Abstract
The mechanical resistance of a folded domain in a polyprotein of five mutant I27 domains (C47S, C63S I27)5is shown to depend on the unfolding history of the protein. This observation can be understood on the basis of competition between two effects, that of the changing number of domains attempting to unfold, and the progressive increase in the compliance of the polyprotein as domains unfold. We present Monte Carlo simulations that show the effect and experimental data that verify these observations. The results are confirmed using an analytical model based on transition state theory. The model and simulations also predict that the mechanical resistance of a domain depends on the stiffness of the surrounding scaffold that holds the domain in vivo, and on the length of the unfolded domain. Together, these additional factors that influence the mechanical resistance of proteins have important consequences for our understanding of natural proteins that have evolved to withstand force.
Keywords: Mechanical unfolding of proteins, I27, Monte Carlo, concatamer, worm-like chain, mechanical resistance, transition state theory
Proteins may be mechanically unfolded using laser tweezers (Kellermayer et al. 1997; Tskhovrebova et al. 1997) or the atomic force microscope (AFM; Mitsui et al. 1996; Rief et al. 1997; Carrion-Vazquez et al. 1999; Best et al. 2001; Brockwell et al. 2002). The first polymeric protein to be mechanically unfolded was the muscle protein titin (Rief et al. 1997; Tskhovrebova et al. 1997). This protein consists of ∼300 immunoglobulin (Ig) and fibronectin type III domains as well as a 163–2174-residue disordered region rich in P, E, V, and K amino acids (Labeit and Kolmerer 1995) thought to be critically important to the mechanical properties of the polymer (Linke et al. 1998; Li et al. 2001). When stretching the protein using laser tweezers or an AFM, individual domains were observed to unfold abruptly at a critical unfolding force in the range 50–300 pN. The result is a now characteristic saw-tooth force–extension pattern (Rief et al. 1997; Tskhovrebova et al. 1997).
Only limited information can be obtained from mechanical unfolding experiments on a heterogeneous protein such as titin. To determine the mechanical unfolding properties of an individual domain, polyproteins or concatamers of controlled composition have been constructed that contain 5–25 identical domains, joined by amino-acid linkers (Carrion-Vazquez et al. 1999; Best et al. 2001; Brockwell et al. 2002) or disulfide bridges (Yang et al. 2000). The 27th Ig domain of titin (I27), comprising 89 amino acids, has been extensively studied using this approach. By analyzing the dependence of the unfolding force on the pulling speed (Merkel et al. 1999), the intrinsic unfolding rate constant ku0, and the placement xu of the mechanical unfolding transition state relative to the native state have been determined (Carrion-Vazquez et al. 1999). Interestingly, although early work on this domain indicated that chemical denaturation and mechanical unfolding follow the same unfolding pathway (Carrion-Vazquez et al. 1999), this has recently been shown not to be the case, at least for mutant I27 domains (Brockwell et al. 2002; Fowler et al. 2002).
It has been widely assumed that (1) in a heterogeneous polyprotein, the domain with the fastest ku0 must unfold first under an applied load (Li et al. 2000), and (2) in a homogeneous polyprotein, all unfolding forces are equivalent within the limits of thermal fluctuations (Carrion-Vazquez et al. 1999; Yang et al. 2000; Best et al. 2001; Brockwell et al. 2002). Here we report simulations, experiments, and theory that show that these assumptions are not always valid. We demonstrate that the unfolding force of a given unfolding event depends subtly on the history of the unfolding process (i.e., the number of domains that remain folded) and on the stiffness of the scaffold that holds the domain during extension (i.e., the compliance of the folded/unfolded chain and the cantilever). The results cast new light on our understanding of the physical characteristics of mechanical unfolding and have important implications for understanding the mechanical resistance of biopolymers in vivo.
Results and Discussion
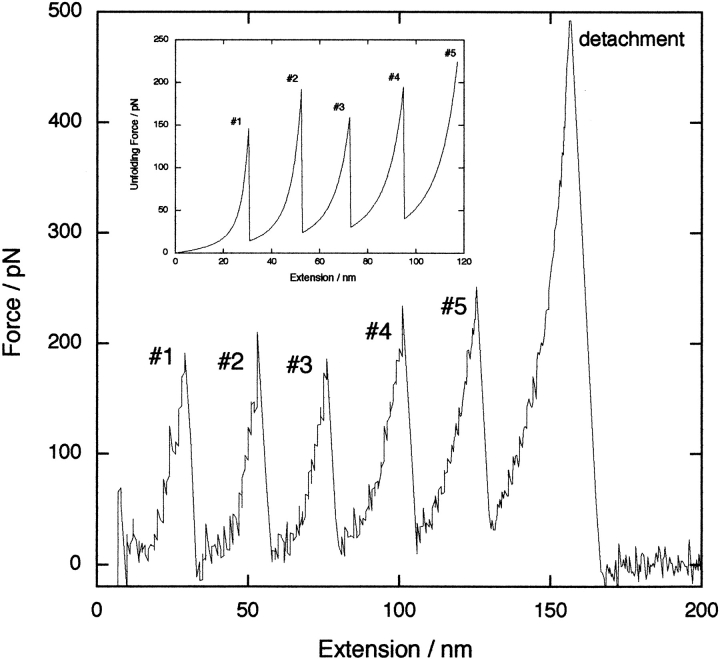
Conventionally, force–extension data acquired using an AFM, such as those shown in Figure 1 ▶, are analyzed in the following manner. The unfolding forces (i.e., the critical force at which a domain unfolds) from many successful approach/retract cycles at a particular tip retraction rate are collated into a force–frequency histogram (Carrion-Vazquez et al. 1999; Yang et al. 2000; Brockwell et al. 2002). This is then repeated at a variety of pulling speeds v (e.g., 70–4000 nm/sec). A plot of the mean (or mode) unfolding force versus ln(v) can then be used to extract the parameters of interest ku0 and xu. However, we (Brockwell et al. 2002) and others (Makarov et al. 2001) have shown that increasing the number of domains decreases the mean unfolding force. To obtain parameters appropriate for a monomer it is necessary to fit the speed dependence of the measured unfolding force using a Monte Carlo simulation based on, for example, an elastically coupled two-level system (Rief et al. 1998) with the same number of domains in the concatamer as is used experimentally.
Fig. 1.
A typical mechanical unfolding force–extension data set of (C47S, C63S I27)5 by experiment (main curve) and simulation (inset). Both the experimental and simulated data were obtained with cantilevers of spring constant, kc = 50 pN/nm and at a pulling speed of 700 nm/sec. The Monte Carlo data are obtained with an unfolded domain contour length of Lu = 28 nm, identical to that of the I27 domain (Carrion-Vazquez et al. 1999; Brockwell et al. 2002). Other parameters in the simulations were: ku0 = 2 × 10−3 sec−1, persistence length of the unfolded domains p = 0.39 nm, and xu = 0.3 nm.
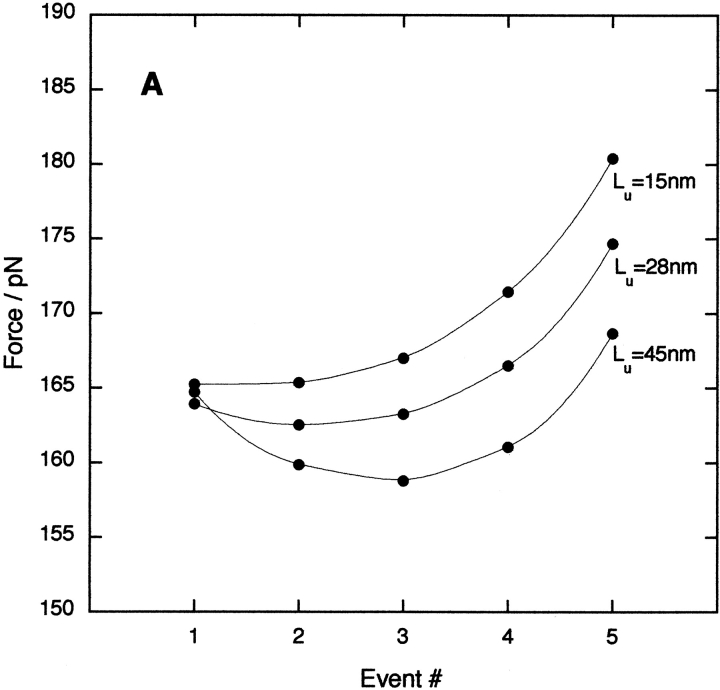
During our Monte Carlo simulations it became clear that combining all unfolding peaks from many force–extension experiments (at the same pulling speed) into a single histogram as described above obscures subtleties in the mechanical unfolding data. To investigate further, we simulated the mechanical unfolding behavior of a polyprotein comprising five identical copies of a mutant of I27 (C47S, C63S I27)5 (Brockwell et al. 2002) and constructed force–frequency histograms for each individual unfolding event (#; Fig. 1 ▶, inset). In this manner, each unfolding event can be considered in the context of the state of the entire polyprotein. The data reveal that each unfolding event is not equivalent within the thermal noise. Instead, the dependence of the force on the event number is a more complex, nonlinear, function (Fig. 2 ▶). To determine if this effect is observed experimentally, (C47S, C63S I27)5 was constructed, purified, and mechanically unfolded (see Materials and Methods). A typical force–extension curve is shown in Figure 1 ▶. The mean unfolding force, analyzed by event number, is shown in Figure 2 ▶. The data accord reasonably well with the simulation, demonstrating a significant increase in force between events #3 and #4 (12.6 ± 3.0 pN), and #4 and #5 (7.9 ± 3.6 pN), but no significant difference between events #2 and #3 (1.8 ± 3.3 pN). The experimental and simulated data deviate significantly at event #1. The relative-force–frequency histogram for this event is broader and more skewed than that of other events (the distribution of events #2–#5 have a half-height half-width of 2% ± 0.1%, whereas that of event #1 is 3%; see Fig. 2 ▶, insets). These effects presumably arise from inclusion in the data set of low-force events owing to nonspecific protein–protein and domain–surface interactions (despite our rigorous selection criteria for an acceptable trace; see Materials and Methods). The weighted mean value obtained for event #1 (Fig. 2 ▶) is, therefore, lower than expected. Consequently, the first distribution was also freely fitted to two Gaussians, of which one had a half-height half-width identical to that of events #2–#5. The result is that the maxima occur at 20% and at 18%. The larger value is now almost identical to that obtained from Monte Carlo and analytical calculations.
Fig. 2.
Monte Carlo simulation (•) and experimental data (□) for unfolding forces of (C47S, C63S I27)5 as a function of the unfolding event number # (see Fig. 1 ▶). Data are expressed as a fraction of the sum of the unfolding forces in order to combine many experimental data sets. Experimental data are expressed as a weighted mean ± weighted standard error of the mean. (Inset) Relative force–frequency histograms for #1 and #3 for all data sets (n = 104). Solid lines are fits to a single (#3) or a double (#1) Gaussian function. The points (×) and (▵) are the modes obtained from the fit to the distribution for #1.
Although the effect of event number on the unfolding force is relatively small for a pentamer, the effect can be observed experimentally because of (1) the high purity of our preparations (which give a single peak in ESI-MS analysis); (2) the low concentration of protein used; and (3) the rigorous quality control of the raw data (see Materials and Methods; Best et al. 2002). More importantly, simulations and calculations show that the effect becomes greater upon increasing the number of domains (Fmax − Fmin = 12 and 25 pN for polymers of 5 and 15 domains, respectively) and becomes more significant because the magnitude of the mean unfolding force decreases.
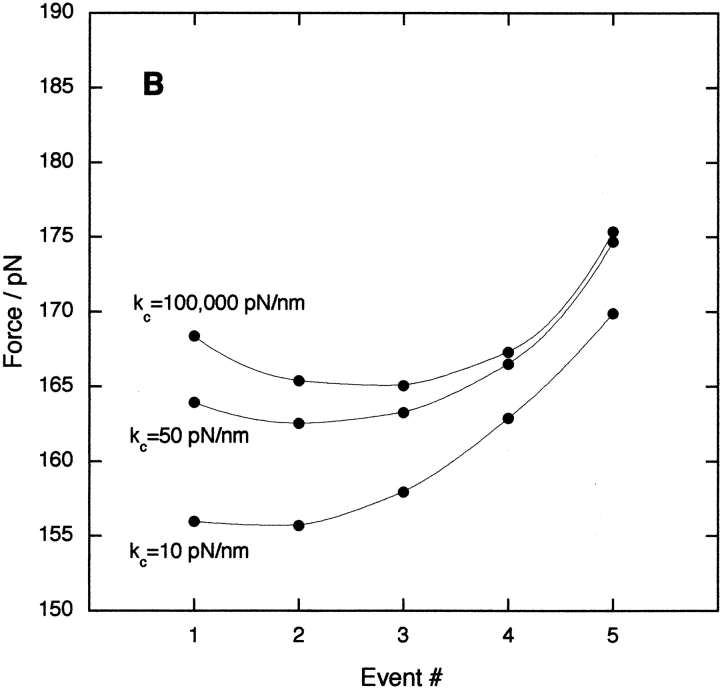
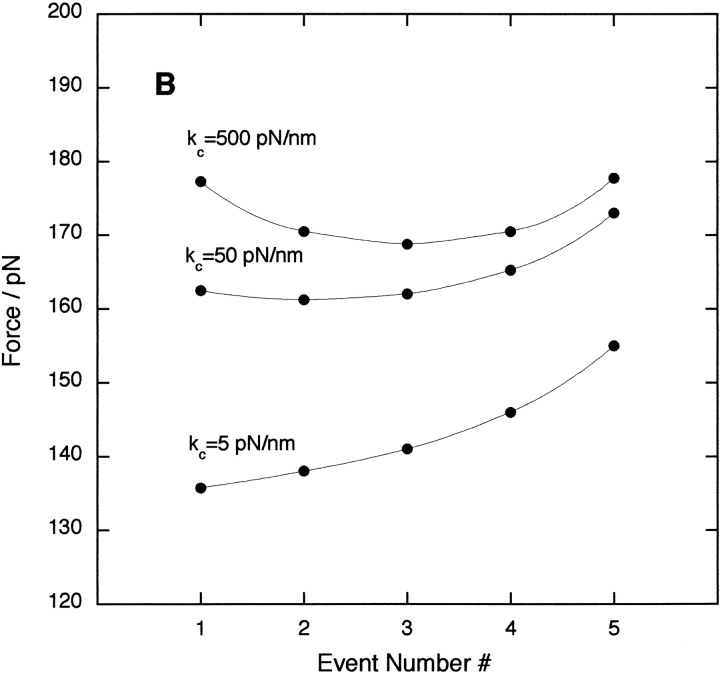
The sensitivity of the measured unfolding force to the polymer length and cantilever stiffness was investigated further by Monte Carlo simulations (Fig. 3 ▶). The effect of varying the length of polypeptide chain when a domain unfolds (Lu) is shown in Figure 3A ▶. The data show that an increase in Lu reduces the unfolding forces and shifts the minimum to higher event numbers. The compliance of the whole assembly is determined by the folded and unfolded domains in the polymer and the flexible AFM cantilever (of spring constant kc). The effect of varying kc on the predicted unfolding forces is shown in Figure 3B ▶: increasing the cantilever stiffness results in larger unfolding forces and movement of the minimum force to higher event numbers.
Fig. 3.
Monte Carlo simulations showing the effect of varying (A) the unfolded contour length Lu and (B) the AFM cantilever spring constant kc on the observed unfolding forces at each event number. In A, kc = 50 pN/nm; and in B, Lu = 28 nm.
Our data demonstrate that the number of domains that have unfolded influences the mechanical resistance of the next unfolding event. This rather surprising unfolding history phenomenon arises from a competition between two effects: the lower unfolding probability at a given force when fewer domains remain folded, and the increased overall compliance J of the concatamer after domains unfold. The compliance is the inverse of an effective spring constant, so that a more compliant system yields a larger extension for a given force than a less compliant one. A decrease in the number of folded domains reduces the number of unfolding attempts and decreases the unfolding probability at any given force or extension. Thus, the measured mean unfolding force rises as more domains unfold. However, as each domain is unfolded, the total length of the concatamer increases, which increases the overall compliance. In a system with a higher compliance J, the loading rate
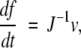 |
(1) |
is reduced, resulting in more thermally driven unfolding attempts per unit time at each extension and thus a lower unfolding force f (Evans and Ritchie 1999). The cantilever spring constant also contributes to the total compliance and therefore affects the loading rate. The net result of the competing effects of number of domains and compliance is a minimum in the unfolding force as a function of the unfolding event.
To test the validity of our interpretation, an analytical model based on the theory of Bell and Evans (Bell 1978; Evans and Ritchie 1997) was developed (Fig. 4 ▶). This model assumes that transition state theory applies to the folding/unfolding transition. Barrier crossing caused by thermal fluctuations becomes more probable as the applied force lowers the activation barrier. We associate the maximum of the unfolding probability distribution with the experimentally measured mean unfolding force. The general equation for the unfolding force is
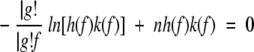 |
(2) |
where n is the current number of folded domains,
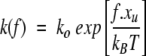 |
(3) |
is the unfolding rate constant at force f, and
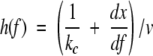 |
(4) |
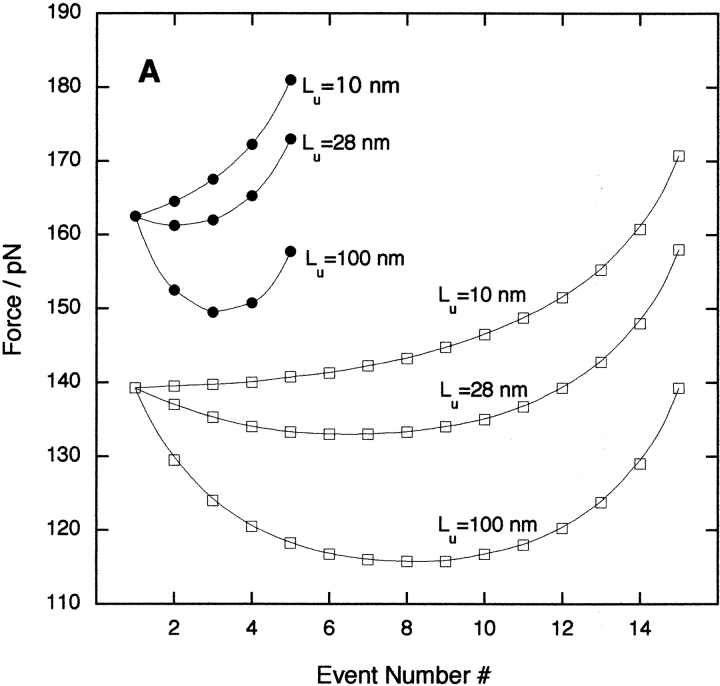
is the compliance of the concatamer and cantilever of spring constant kc, divided by the pulling speed, v. The worm-like-chain model was used to describe the unfolded domain elasticity, f(x). The analytical model replicates the results of the simulations and experiment. Figure 4A ▶ shows that there is a strong dependence of the unfolding force on the unfolding event number and the unfolded chain length for concatamers of 5 and 15 domains. The effect of the cantilever spring constant on the unfolding force for each unfolding event is also confirmed by theory (Fig. 4B ▶).
Fig. 4.
Results of the analytical model using a folded domain length Lf = 4 nm, a pulling speed of 700 nm/sec, and a persistence length p = 0.39 nm. (A) The effect of changing the length of the unfolded polypeptide chain Lu for two concatamers with 5 (•) and 15 (□) domains using a fixed cantilever spring constant kc = 50 pN/nm. For I27, Lu = 28 nm. (B) The effect on a pentamer of varying kc for a fixed Lu = 28 nm.
The effect of domain number and cantilever compliance implies that comparison of experiments performed in different laboratories is extremely difficult, unless not only the same pulling speed is used, but also the experiments are performed with the same cantilever spring constant and on concatamers with the same number of domains. Providing the same length concatamer is used, one could plot the measured unfolding forces as a function of loading rate at the point of unfolding to circumvent these problems. However, the loading rate at point of rupture must be used (which at present is difficult to measure), not the more easily obtained mean loading rate (kcv). Although the differences in concatamer behavior owing to the cantilever spring constant could be large (Figs. 3B, 4B ▶ ▶), in practice the range of spring constants usually used (10–100 pN/nm) would produce only small differences in the average measured unfolding force. In addition, the effect of the number of domains in the polymer on the mean unfolding force can also be accounted for (Brockwell et al. 2002), and experiments that compare the effect of conservative mutations on the behavior of an otherwise identical concatamer effectively circumvent these complexities (Best et al. 2002a,b).
In light of these observations, it is clear that, for a heteropolymer, the simple picture of the domain with the fastest unfolding rate constant unfolding first is not always correct. This has a profound effect in experiments on heteropolymeric proteins. For example, consider a sequence of one domain (labeled W) that unfolds rapidly with rate constant kW, and four (labeled S) that unfold more slowly with rate constant kS. If, for example, kW = 2.5kS, and all other parameters are otherwise identical, W will unfold first in only 38% of experiments. This probability increases to 53% when kW = 4.5kS. In contrast, when kW = 0.5kS, W has probabilities of 11% and 41%, respectively, of unfolding first and last. In the case of a polymer constructed from four pairs of alternating I27 (ku0 = 3.3 × 10−4 sec−1) and I28 (ku0 = 2.5 × 10−5 sec−1) domains, the rate constants are sufficiently different such that all the I27 domains unfold first (Li et al. 2000). However, it is interesting to note that this result depends on the relative number of I27 and I28 domains and not solely on their intrinsic unfolding rate constants.
Our observations have important implications for understanding the mechanical properties of heteropolymers that have evolved naturally to resist force in vivo. The passive effect of unstructured polymers acting as an entropic spring is well known (e.g., the PEVK domain of titin [Linke et al. 1996] and the selectin cell-surface carbohydrate interaction [Fritz et al. 1998]). We have shown that both the superstructure or scaffold in which the polymer is held, as well as the number and length of unfolded domains, influence the mechanical resistance of the remaining folded domains. Thus, effects such as the compliance of the surrounding tissue and the lengths of unstructured regions will play a key role in tailoring the mechanical resistance of folded domains in polyproteins.
The variation in mechanical properties that results from differences in the number and type of folded domains and the nature of the barrier between states has been noted previously (Fritz et al. 1998; Rief et al. 1999; Smith et al. 1999; Brockwell et al. 2002). Sequence variations in domains with identical folds can also significantly alter their mechanical properties (Li et al. 2000). However, the observations we report here indicate that other, more subtle factors, such as domain length and their number, relative folding and unfolding rate constants, the length of naturally unfolded regions, and the compliance of the surrounding scaffold add another level of complexity to the mechanical properties of naturally occurring polyproteins. Relating the macroscopic mechanical properties of natural biopolymers to these coupled microscopic effects is thus a formidable challenge.
Materials and methods
A two-state model was used to simulate the forced extension of the I27 construct as previously reported (Rief et al. 1998; Brockwell et al. 2002), assuming a worm-like chain in series with a cantilever of spring constant kc.
For mechanical unfolding experiments, a homopolymer (C47S, C63S I27)5 was constructed from the heteropolymer (I27)5* (Brockwell et al. 2002) using PCR. (C47S, C63S I27)5 was overexpressed and purified as described (Brockwell et al. 2002). The identity and purity of the homopolymer was verified by ESI-MS (expected mass: 52,219 D, measured mass: 52,218 D).
Mechanical unfolding experiments were carried out as described (Brockwell et al. 2002) using 150 μL of 0.8 μM homopolymer and a retraction speed of 700 nm/sec. For each experimental data set (i.e., when using the same cantilever), data were only binned if they fulfilled several criteria: (1) each retraction must only have 5 unfolding events and 1 pull-off event; (2) the spacing between each peak must be ∼23 nm; (3) each pull must be clean with no nonspecific interactions. The low protein concentration used results in an acceptable trace at a hit rate of ∼1.5% of approach/retract cycles. Each force peak was then normalized to the total of all unfolding forces in the trace,
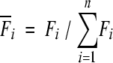 |
(5) |
so that data sets obtained with different cantilevers of the same nominal kccould be combined. For each experimental data set (9 sets containing a total of 104 force–extension curves with 5 unfolding events) the mean was found for each event number, then all the data sets were combined to give a weighted mean (± weighted standard error of the mean) for each event.
Acknowledgments
The authors are grateful to Phil Williams and David Salt for useful discussions. We acknowledge the BBSRC, EPSRC, and The Wellcome Trust for financial support. S.E.R. is a BBSRC Professorial Research fellow. The manuscript is a contribution from the Astbury Centre for Structural Molecular Biology, which is part of the North of England Structural Biology Centre (NESBIC) and is funded by the BBSRC. The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked "advertisement" in accordance with 18 USC section 1734 solely to indicate this fact.
Note added in proof First steps toward understanding the mechanical properties of titin in intact muscle, as a sum of its constituent parts, has recently been published (Li et al. 2002).
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1110/ps.0224602.
References
- Bell, G.I. 1978. Models for the specific adhesion of cells to cells. Science 200 618–627. [DOI] [PubMed] [Google Scholar]
- Best, R.B., Li, B., Steward, A., Daggett, V., and Clarke, J. 2001. Can non-mechanical proteins withstand force? Stretching Barnase by atomic force microscopy and molecular dynamics simulation. Biophys. J. 81 2344–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best, R.B., Brockwell, D.J., Toca-Herrera, J.L., Blake, A.W., Smith, D.A., Radford, S.E., and Clarke, J. 2002a. Force mode AFM as a tool for protein folding studies. Anal. Chim. Acta (in press).
- Best, R.B., Fowler, S.B., Toca-Herrera, J.L., Clarke, J. 2002. A simple method for probing the mechanical unfolding pathway of proteins in detail. Proc. Natl. Acad. Sci. USA. 99 12143–12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockwell, D.J., Beddard, G.S., Clarkson, J., Zinober, R.C., Blake, A.W., Trinick, J., Olmsted, P.D., Smith, D.A., and Radford, S.E. 2002. The effect of core destabilisation on the mechanical resistance of I27. Biophys. J. 83 458–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion-Vazquez, M., Oberhauser, A.F., Fowler, S.B., Marszalek, P.E., Broedel, S.E., Clarke, J., and Fernandez, J.M. 1999. Mechanical and chemical unfolding of a single protein: A comparison. Proc. Natl. Acad. Sci. 96 3694–3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans, E. and Ritchie, K. 1997. Dynamic strength of molecular adhesion bonds. Biophys. J. 72 1541–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ———. 1999. Strength of a weak bond connecting flexible polymer chains. Biophys. J. 76 2439–2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler, S.B., Best, R.B., Toca-Herrera, J.L., Rutherford, T.J., Steward, A., Paci, E., Karplus, M., Clarke, J. 2002. Mechanical unfolding of a titin Ig domain: Structure of unfolding intermediate revealed by combining AFM, molecular dynamics simulations, NMR, and protein engineering. J. Mol. Biol.. 322 841. [DOI] [PubMed] [Google Scholar]
- Fritz, J., Katopodis, A.G., Kolbinger, F., and Anselmetti, D. 1998. Force-mediated kinetics of single P-selectin/ligand complexes observed by atomic force microscopy. Proc. Natl. Acad. Sci. 95 12283–12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellermayer, M.S.Z., Smith, S.B., Granzier, H.L., and Bustamante, C. 1997. Folding–unfolding transitions in single titin molecules characterized with laser tweezers. Science 276 1112–1116. [DOI] [PubMed] [Google Scholar]
- Labeit, S. and Kolmerer, B. 1995. Titins: Giant proteins in charge of muscle ultrastructure and elasticity. Science 270 293–296. [DOI] [PubMed] [Google Scholar]
- Li, H., Oberhauser, A.F., Fowler, S.B., Clarke, J., and Fernandez, J.M. 2000. Atomic force microscopy reveals the mechanical design of a modular protein. Proc. Natl. Acad. Sci. 97 6527–6531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H., Oberhauser, A.F., Redick, S.D., Carrion-Vazquez, M., Erickson, H.P., and Fernandez, J.M. 2001. Multiple conformations of PEVK proteins detected by single-molecule techniques. Proc. Natl. Acad. Sci. 98 10682–10686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H., Linke, W.A., Oberhauser, A.F., Carrion-Vasquez, M., Kerkvliet, J.G., Lu, H., Marszatek, P.F., Fernandez, J.M. 2002. Nature 418 998–1002. [DOI] [PubMed] [Google Scholar]
- Linke, W.A., Ivemeyer, M., Olivieri, N., Kolmerer, B., Casper Rüegg, J., and Labeit, S. 1996. Towards a molecular understanding of the elasticity of Titin. J. Mol. Biol. 261 62–71. [DOI] [PubMed] [Google Scholar]
- Linke, W.A., Ivemeyer, M., Mundel, P., Stockmeier, M.R., and Kolmerer, B. 1998. Nature of PEVK-titin elasticity in skeletal muscle. Proc. Natl. Acad. Sci. 95 8052–8057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarov, D.E., Hansma, P.K., and Metiu, H. 2001. Kinetic Monte Carlo simulation of titin unfolding. J. Chem. Phys. 114 9663–9673. [Google Scholar]
- Merkel, R., Nassoy, P., Leung, A., Ritchie, K., and Evans, E. 1999. Energy landscapes of receptor–ligand bonds explored with dynamic force spectroscopy. Nature 397 50–53. [DOI] [PubMed] [Google Scholar]
- Mitsui, K., Hara, M., and Ikai, A. 1996. Mechanical unfolding of α2-macroglobulin molecules with atomic force microscope. FEBS Lett. 385 29–33. [DOI] [PubMed] [Google Scholar]
- Rief, M., Gautel, M., Oesterhelt, F., Fernandez, J.M., and Gaub, H.E. 1997. Reversible unfolding of individual titin immunoglobulin domains by AFM. Science 276 1109–1111. [DOI] [PubMed] [Google Scholar]
- Rief, M., Fernandez, J.M., and Gaub, H.E. 1998. Elastically coupled two-level systems as a model for biopolymer extensibility. Phys. Rev. Lett. 81 4764–4767. [Google Scholar]
- Rief, M., Pascual, J., Saraste, M., and Gaub, H.E. 1999. Single molecule force spectroscopy of spectrin repeats: Low unfolding forces in helix bundles. J. Mol. Biol. 286 553–561. [DOI] [PubMed] [Google Scholar]
- Smith, B.L., Schäffer, T.E., Viani, M., Thompson, J.B., Frederick, N.A., Kindt, J., Belcher, A., Stucky, G.D., Morse, D.E., and Hansma, P.K. 1999. Molecular mechanistic origin of the origin of the toughness of natural adhesives, fibres and composites. Nature 399 761–763. [Google Scholar]
- Tskhovrebova, L., Trinick, J., Sleep, J.A., and Simmons, R.M. 1997. Elasticity and unfolding of single molecules of the giant muscle protein titin. Nature 387 308–312. [DOI] [PubMed] [Google Scholar]
- Yang, G., Cecconi, C., Baase, W.A., Vetter, I.R., Breyer, W.A., Haack, J.A., Matthews, B.W., Dahlquist, F.W., and Bustamante, C. 2000. Solid-state synthesis and mechanical unfolding of polymers of T4 lysozyme. Proc. Natl. Acad. Sci. 97 139–144. [DOI] [PMC free article] [PubMed] [Google Scholar]