorylation of Tie-2 receptor peptides. These results suggest that tyrosine dephosphorylation may be regulated by surrounding serine/threonine phosphorylation events.
Fig. 6.
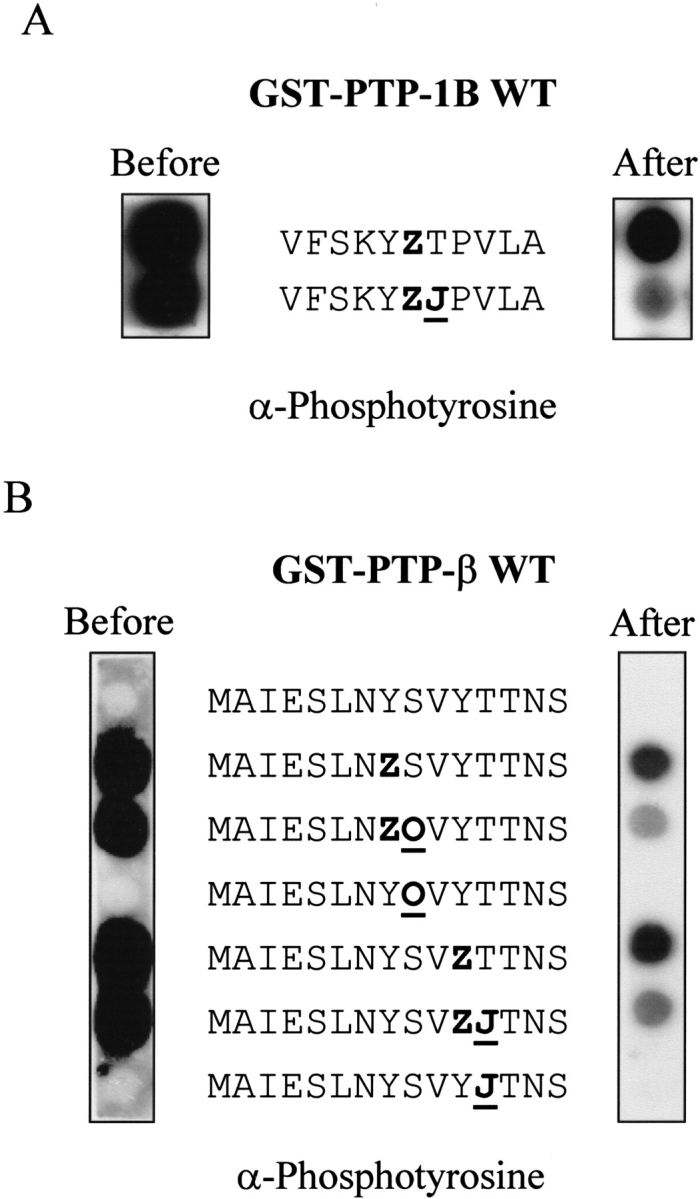
Phosphoserine or phosphothreonine at +1 enhanced dephosphorylation by PTP-1B and PTP-β. (A) Human STAT 5A peptides were synthesized on two SPOT membranes with and without phosphothreonine (J). Only the right panel membrane was incubated with wild-type PTP-1B. Phosphotyrosines were revealed by ECL using the 4G10 antiphosphotyrosine antibody. (B) Human Tie-2 peptides were synthesized with phosphothreonine (J) and phosphoserine (O) on SPOT and incubated with or without PTP-β.
Discussion
Using PTP-1B/IR and PTP-β/Tie2 receptor phosphopeptides as models, we found that the SPOT technique is a powerful tool for the detailed study of PTP binding and dephosphorylation specificities. Although we reproduced binding by a PTP1B trapping mutant, we found different optimal sequences when testing peptide libraries. Our data indicate that (Y/F)ZM-containing peptides are the best binders for trapping mutant PTP1B, whereas an optimal dephosphorylation motif is obtained with an arginine at position +1. It is possible that too strong an interaction may reduce the overall dephosphorylation rate. The DYZR motif within the IR is a bona fide substrate of PTP-1B, because it is a good binder due to the double tyrosines and a good substrate with the presence of an arginine at +1. The ability of PTP-1B to bind DYZR motifs from other tyrosine kinases strongly suggests a common mechanism of dephosphorylation mediated by PTP-1B or related proteins, such as TC-PTP, its closest homolog. We conclude that the PTP primary sequence directs a specific substrate preference; consistent with this, substrate-trapping TC-PTP D182A showed on IR SPOT membranes binding preferences similar to those of PTP-1B D181A, whereas PTP-Sap1 binds to a SPOT library motif that differs both at −1 and +1 positions (data not shown).
Thus far, there is no firm evidence that STAT 5 is phosphorylated at the YY motif, but (1) this sequence shows similarity with the IR autophosphorylation site, (2) direct phosphorylation of STAT 5B by IR has been demonstrated (Chen et al. 1997; Storz et al. 1999), and (3) the STAT 5 phosphopeptide interacted with PTP-1B (Fig. 2 ▶; Aoki and Matsuda 2000), suggesting that these tyrosines may be phosphorylated. Interestingly, another STAT 5 peptide that contains the phosphotyrosine 649 (STAT 5A) or 699 (5B) responsible for STAT 5 activation by IL-2 (Lin et al. 1996) did not bind PTP-1B D181A (data not shown).
Interestingly, the STAT 5 peptide AKAVDGZVKPQIK, with phosphotyrosines 694 and 699 (in 5A and 5B, respectively), which are responsible for STAT 5 activation by IL-2, did not bind PTP-1B D181A (data not shown). These STAT 5A and 5B motifs have not an arginine at +1 but a threonine, which probably makes them poor PTP-1B substrates. However, phosphorylation of this threonine could convert these peptides into good PTP-1B substrates (Fig. 6A ▶). The same result was obtained with Tie-2 and PTP-β, suggesting that tyrosine dephosphorylation can be regulated by additional phosphorylation events.
Our binding studies confirm earlier peptide library-based approaches, where wild-type PTP-1B preferably bound and dephosphorylated peptides carrying the E(Y/F/D)ZM motif (Huyer et al. 1998; Pellegrini et al. 1998; Vetter et al. 2000). TC-PTP, which is closely related to PTP-1B, also strongly interacted with the (E/D/Y)Z motif within the EGF receptor peptide (Asante-Appiah et al. 2001). However, another study found that PTP-1B dephosphorylated the ZZ sequence more efficiently than YZ or ZY motifs in IR peptides (Salmeen et al. 2000). In our dephosphorylation assays the ZZ sequence is also very well dephosphorylated, especially when taking into account that two phosphates need to be removed to reduce antibody reactivity. Therefore ZZ dephosphorylation may be as efficient as for the YZ motif. The cited study (Salmeen et al. 2000) did not look at direct interaction, but performed competition assays and showed that the IR ZZ motif was more efficient than ZY or YZ sequences in displacing an EGF peptide bound to PTP-1B D181A. Crystallographic studies with PTP-1B C215A also suggested a pocket for the binding of phosphotyrosine 1163 (Salmeen et al. 2000). This observation is supported by the finding that peptidomimetics that carry two phosphotyrosines are potent PTP-1B inhibitors (Jia et al. 2001). Besides the difference between the substrate trapping mutants, this discrepancy with our results could be explained by the fact that buffer conditions, especially pH conditions, were not the same. Dephosphorylation and competitive assays in one of the cited studies (Salmeen et al. 2000) were performed at pH 6.5 and pH 7 respectively, whereas all assays in the present study were performed at pH 7.45. This difference may be relevant, since pH variations greatly affected, for example, the Kcat of a Yersinia PTP (Zhang et al. 1994). These data suggest that PTP binding specificity may change according to pH conditions. In either case, it is clear that PTP1B substrate recognition involves more than just the 15-aa substrate IR motif; for instance, the N-terminus of PTP-1B is involved in an additional interaction with the IR (Dadke et al. 2000).
We have shown here that phosphopeptides synthesized on filters can be used in two ways to probe PTP substrate specificity. One method consists of binding with substrate-trapping mutant PTPs. The second method involves dephosphorylation of phosphopeptides with wild-type PTP, whereby an antibody is used to measure remaining phosphate on the spots. As we have shown here, this second approach is not feasible when the peptides carry a ZC motif, because commercial antibodies poorly recognize their phosphotyrosine antigen in this context. Therefore, appropriate care and controls should be included when investigating unknown motifs using this approach.
In summary, we have validated the SPOT membrane approach as a useful tool in finding and testing PTP substrate specificities. Especially when this approach is combined with a robotized synthesis procedure, one can systematically test hundreds of specific targets and even use degenerate sequences. Using two-dimensional grids such as the one we used here, one may undertake an incremental approach to finding “optimal” phosphopeptides; for example, by systematically varying the −2 and +2, and then the −3 and +3 aa positions around a fixed YZM motif for PTP-1B. The potential for these "optimal" motifs to predict physiological substrates is probably limited. However, such "optimal motifs" for initial PTP recognition and overall dephosphorylation may be used as potent and specific peptidomimetic PTP inhibitors.
Materials and methods
DNA constructs
The pGEX-4TK3 vector corresponds to pGEX-2TK (Amersham Pharmacia Biotech) with multicloning sites from the pGEX-4T3 vector (Amersham Pharmacia Biotech). Wild-type and substrate-trapping pGEX-4TK3-PTP-1B encode the GST-human PTP-1B fusion protein from amino acids 1 to 289. The mutation exchanges Asp 181 (WPD) for Ala (WPA) (Wälchli et al. 2000). Wild-type pGEX-4TK3-PTP-β encodes GST fused to the human PTP-β from amino acids 1712 to 2030 (end of the protein; Wälchli et al. 2000).
GST production and labeling
Transformed bacteria were grown at 37°C in LB medium in the presence of ampicillin (0.1 mg/mL). For the induction of GST constructs, IPTG (1mM) was added when bacteria were in the exponential growth phase. Cells were incubated at 30°C in a shaker for a further 2 h. After centrifugation, cell pellets were lysed by sonication in PBS plus 1% Triton X-100 in the presence of a proteinase inhibitor cocktail, (complete™; Roche Molecular Biochemicals).
Two to 5 μg of GST fusion proteins were bound to glutathione-sepharose beads (Amersham Pharmacia Biotech) at 4°C. After several washes with PBS, beads were incubated with 50 units of protein kinase A from bovine heart (Sigma) in kinase buffer (20 mM Tris, pH 7.5, 100 mM NaCl, 12 mM MgCl2, 1mM DTT) with 35 μCi of [γ-32P] ATP for 30 min on ice, as specified by the supplier. Probes were eluted from beads in 10 mM free glutathione in 50 mM Tris, pH 8. We verified that protein kinase A was not able to significantly phosphorylate GST or GST-PTP-1B, when the kinase phosphorylation site was absent (data not shown).
SPOT synthesis and membrane probing
Peptides were manually synthesized on derivatized cellulose membrane and 20 Fmoc-amino acids active esters provided by Sigma-Genosys. Fmoc-phosphotyrosine, Fmoc-phosphoserine, and Fmoc-phosphothreonine from Novabiochem (#04-12-1156, #04-12-1151, and #04-12-1155, respectively) were incorporated in the presence of the coupling reagent, N,N′-diisopropylcarbodiimide (DIC; Sigma) and hydroxybenzotriale (HOBt; Fluka). For further details see Frank and coworkers (Frank and Doring 1988; Blankenmeyer-Menge et al. 1990). The SPOT library was made with the SPOT robot ASP222 from Intavis.
Membranes were blocked (at least 2 h) and probed at 4°C in Western wash buffer (10 mM Tris, pH 7.4, 0.1% Triton X-100, 1 mM DTT and 150 mM NaCl) in SPOT blocking buffer (Sigma-Genosys). After 2 h, membranes were washed several times in Western wash buffer.
Dephosphorylation studies
SPOT membranes were incubated for at least 2 h in dephosphorylation buffer (Western wash buffer, SPOT blocking buffer, and 1 mM DTT). GST-PTP-1B or GST-PTPβ wild type was incubated with the SPOT membranes in dephosphorylation buffer. After variable times of dephosphorylation, GST-PTPs were removed by extensive washes in PBS + 0.1% Tween-20.
Western blots
SPOT membranes were blocked 1 h at room temperature in blocking solution (PBS, 0.1% Tween-20, and 3% bovine serum albumin). The antiphosphotyrosine antibody mix, 4G10 (1:2000; Upstate), PY 20 (1:1000; Transduction Laboratories), PY 69 (1:1000; Transduction Laboratories), and P-Tyr-100 (1:2000; Biolabs), was incubated for 1 h at room temperature with agitation. After washes with PBS plus 0.1% Tween-20, the horseradish peroxidase conjugated antimouse antibody (Dako) was added at 1:2000 dilution for 1 h. The antibody-enzyme conjugate was visualized using an enhanced chemiluminescence kit (ECL™, Amersham Pharmacia Biotech).
Acknowledgments
We thank Sébastien Wälchli and Axel Harrenga for their stimulating discussions, and Thomas Rückle for the help in the phosphopeptide syntheses.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked "advertisement" in accordance with 18 USC section 1734 solely to indicate this fact.
Abbreviations
aa, amino acid(s)
GST, glutathione-S-transferase
IR, insulin receptor
PBS, phosphate-buffered saline
PTP, protein tyrosine phosphatase
STAT, signal transducer and activator of transcription
TC-PTP, T-cell protein tyrosine phosphatase
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1110/ps.0213402.
References
- Aoki, N. and Matsuda, T. 2000. A cytosolic protein-tyrosine phosphatase PTP1B specifically dephosphorylates and deactivates prolactin-activated STAT5a and STAT5b.J. Biol. Chem. 275 39718–39726. [DOI] [PubMed] [Google Scholar]
- Asante-Appiah, E., Ball, K., Bateman, K., Skorey, K., Friesen, R., Desponts, C., Payette, P., Bayly, C., Zamboni, R., Scapin, G., et al. 2001. The YRD motif is a major determinant of substrate and inhibitor specificity in T-cell protein-tyrosine phosphatase.J. Biol. Chem. 276 26036–26043. [DOI] [PubMed] [Google Scholar]
- Blankenmeyer-Menge, B., Nimitz, B., and Frank, R. 1990. An efficient method for anchoring Fmoc-amino acids to hydroxyl-functionalised solid supports.Tetrahedron 31 1701–1704. [Google Scholar]
- Blom, N., Gammeltoft, S., and Brunak, S. 1999. Sequence and structure-based prediction of eukaryotic protein phosphorylation sites.J Mol. Biol 294 1351–1362. [DOI] [PubMed] [Google Scholar]
- Chen, J., Sadowski, H.B., Kohanski, R.A., and Wang, L.H. 1997. Stat5 is a physiological substrate of the insulin receptor.Proc. Natl. Acad. Sci. 94 2295–2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadke, S., Kusari, J., and Chernoff, J. 2000. Down-regulation of insulin signaling by protein-tyrosine phosphatase 1B is mediated by an N-terminal binding region.J. Biol. Chem. 275 23642–23647. [DOI] [PubMed] [Google Scholar]
- Ebina, Y., Ellis, L., Jarnagin, K., Edery, M., Graf, L., Clauser, E., Ou, J.H., Masiarz, F., Kan, Y.W., Goldfine, I.D., et al. 1985. The human insulin receptor cDNA: The structural basis for hormone-activated transmembrane signalling.Cell 40 747–758. [DOI] [PubMed] [Google Scholar]
- Elchebly, M., Payette, P., Michaliszyn, E., Cromlish, W., Collins, S., Loy, A.L., Normandin, D., Cheng, A., Himms-Hagen, J., Chan, C.C., et al. 1999. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene.Science 283 1544–1548. [DOI] [PubMed] [Google Scholar]
- Espanel, X. and Sudol, M. 2001. Yes-associated protein and p53-binding protein-2 interact through their WW and SH3 domains.J. Biol. Chem. 276 14514–14523. [DOI] [PubMed] [Google Scholar]
- Espanel, X., Wälchli, S., Pescini Gobert, R., El Alama, M., Curchod, M.-L., Gullu-Isler, N., and Hooft van Huijsduijnen, R. 2001. Pulling strings below the surface: Hormone receptor signaling through inhibition of protein tyrosine phosphatases.Endocrine 15 19–28. [DOI] [PubMed] [Google Scholar]
- Fachinger, G., Deutsch, U., and Risau, W. 1999. Functional interaction of vascular endothelial-protein-tyrosine phosphatase with the angiopoietin receptor Tie-2.Oncogene 18 5948–5953. [DOI] [PubMed] [Google Scholar]
- Flint, A.J., Tiganis, T., Barford, D., and Tonks, N.K. 1997. Development of "substrate-trapping" mutants to identify physiological substrates of protein tyrosine phosphatases.Proc. National. Acad. Sci. 94 1680–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank, R. and Doring, R. 1988. Simultaneous multiple peptide synthesis under continuous flow conditions on cellulose paper discs as segmental solid synthesis.Tetrahedron 44 6031–6040. [Google Scholar]
- Frank, R. and Overwin, H. 1996. SPOT synthesis. Epitope analysis with arrays of synthetic peptides prepared on cellulose membranes.Methods Mol. Biol. 66 149–169. [DOI] [PubMed] [Google Scholar]
- Garton, A.J., Flint, A.J., and Tonks, N.K. 1996. Identification of p130(cas) as a substrate for the cytosolic protein tyrosine phosphatase PTP-PEST.Mol. Cell. Biol. 16 6408–6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huyer, G., Kelly, J., Moffat, J., Zamboni, R., Jia, Z., Gresser, M.J., and Ramachandran, C. 1998. Affinity selection from peptide libraries to determine substrate specificity of protein tyrosine phosphatases.Anal. Biochem. 258 19–30. [DOI] [PubMed] [Google Scholar]
- Jia, Z., Ye, Q., Dinaut, A.N., Wang, Q., Waddleton, D., Payette, P., Ramachandran, C., Kennedy, B., Hum, G., and Taylor, S.D. 2001. Structure of protein tyrosine phosphatase 1B in complex with inhibitors bearing two phosphotyrosine mimetics.J. Med. Chem. 44 4584–4594. [DOI] [PubMed] [Google Scholar]
- Klaman, L.D., Boss, O., Peroni, O.D., Kim, J.K., Martino, J.L., Zabolotny, J.M., Moghal, N., Lubkin, M., Kim, Y.B., Sharpe, A.H., et al. 2000. Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice.Mol. Cell. Biol. 20 5479–5489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer, A., Volkmer-Engert, R., Malin, R., Reineke, U., and Schneider-Mergener, J. 1993. Simultaneous synthesis of peptide libraries on single resin and continuous cellulose membrane supports: Examples for the identification of protein, metal and DNA binding peptide mixtures.Pept. Res. 6 314–319. [PubMed] [Google Scholar]
- Lander, E.S., Linton, L.M., Birren, B., Nusbaum, C., Zody, M.C., Baldwin, J., Devon, K., Dewar, K., Doyle, M., FitzHugh, W., et al. 2001. Initial sequencing and analysis of the human genome.Nature 409 860–921. [DOI] [PubMed] [Google Scholar]
- Lin, J.X., Mietz, J., Modi, W.S., John, S., and Leonard, W.J. 1996. Cloning of human Stat5B. Reconstitution of interleukin-2-induced Stat5A and Stat5B DNA binding activity in COS-7 cells.J. Biol. Chem. 271 10738–10744. [PubMed] [Google Scholar]
- Martens, W., Greiser-Wilke, I., Harder, T.C., Dittmar, K., Frank, R., Orvell, C., Moennig, V., and Liess, B. 1995. Spot synthesis of overlapping peptides on paper membrane supports enables the identification of linear monoclonal antibody binding determinants on morbillivirus phosphoproteins.Vet. Microbiol. 44 289–298. [DOI] [PubMed] [Google Scholar]
- Myers, M.P., Andersen, J.N., Cheng, A., Tremblay, M.L., Horvath, C.M., Parisien, J.P., Salmeen, A., Barford, D., and Tonks, N.K. 2001. TYK2 and JAK2 are substrates of protein-tyrosine phosphatase 1B.J. Biol. Chem. 276 47771–47774. [DOI] [PubMed] [Google Scholar]
- Pasquali, C., Vilbois, F., Curchod, M.L., Hooft van Huijsduijnen, R., and Arigoni, F. 2000. Mapping and identification of protein-protein interactions by two-dimensional far-Western immunoblotting.Electrophoresis 21 3357–3368. [DOI] [PubMed] [Google Scholar]
- Pellegrini, M.C., Liang, H., Mandiyan, S., Wang, K., Yuryev, A., Vlattas, I., Sytwu, T., Li, Y.C., and Wennogle, L.P. 1998. Mapping the subsite preferences of protein tyrosine phosphatase PTP-1B using combinatorial chemistry approaches.Biochemistry 37 15598–15606. [DOI] [PubMed] [Google Scholar]
- Salmeen, A., Andersen, J.N., Myers, M.P., Tonks, N.K., and Barford, D. 2000. Molecular basis for the dephosphorylation of the activation segment of the insulin receptor by protein tyrosine phosphatase 1B.Mol. Cell 6 1401–1412. [DOI] [PubMed] [Google Scholar]
- Storz, P., Doppler, H., Pfizenmaier, K., and Muller, G. 1999. Insulin selectively activates STAT5b, but not STAT5a, via a JAK2-independent signalling pathway in Kym-1 rhabdomyosarcoma cells.FEBS Lett 464 159–163. [DOI] [PubMed] [Google Scholar]
- Taddei, N., Chiarugi, P., Cirri, P., Fiaschi, T., Stefani, M., Camici, G., Raugei, G., and Ramponi, G. 1994. Aspartic-129 is an essential residue in the catalytic mechanism of the low M(r) phosphotyrosine protein phosphatase.FEBS Lett. 350 328–332. [DOI] [PubMed] [Google Scholar]
- Venter, J.C., Adams, M.D., Myers, E.W., Li, P.W., Mural, R.J., Sutton, G.G., Smith, H.O., Yandell, M., Evans, C.A., Holt, R.A., et al. 2001. The sequence of the human genome.Science 291 1304–1351. [DOI] [PubMed] [Google Scholar]
- Vetter, S.W., Keng, Y.F., Lawrence, D.S., and Zhang, Z.Y. 2000. Assessment of protein-tyrosine phosphatase 1B substrate specificity using "inverse alanine scanning".J. Biol. Chem. 275 2265–2268. [DOI] [PubMed] [Google Scholar]
- Wälchli, S., Curchod, M.L., Gobert, R.P., Arkinstall, S., and Hooft van Huijsduijnen, R. 2000. Identification of tyrosine phosphatases that dephosphorylate the insulin receptor. A brute force approach based on "substrate-trapping" mutants.J. Biol. Chem. 275 9792–9796. [DOI] [PubMed] [Google Scholar]
- Zhang, S.H., Liu, J., Kobayashi, R., and Tonks, N.K. 1999. Identification of the cell cycle regulator VCP (p97/CDC48) as a substrate of the band 4.1-related protein-tyrosine phosphatase PTPH1.J. Biol. Chem. 274 17806–17812. [DOI] [PubMed] [Google Scholar]
- Zhang, Z.Y., Thieme-Sefler, A.M., Maclean, D., McNamara, D.J., Dobrusin, E.M., Sawyer, T.K., and Dixon, J.E. 1993. Substrate specificity of the protein tyrosine phosphatases.Proc. Natl. Acad. Sci. 90 4446–4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Z.Y., Wang, Y., and Dixon, J.E. 1994. Dissecting the catalytic mechanism of protein-tyrosine phosphatases.Proc. Natl. Acad. Sci. 91 1624–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng, X.M., Wang, Y., and Pallen, C.J. 1992. Cell transformation and activation of pp60c-src by overexpression of a protein tyrosine phosphatase.Nature 359 336–339. [DOI] [PubMed] [Google Scholar]