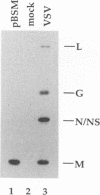
Abstract
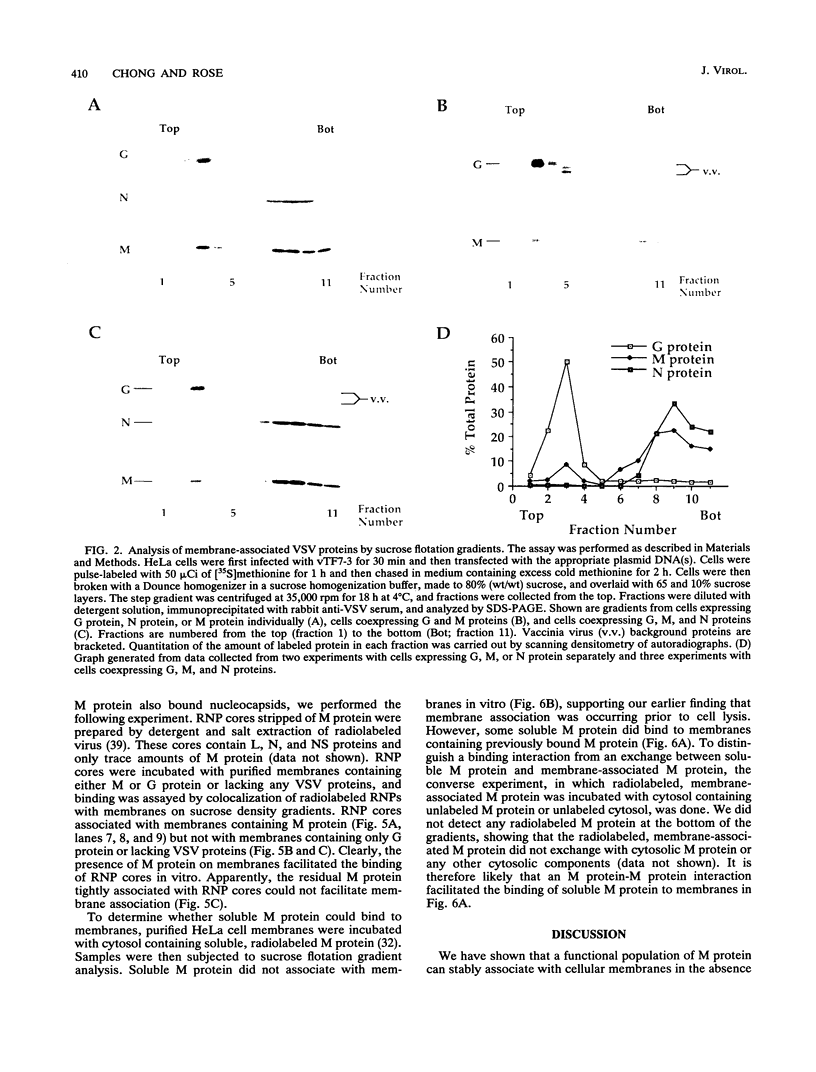
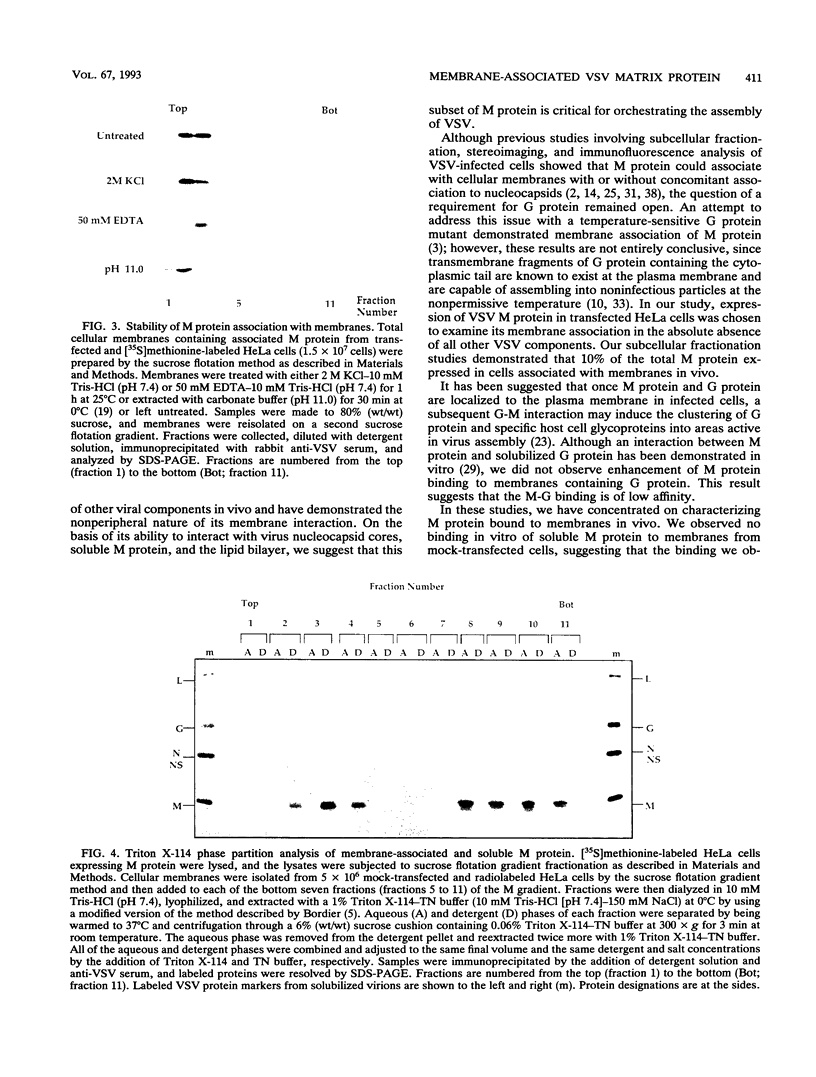
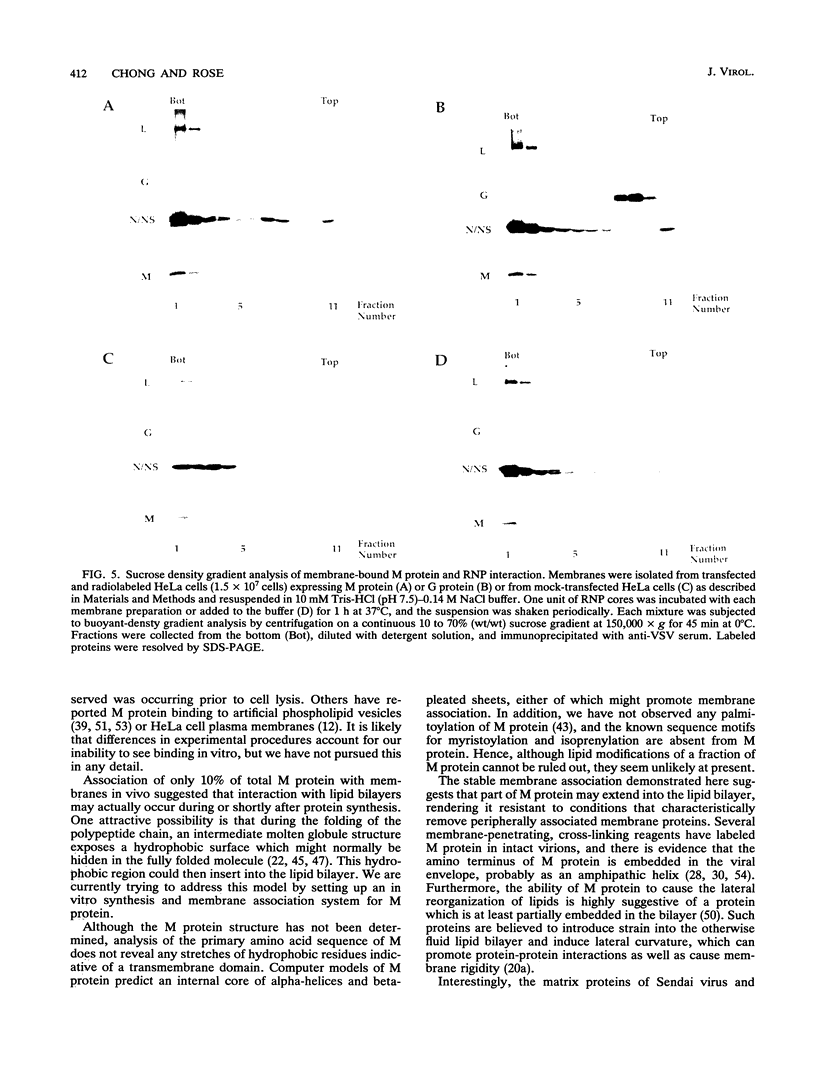
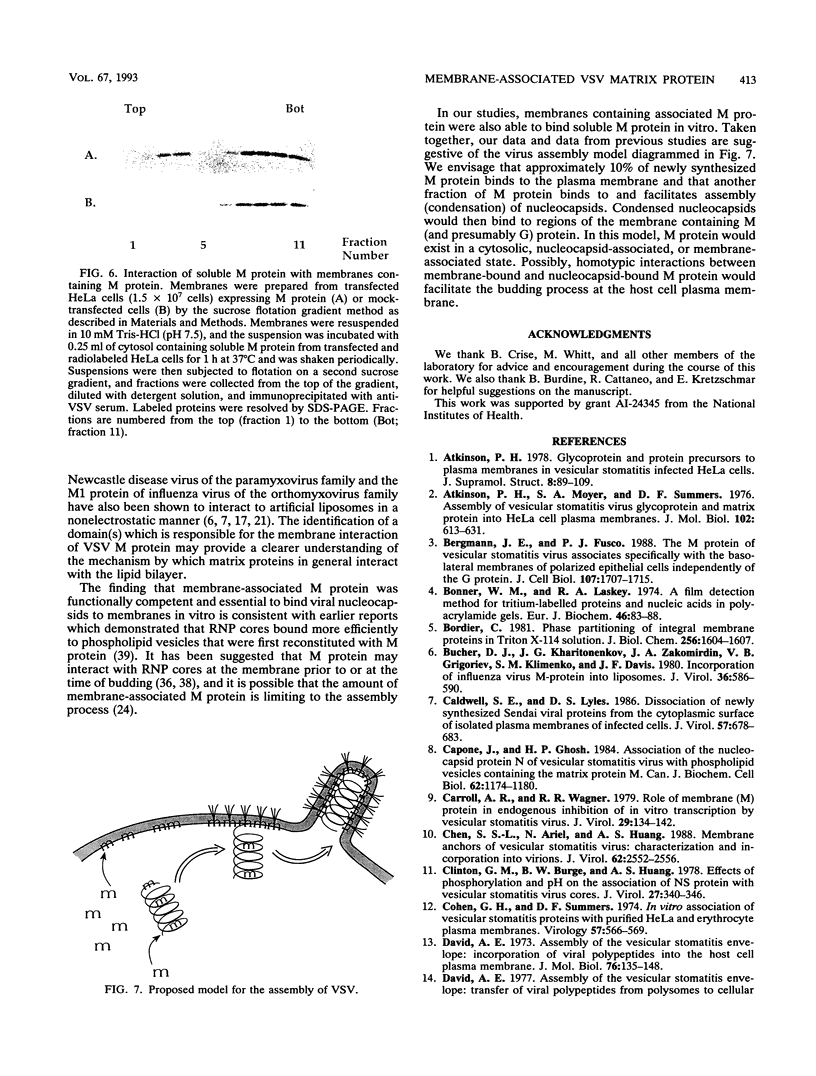
The matrix (M) protein of vesicular stomatitis virus (VSV) is a major structural component of the virion which is generally believed to bridge between the membrane envelope and the ribonucleocapsid (RNP) core. To investigate the interaction of M protein with cellular membranes in the absence of other VSV proteins, we examined its distribution by subcellular fractionation after expression in HeLa cells. Approximately 90% of M protein, expressed without other viral proteins, was soluble, whereas the remaining 10% was tightly associated with membranes. A similar distribution in VSV-infected cells has been observed previously. Conditions known to release peripherally associated membrane proteins did not detach M protein from isolated membranes. Membrane-associated M protein was soluble in the detergent Triton X-114, whereas soluble M protein was not, suggesting a chemical or conformational difference between the two forms. Membranes containing associated M protein were able to bind RNP cores, whereas membranes lacking M protein were not. We suggest that this membrane-bound M fraction constitutes a functional subset of M protein molecules required for the attachment of RNP cores to membranes during normal virus budding.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson P. H. Gycoprotein and protein precursors to plasma membranes in vesicular stomatitis virus infected HeLa cells. J Supramol Struct. 1978;8(1):89–109. doi: 10.1002/jss.400080108. [DOI] [PubMed] [Google Scholar]
- Atkinson P. H., Moyer S. A., Summers D. F. Assembly of vesicular stomatitis virus glycoprotein and matrix protein into HeLa cell plasma membranes. J Mol Biol. 1976 Apr 15;102(3):613–631. doi: 10.1016/0022-2836(76)90338-7. [DOI] [PubMed] [Google Scholar]
- Bergmann J. E., Fusco P. J. The M protein of vesicular stomatitis virus associates specifically with the basolateral membranes of polarized epithelial cells independently of the G protein. J Cell Biol. 1988 Nov;107(5):1707–1715. doi: 10.1083/jcb.107.5.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Bucher D. J., Kharitonenkov I. G., Zakomirdin J. A., Grigoriev V. B., Klimenko S. M., Davis J. F. Incorporation of influenza virus M-protein into liposomes. J Virol. 1980 Nov;36(2):586–590. doi: 10.1128/jvi.36.2.586-590.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell S. E., Lyles D. S. Dissociation of newly synthesized Sendai viral proteins from the cytoplasmic surface of isolated plasma membranes of infected cells. J Virol. 1986 Feb;57(2):678–683. doi: 10.1128/jvi.57.2.678-683.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capone J., Ghosh H. P. Association of the nucleocapsid protein N of vesicular stomatitis virus with phospholipid vesicles containing the matrix protein M. Can J Biochem Cell Biol. 1984 Nov;62(11):1174–1180. doi: 10.1139/o84-151. [DOI] [PubMed] [Google Scholar]
- Carroll A. R., Wagner R. R. Role of the membrane (M) protein in endogenous inhibition of in vitro transcription by vesicular stomatitis virus. J Virol. 1979 Jan;29(1):134–142. doi: 10.1128/jvi.29.1.134-142.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. S., Ariel N., Huang A. S. Membrane anchors of vesicular stomatitis virus: characterization and incorporation into virions. J Virol. 1988 Aug;62(8):2552–2556. doi: 10.1128/jvi.62.8.2552-2556.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton G. M., Burge B. W., Huang A. S. Effects of phosphorylation and pH on the association of NS protein with vesicular stomatitis virus cores. J Virol. 1978 Aug;27(2):340–346. doi: 10.1128/jvi.27.2.340-346.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G. H., Summers D. F. In vitro association of vesicular stomatitis virus proteins with purified HeLa and erythrocyte plasma membranes. Virology. 1974 Feb;57(2):566–569. doi: 10.1016/0042-6822(74)90195-0. [DOI] [PubMed] [Google Scholar]
- David A. E. Assembly of the vesicular stomatitis virus envelope: incorporation of viral polypeptides into the host plasma membrane. J Mol Biol. 1973 May 5;76(1):135–148. doi: 10.1016/0022-2836(73)90085-5. [DOI] [PubMed] [Google Scholar]
- De B. P., Thornton G. B., Luk D., Banerjee A. K. Purified matrix protein of vesicular stomatitis virus blocks viral transcription in vitro. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7137–7141. doi: 10.1073/pnas.79.23.7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubovi E. J., Wagner R. R. Spatial relationships of the proteins of vesicular stomatitis virus: induction of reversible oligomers by cleavable protein cross-linkers and oxidation. J Virol. 1977 May;22(2):500–509. doi: 10.1128/jvi.22.2.500-509.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faaberg K. S., Peeples M. E. Association of soluble matrix protein of Newcastle disease virus with liposomes is independent of ionic conditions. Virology. 1988 Sep;166(1):123–132. doi: 10.1016/0042-6822(88)90153-5. [DOI] [PubMed] [Google Scholar]
- Fuerst T. R., Niles E. G., Studier F. W., Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki Y., Hubbard A. L., Fowler S., Lazarow P. B. Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J Cell Biol. 1982 Apr;93(1):97–102. doi: 10.1083/jcb.93.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoriades A. Interaction of influenza M protein with viral lipid and phosphatidylcholine vesicles. J Virol. 1980 Nov;36(2):470–479. doi: 10.1128/jvi.36.2.470-479.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua Q. X., Kochoyan M., Weiss M. A. Structure and dynamics of des-pentapeptide-insulin in solution: the molten-globule hypothesis. Proc Natl Acad Sci U S A. 1992 Mar 15;89(6):2379–2383. doi: 10.1073/pnas.89.6.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs B. L., Penhoet E. E. Assembly of vesicular stomatitis virus: distribution of the glycoprotein on the surface of infected cells. J Virol. 1982 Dec;44(3):1047–1055. doi: 10.1128/jvi.44.3.1047-1055.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S. R., Lazzarini R. A. The relationship between autointerference and the replication of defective interfering particle. Virology. 1977 Mar;77(1):189–201. doi: 10.1016/0042-6822(77)90417-2. [DOI] [PubMed] [Google Scholar]
- Knipe D. M., Baltimore D., Lodish H. F. Maturation of viral proteins in cells infected with temperature-sensitive mutants of vesicular stomatitis virus. J Virol. 1977 Mar;21(3):1149–1158. doi: 10.1128/jvi.21.3.1149-1158.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe D. M., Baltimore D., Lodish H. F. Separate pathways of maturation of the major structural proteins of vesicular stomatitis virus. J Virol. 1977 Mar;21(3):1128–1139. doi: 10.1128/jvi.21.3.1128-1139.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lenard J., Vanderoef R. Localization of the membrane-associated region of vesicular stomatitis virus M protein at the N terminus, using the hydrophobic, photoreactive probe 125I-TID. J Virol. 1990 Jul;64(7):3486–3491. doi: 10.1128/jvi.64.7.3486-3491.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyles D. S., McKenzie M., Parce J. W. Subunit interactions of vesicular stomatitis virus envelope glycoprotein stabilized by binding to viral matrix protein. J Virol. 1992 Jan;66(1):349–358. doi: 10.1128/jvi.66.1.349-358.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancarella D. A., Lenard J. Interactions of wild-type and mutant M protein of vesicular stomatitis virus with viral nucleocapsid and envelope in intact virions. Evidence from [125I]iodonaphthyl azide labeling and specific cross-linking. Biochemistry. 1981 Nov 24;20(24):6872–6877. doi: 10.1021/bi00527a020. [DOI] [PubMed] [Google Scholar]
- McCreedy B. J., Jr, Lyles D. S. Distribution of M protein and nucleocapsid protein of vesicular stomatitis virus in infected cell plasma membranes. Virus Res. 1989 Nov;14(3):189–205. doi: 10.1016/0168-1702(89)90001-4. [DOI] [PubMed] [Google Scholar]
- McCreedy B. J., Jr, McKinnon K. P., Lyles D. S. Solubility of vesicular stomatitis virus M protein in the cytosol of infected cells or isolated from virions. J Virol. 1990 Feb;64(2):902–906. doi: 10.1128/jvi.64.2.902-906.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metsikkö K., Simons K. The budding mechanism of spikeless vesicular stomatitis virus particles. EMBO J. 1986 Aug;5(8):1913–1920. doi: 10.1002/j.1460-2075.1986.tb04444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison T. G., McQuain C. O. Assembly of viral membranes: nature of the association of vesicular stomatitis virus proteins to membranes. J Virol. 1978 Apr;26(1):115–125. doi: 10.1128/jvi.26.1.115-125.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudd J. A., Swanson R. E. In situ cross-linking of vesicular stomatitis virus proteins with reversible agents. Virology. 1978 Jul 15;88(2):263–280. doi: 10.1016/0042-6822(78)90284-2. [DOI] [PubMed] [Google Scholar]
- Newcomb W. W., Brown J. C. Role of the vesicular stomatitis virus matrix protein in maintaining the viral nucleocapsid in the condensed form found in native virions. J Virol. 1981 Jul;39(1):295–299. doi: 10.1128/jvi.39.1.295-299.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb W. W., Tobin G. J., McGowan J. J., Brown J. C. In vitro reassembly of vesicular stomatitis virus skeletons. J Virol. 1982 Mar;41(3):1055–1062. doi: 10.1128/jvi.41.3.1055-1062.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odenwald W. F., Arnheiter H., Dubois-Dalcq M., Lazzarini R. A. Stereo images of vesicular stomatitis virus assembly. J Virol. 1986 Mar;57(3):922–932. doi: 10.1128/jvi.57.3.922-932.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden J. R., Pal R., Wagner R. R. Mapping regions of the matrix protein of vesicular stomatitis virus which bind to ribonucleocapsids, liposomes, and monoclonal antibodies. J Virol. 1986 Jun;58(3):860–868. doi: 10.1128/jvi.58.3.860-868.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K., Dubois-Dalcq M. E., Schubert M., Lazzarini R. A. A mutated membrane protein of vesicular stomatitis virus has an abnormal distribution within the infected cell and causes defective budding. J Virol. 1987 May;61(5):1332–1341. doi: 10.1128/jvi.61.5.1332-1341.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidler J. A., Keller P. M., Elson E. L., Lenard J. A fluorescence photobleaching study of vesicular stomatitis virus infected BHK cells. Modulation of G protein mobility by M protein. Biochemistry. 1981 Mar 3;20(5):1345–1349. doi: 10.1021/bi00508a047. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Bergmann J. E. Altered cytoplasmic domains affect intracellular transport of the vesicular stomatitis virus glycoprotein. Cell. 1983 Sep;34(2):513–524. doi: 10.1016/0092-8674(83)90384-7. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Gallione C. J. Nucleotide sequences of the mRNA's encoding the vesicular stomatitis virus G and M proteins determined from cDNA clones containing the complete coding regions. J Virol. 1981 Aug;39(2):519–528. doi: 10.1128/jvi.39.2.519-528.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague J., Condra J. H., Arnheiter H., Lazzarini R. A. Expression of a recombinant DNA gene coding for the vesicular stomatitis virus nucleocapsid protein. J Virol. 1983 Feb;45(2):773–781. doi: 10.1128/jvi.45.2.773-781.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stigter D., Alonso D. O., Dill K. A. Protein stability: electrostatics and compact denatured states. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4176–4180. doi: 10.1073/pnas.88.10.4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D., Newcomb W. W., Brown J. C., Wall J. S., Hainfeld J. F., Trus B. L., Steven A. C. Mass and molecular composition of vesicular stomatitis virus: a scanning transmission electron microscopy analysis. J Virol. 1985 May;54(2):598–607. doi: 10.1128/jvi.54.2.598-607.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. A., Bennett P. L. Assembly of membrane glycoproteins studied by phenotypic mixing between mutants of vesicular stomatitis virus and retroviruses. Virology. 1980 Jan 30;100(2):252–274. doi: 10.1016/0042-6822(80)90518-8. [DOI] [PubMed] [Google Scholar]
- Whitt M. A., Chong L., Rose J. K. Glycoprotein cytoplasmic domain sequences required for rescue of a vesicular stomatitis virus glycoprotein mutant. J Virol. 1989 Sep;63(9):3569–3578. doi: 10.1128/jvi.63.9.3569-3578.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener J. R., Pal R., Barenholz Y., Wagner R. R. Effect of the vesicular stomatitis virus matrix protein on the lateral organization of lipid bilayers containing phosphatidylglycerol: use of fluorescent phospholipid analogues. Biochemistry. 1985 Dec 17;24(26):7651–7658. doi: 10.1021/bi00347a023. [DOI] [PubMed] [Google Scholar]
- Wiener J. R., Pal R., Barenholz Y., Wagner R. R. Influence of the peripheral matrix protein of vesicular stomatitis virus on the membrane dynamics of mixed phospholipid vesicles: fluorescence studies. Biochemistry. 1983 Apr 26;22(9):2162–2170. doi: 10.1021/bi00278a017. [DOI] [PubMed] [Google Scholar]
- Wilson T., Lenard J. Interaction of wild-type and mutant M protein vesicular stomatitis virus with nucleocapsids in vitro. Biochemistry. 1981 Mar 3;20(5):1349–1354. doi: 10.1021/bi00508a048. [DOI] [PubMed] [Google Scholar]
- Zakowski J. J., Petri W. A., Jr, Wagner R. R. Role of matrix protein in assembling the membrane of vesicular stomatitis virus: reconstitution of matrix protein with negatively charged phospholipid vesicles. Biochemistry. 1981 Jun 23;20(13):3902–3907. doi: 10.1021/bi00516a037. [DOI] [PubMed] [Google Scholar]
- Zakowski J. J., Wagner R. R. Localization of membrane-associated proteins in vesicular stomatitis virus by use of hydrophobic membrane probes and cross-linking reagents. J Virol. 1980 Oct;36(1):93–102. doi: 10.1128/jvi.36.1.93-102.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Goot F. G., González-Mañas J. M., Lakey J. H., Pattus F. A 'molten-globule' membrane-insertion intermediate of the pore-forming domain of colicin A. Nature. 1991 Dec 5;354(6352):408–410. doi: 10.1038/354408a0. [DOI] [PubMed] [Google Scholar]