Abstract
Initial rates of ATP hydrolysis by the chaperonin containing TCP-1 (CCT) from bovine testis were measured as a function of ATP concentration. Two allosteric transitions are observed: one at relatively low concentrations of ATP (<100 μM) and the second at higher concentrations of ATP. The data suggest that CCT has positive intra-ring cooperativity and negative inter-ring cooperativity in ATP hydrolysis, with respect to ATP, as previously observed in the case of GroEL. It is shown that the relatively weak positive intra-ring cooperativity found in the case of CCT may be due to heterogeneity in its subunit composition. Our results suggest that nested allosteric behavior may be common to chaperone double-ring systems.
Keywords: Protein folding, chaperonins, CCT, cooperativity, allostery
Molecular chaperones are required for protein folding, transport, and degradation in the cell. An essential family of molecular chaperones, the chaperonins, can be divided into two types: type I is found in eubacteria, mitochondria, and chloroplasts (Ranson et al. 1998; Sigler et al. 1998), and type II is found in archaea and the eukaryotic cytosol (Gutsche et al. 1999). Chaperonins are oligomeric proteins that consist of two rings, stacked back to back, with a cavity at each end (Ranson et al. 1998; Sigler et al. 1998; Gutsche et al. 1999). The extent of symmetry in their structure depends on the number and composition of subunits in each ring. Type I chaperonins, such as GroEL from Escherichia coli and mitochondrial hsp60, consist of 14 identical subunits that form two heptameric rings (Ranson et al. 1998; Sigler et al. 1998). Type II chaperonins consist of two eight- or nine-membered rings that are made up of two types of subunits in the case of the archaeal thermosome or eight different subunits in the case of the cytoplasmic eukaryotic chaperonin containing TCP-1 (CCT) (Gutsche et al. 1999). Cross-linking studies have shown that the eight different subunits of CCT rings are arranged in a fixed permutation (Liou and Willison 1997). The crystal structures of GroEL (Braig et al. 1994) and the thermosome from Thermoplasma acidophilum (Ditzel et al. 1998) show that they share a similar domain arrangement. Each subunit consists of three domains: (1) an equatorial domain that contains an ATP-binding site, (2) an apical domain that forms the opening of the central cavity, and (3) an intermediate domain that connects the apical and equatorial domains.
Type I chaperonins, as exemplified by GroEL, are known to mediate the in vivo folding of a large number of different proteins (Houry et al. 1999), whereas CCT is thought to be involved mainly, or perhaps exclusively, in the folding of actin and tubulin (Gao et al. 1992; Yaffe et al. 1992). Both type I and type II chaperonin-mediated protein folding are MgATP dependent (Ranson et al. 1998; Sigler et al. 1998; Gutsche et al. 1999). ATP binding and hydrolysis switch CCT (Melki and Cowan 1994) and GroEL (Staniforth et al. 1994; Yifrach and Horovitz 1996) rings between different conformations with either low or high affinity for unfolded polypeptide substrates. The ATP-induced conformational changes of GroEL (Roseman et al. 1996) are reflected in binding of ATP with positive cooperativity within rings (Gray and Fersht 1991; Bochkareva et al. 1992; Jackson et al. 1993) and negative cooperativity between rings (Yifrach and Horovitz 1994, 1995). ATP-induced conformational changes of CCT have been monitored by changes in fluorescence (Melki et al. 1997); more recently, they have been visualized at 28 Å resolution by electron cryo-microscopy and single-particle reconstruction (Llorca et al. 1999). Binding of ATP to CCT was found to generate an asymmetric particle in which one ring has a slightly different conformation from the apo-ring and the other ring has undergone substantial movements in the apical and equatorial domains (Llorca et al. 1999). The structural data for the ATP-induced conformational changes in CCT and the similarities between CCT and GroEL suggest that ATP binding to CCT may also be cooperative. Kinetic evidence for cooperativity in ATP binding/hydrolysis by CCT has, however, not been reported. Here, we show that CCT undergoes two ATP-induced allosteric transitions. The data are consistent with the presence of positive intra-ring cooperativity and negative inter-ring cooperativity in ATP hydrolysis by CCT. The results are discussed in the context of the allosteric mechanism of GroEL.
Results and discussion
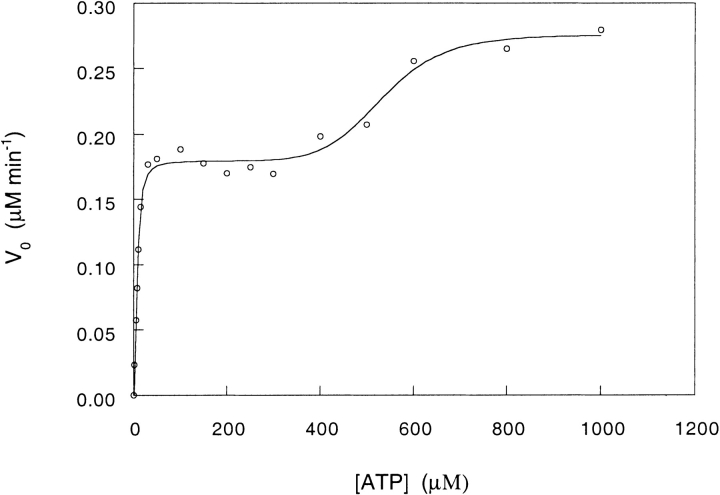
Initial rates of ATP hydrolysis by CCT were measured at different concentrations of ATP from 0 to 1 mM. Two allosteric transitions were observed: one at relatively low ATP concentrations (<100 μM) and the second at higher concentrations of ATP (Fig. 1 ▶). The allosteric transitions observed most likely reflect ATP-induced conformational changes in CCT and not its assembly or disassembly (Roobol et al. 1999; Dobrzynski et al. 2000). In the analysis here, we assume that the two transitions correspond to the ATP-induced allosteric switch of one ring and then the other ring of CCT. The data were fitted to a Hill-type equation for two sequential allosteric transitions. The values of the apparent ATP-binding constants of the first and second rings, K1 and K2, are found to be 7.6 (± 0.4) and 533 (± 22) μM, respectively. The two orders of magnitude difference in the affinity of the first and second rings for ATP are consistent with negative cooperativity between rings, as observed in the case of GroEL (Yifrach and Horovitz 1994, 1995). Previous estimates of the values of ATP-binding constants of type II chaperonins range from 5.6 μM for the Pyrodictium occultum (Phipps et al. 1991) and the Pyrococcus kodakaraensis KOD1 (Yan et al. 1997) thermosomes to 115 and 150 μM for TRiC (Martin and Cruetz 1990) and the Methanopyrus kandleri thermosome (Andra et al. 1998), respectively. In these earlier studies, no distinction was made between two classes of ATP-binding sites. The values of kcat for ATP hydrolysis by one ring and by both rings of CCT are 0.0119 (± 0.0002) sec−1 and 0.0183 (± 0.0005) sec−1, respectively. CCT is therefore a considerably more sluggish ATPase than GroEL, which has values of kcat for ATP hydrolysis of 0.40 and 0.27 sec−1 by one ring and both rings, respectively (Yifrach and Horovitz 1995). The value of Vmax for ATP hydrolysis by both rings of CCT is nearly double the value of Vmax for ATP hydrolysis by one ring, thus indicating that in contrast with GroEL (Yifrach and Horovitz 1995), inter-ring communication in CCT has little effect on the rate of ATP hydrolysis.
Fig. 1.
Initial velocity of ATP hydrolysis by CCT at different ATP concentrations. The data were fitted to equation 4. The oligomer concentration of CCT is 250 nM.
The value of the Hill coefficient n for the first allosteric transition of CCT, in the presence of 50 mM K+ ions, is found to be 2.00 (±0.25), which is lower than the value of 2.41 (± 0.13) determined for GroEL under the same conditions (Yifrach and Horovitz 1996). These values of the Hill coefficient are relatively low for assemblies of seven or eight subunits. The value of the Hill coefficient m for the second allosteric transition of CCT, in the presence of 50 mM K+ ions, is found to be 8.52 (± 2.78). This value is close to the upper limit value of the Hill coefficient, which is equal to the total number of ATP-binding sites in a ring. In contrast, the value of the Hill coefficient for the second allosteric transition of GroEL, in the presence of 50 mM K+ ions, was previously found to be 2.54 (± 0.56), which is considerably lower (O. Yifrach and A. Horovitz, unpubl.). The much larger value of m relative to n reflects negative cooperativity between rings, which seems to be much stronger in CCT than in GroEL. This finding is in agreement with the electron cryo-microscopy observation of an ATP-bound symmetric conformation of GroEL (White et al. 1997) but not CCT (Llorca et al. 1999) in the presence of a high ATP concentration.
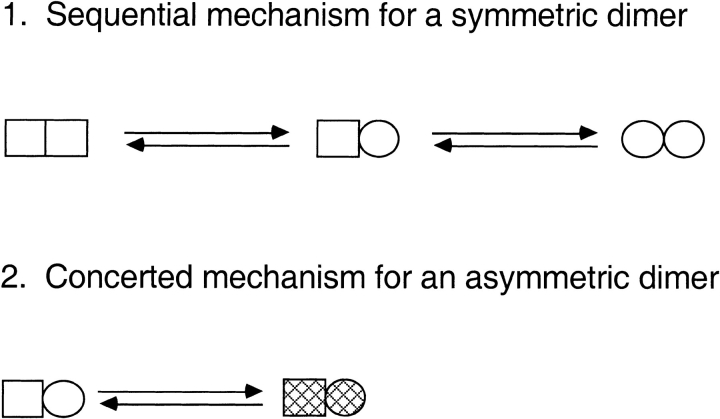
Recently, we showed that one possible reason for the relatively low value of the Hill coefficient of GroEL is that the observed rate of protein folding is slower when intra-ring positive cooperativity with respect to ATP is stronger (Yifrach and Horovitz 2000). In the case of CCT, a second reason for the relatively low value of the Hill coefficient for the first transition may be differences in the intrinsic affinities for ATP of the different subunits in a ring. To explain this, let us consider, for simplicity, a heterodimer with low- and high-affinity sites (K1 > K2) for a substrate (ATP) that is in equilibrium between T and R states (L = [T] / [R]) (Fig. 2 ▶.2). In the R state, both sites have relatively high affinity for substrate in comparison with the T state (K1R > K1T and K2R > K2T). In the case of this model, the Hill coefficient at 50% saturation (for exclusive binding to the R state) is given by
Fig. 2.
Models for conformational changes in CCT. Two models for conformational changes that may account for intra-ring positive cooperativity in CCT are shown, for simplicity, for a dimer. (1) The subunits of an unliganded symmetric dimer undergo sequential ATP-induced transitions from a low-affinity (square) to a high-affinity (circle) state; (2) the subunits of an unliganded asymmetric dimer with low-affinity (square) and high-affinity (circle) sites undergo a concerted ATP-induced transition from a low-affinity (empty) to a high-affinity (hatched) state. The models can be extended for the eight sites in CCT rings.
 |
1 |
Two extreme cases of this model are (1) a heterodimer with low- and high-affinity sites for a substrate that is found predominantly in one state and (2) a homodimer with identical sites that is in equilibrium between T and R states. In the first case (L → 0), the Hill coefficient at 50% saturation is given by the following equation (Levitzki 1978):
 |
2 |
The value of the Hill coefficient n50 is <1 when K1 > K2, thus reflecting the fact that site heterogeneity cannot be distinguished from negative cooperativity. In the second case (K1R = K2R), the Hill coefficient at 50% saturation (for exclusive binding to the R state) is given by the following:
 |
3 |
In this case, the value of n50 is between 1 and 2, and negative cooperativity is never observed. The above analysis shows, therefore, that positive cooperativity in ligand binding which results from a shift in equilibrium from a low- to a high-affinity state of the protein can be partially or fully masked by site heterogeneity. That may explain why CCT with eight ATP-binding sites per ring has a Hill coefficient with a value even lower than that of GroEL with seven sites per ring. It may also explain why slight negative cooperativity is observed in the case of the archaeal chaperonin (Gutsche et al. 2000).
In conclusion, our results indicate that there is positive intra-ring cooperativity and negative inter-ring cooperativity in ATP hydrolysis by CCT, as previously found for GroEL (Yifrach and Horovitz 1994, 1995). It was suggested that positive intra-ring cooperativity in GroEL is the result of an equilibrium of each ring between a tense T state with low affinity for ATP and high affinity for nonfolded proteins and a relaxed R state with high affinity for ATP and low affinity for nonfolded proteins (Yifrach and Horovitz 1995Yifrach and Horovitz 1996), in accordance with the Monod-Wyman-Changeux model (Monod et al. 1965). Evidence supporting the concerted nature of the intra-ring transitions in GroEL has recently been obtained (Ma and Karplus 1998; Yifrach and Horovitz 1998; Horovitz and Yifrach 2000). In the case of CCT, it has been suggested (Lin and Sherman 1997) on the basis of genetic analysis that the allosteric transition of each ring takes place in a sequential manner, in accordance with the Koshland-Némethy-Filmer (KNF) model (Koshland et al. 1966). The interaction between rings in both GroEL and CCT requires invoking the KNF model because of the presence of inter-ring negative cooperativity. Cooperativity in CCT can therefore be described by a model in which KNF-type transitions within a ring are nested inside inter-ring KNF-type transitions (Fig. 2.1 ▶). Alternatively, the intra-ring cooperativity in CCT may reflect the combined effect of a concerted structural change and site heterogeneity (Fig. 2.2 ▶), as shown above. Our results suggest that nested allosteric behavior may be common to chaperone double-ring systems.
Materials and methods
Materials
[γ-32P]ATP was purchased from Amersham; all other reagents were purchased from Sigma. CCT was purified from tubules of bovine testis (10 g) that were separated from the tunica albuginea by dissection and added to 4–8 mL of buffer A (100 mM triethanolamine at pH 7.4, 5 mM MgCl2, and 5 mM β-mercaptoethanol) containing 5 μg/mL chymostatin, 10 μg/mL leupeptin, 5 μg/mL antipain, 5 μg/mL pepstatin, 8.2 TIU aprotinin. The tubules were homogenized and centrifuged at 14,000 rpm (SA-600 rotor, Sorvall) for 30 min at 4°C. Supernatant was removed and ultracentrifuged at 54,000 rpm (Ti-60 rotor, Beckman) for 1 h at 4°C. The supernatant was separated on a 10%–40% sucrose gradient in 100 mM triethanolamine buffer (pH 7.4) containing 5 mM MgCl2 at 24,000 rpm (SW-28 rotor, Beckman) for 17 h at 4°C. Fractions were collected, starting at the bottom of the tube, and analyzed by Western blotting using rabbit polyclonal anti-CCT serum UM1, as previously described (Hynes et al. 1995). CCT-containing fractions were combined and incubated at room temperature for 10 min with 1 mM ATP and 10 mM KCl and then loaded on a Mono Q HR 5/5 column after twofold dilution with buffer A. CCT was eluted using a 20-mL gradient of 0–0.6 M NaCl in buffer A containing 2 mM β-mercaptoethanol (buffer B). It was found to elute at 0.1–0.3 NaCl. CCT-containing fractions were combined and loaded on a 5-mL HiTrap heparin column (Pharmacia) equilibrated with buffer A. CCT was eluted using a 100-mL gradient of 0–0.8 M NaCl in buffer B. It was found to elute at 0.4–0.6 NaCl. Pure CCT fractions were combined, concentrated by Centriprep-30, and desalted using a PD-10 Sephadex column equilibrated with buffer A. Pure CCT was flash-frozen in liquid N2 and stored at −80°C in aliquots. The concentration of the purified CCT was determined using amino acid analysis.
ATPase assays
The ATPase activity of CCT was measured as described (Horovitz et al. 1993). The reactions were started by mixing 10 μL of different concentrations of [γ-32P]ATP with a 40-μL solution containing 300 μg/mL CCT in 50 mM Tris-HCl buffer (pH 7.5) containing 10 mM MgCl2, 50 mM KCl, 1 mM dithiothreitol. Both solutions were incubated for 7 min at 25°C before mixing. The reactions were performed at 25°C and terminated after 1, 2, 3, and 4 min (for [ATP] ≤15 μM) or 2, 4, 6 and 8 min (for [ATP] >15 μM) by removing a 10-μL aliquot to 70 μL of pre-cooled stop solution containing 1 M perchloric acid and 1 mM KH2PO4.
Data analysis
Analysis of cooperativity in ATP hydrolysis by CCT was performed by directly fitting data of initial ATPase velocities at different ATP concentrations by using Kaleidagraph (version 2.1 Synergy Software [PCS] Inc.) to
 |
4 |
where V0 is the observed initial rate of ATP hydrolysis, [S] is the substrate (ATP) concentration, Vmax(1) and Vmax(2) are the respective maximal initial rates of ATP hydrolysis by a single ring and by both rings of CCT, n and m are the respective Hill coefficients for ATP binding to the first and second rings, and K1 and K2 are the respective apparent binding constants of ATP for the first and second rings.
Acknowledgments
This work was supported by the Israel Science Foundation, administered by The Israel Academy of Sciences and Humanities; by the MINERVA Foundation, Germany; and by the Asher and Jeannette Alhadeff research award. A.H. is an incumbent of the Carl and Dorothy Bennett Professorial Chair in Biochemistry.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked "advertisement" in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at www.proteinscience.org/cgi/doi/10.1110/ps.44401
References
- Andra, S., Frey, G., Jaenicke, R., and Stetter, K.O. 1998. The thermosome from Methanopyrus kandleri possesses an NH4+-dependent ATPase activity. Eur. J. Biochem. 255 93–99. [DOI] [PubMed] [Google Scholar]
- Bochkareva, E.S., Lissin, N.M., Flynn, G.C., Rothman, J.E., and Girshovich, A.S. 1992. Positive cooperativity in the functioning of molecular chaperone GroEL. J. Biol. Chem. 267 6796–6800. [PubMed] [Google Scholar]
- Braig, K., Otwinowski, Z., Hegde, R., Boisvert, D.C., Joachimiak, A., Horwich, A.L., and Sigler, P.B. 1994. The crystal structure of the bacterial chaperonin GroEL at 2.8 Å. Nature 371 578–586. [DOI] [PubMed] [Google Scholar]
- Ditzel, L., Lowe, J., Stock, D., Stetter, K.O., Huber, H., Huber, R., and Steinbacher, S. 1998. Crystal structure of the thermosome, the archaeal chaperonin and homolog of CCT. Cell 93 125–138. [DOI] [PubMed] [Google Scholar]
- Dobrzynski, J.K., Sternlicht, M.L., Peng, I., Farr, G.W., and Sternlicht, H. 2000. Evidence that β-tubulin induces a conformation change in the cytosolic chaperonin which stabilizes binding: Implications for the mechanism of action. Biochemistry 39 3988–4003. [DOI] [PubMed] [Google Scholar]
- Gao, Y., Thomas, J.O., Chow, R.L., Lee, G.H., and Cowan, N.J. 1992. A cytoplasmic chaperonin that catalyzes β-actin folding. Cell 69 1043–1050. [DOI] [PubMed] [Google Scholar]
- Gray, T.E and Fersht, A.R. 1991. Cooperativity in ATP hydrolysis by GroEL is increased by GroES. FEBS Lett. 292 254–258. [DOI] [PubMed] [Google Scholar]
- Gutsche, I., Essen, L.O., and Baumeister, W. 1999. Group II chaperonins: New TRic(k)s and turns of a protein folding machine. J. Mol. Biol. 293 295–312. [DOI] [PubMed] [Google Scholar]
- Gutsche, I., Mihalache, O., and Baumeister, W. 2000. ATPase cycle of an archaeal chaperonin. J. Mol. Biol. 300 187–196. [DOI] [PubMed] [Google Scholar]
- Horovitz, A. and Yifrach, O. 2000. On the relationship between the Hill coefficients for steady-state and transient kinetic data: A criterion for concerted transitions in allosteric proteins. Bull. Math. Biol. 62 241–246. [DOI] [PubMed] [Google Scholar]
- Horovitz, A., Bochkareva, E.S., Kovalenko, O., and Girshovich, A.S. 1993. Mutation Ala2→Ser destabilizes intersubunit interactions in the molecular chaperone GroEL. J. Mol. Biol. 231 58–64. [DOI] [PubMed] [Google Scholar]
- Houry, W.A., Frishman, D., Eckerskorn, C., Lottspeich, F., and Hartl, F.U. 1999. Identification of in vivo substrates of the chaperonin GroEL. Nature 402 147–154. [DOI] [PubMed] [Google Scholar]
- Hynes, G., Kubota, H., and Willison, K.R. 1995. Antibody characterisation of two distinct conformations of the chaperonin-containing TCP-1 from mouse testis. FEBS Lett. 358 129–132. [DOI] [PubMed] [Google Scholar]
- Jackson, G.S., Staniforth, R.A., Halsall, D.J., Atkinson, T., Holbrook, J.J., Clarke, A.R., and Burston, S.G. 1993. Binding and hydrolysis of nucleotides in the chaperonin catalytic cycle: Implications for the mechanism of assisted protein folding. Biochemistry 32 2554–2563. [DOI] [PubMed] [Google Scholar]
- Koshland, D.E., Jr., Némethy, G., and Filmer, D. 1966. Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry 5 365–385. [DOI] [PubMed] [Google Scholar]
- Levitzki, A. 1978. Quantitative aspects of allosteric mechanisms. Springer-Verlag, Berlin. [DOI] [PubMed]
- Lin, P. and Sherman, F. 1997. The unique hetero-oligomeric nature of the subunits in the catalytic cooperativity of the yeast Cct chaperonin complex. Proc. Natl. Acad. Sci. 94 10780–10785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou, A.K.F and Willison, K.R. 1997. Elucidation of the subunit orientation in CCT (chaperonin containing TCP1) from the subunit composition of CCT micro-complexes. EMBO J. 16 4311–4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorca, O., Smyth, M.G., Carrascosa, J.L., Willison, K.R., Radermacher, M., Steinbacher, S., and Valpuesta, J.M. 1999. 3D reconstruction of the ATP-bound form of CCT reveals the asymmetric folding conformation of a type II chaperonin. Nat. Struct. Biol. 6 639–642. [DOI] [PubMed] [Google Scholar]
- Ma, J. and Karplus, M. 1998. The allosteric mechanism of the chaperonin GroEL: A dynamic analysis. Proc. Natl. Acad. Sci. 95 8502–8507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, W.H. and Cruetz, C.E. 1990. Interactions of the complex secretory vesicle binding protein chromobindin A with nucleotides. J. Neurochem. 54 612–619. [DOI] [PubMed] [Google Scholar]
- Melki, R. and Cowan, N.J. 1994. Facilitated folding of actins and tubulins occurs via a nucleotide-dependent interaction between cytoplasmic chaperonin and distinctive folding intermediates. Mol. Cell. Biol. 14 2895–2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melki, R., Batelier, G., Soulié, S., and Williams, R.C., Jr. 1997. Cytoplasmic chaperonin containing TCP-1: Structural and functional characterization. Biochemistry 36 5817–5826. [DOI] [PubMed] [Google Scholar]
- Monod, J., Wyman, J., and Changeux, J.P. 1965. On the nature of allosteric transitions: A plausible model. J. Mol. Biol. 12 88–118. [DOI] [PubMed] [Google Scholar]
- Phipps, B.M., Hoffmann, A., Stetter, K.O., and Baumeister, W. 1991. A novel ATPase complex selectively accumulated upon heat shock is a major cellular component of thermophilic archaebacteria. EMBO J. 10 1711–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranson, N.A., White, H.E., and Saibil, H.R. 1998. Chaperonins. Biochem. J. 333 233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roobol, A., Grantham, J., Whitaker, H.C., and Carden, M.J. 1999. Disassembly of the cytosolic chaperonin in mammalian cell extracts at intracellular levels of K+ and ATP. J. Biol. Chem. 274 19220–19227. [DOI] [PubMed] [Google Scholar]
- Roseman, A.M., Chen, S., White, H., Braig, K., and Saibil, H.R. 1996. The chaperonin ATPase cycle: Mechanism of allosteric switching and movements of substrate-binding domains in GroEL. Cell 87 241–251. [DOI] [PubMed] [Google Scholar]
- Sigler, P.B., Xu, Z., Rye, H.S., Burston, S.G., Fenton, W.A., and Horwich, A.L. 1998. Structure and function in GroEL-mediated protein folding. Annu. Rev. Biochem. 67 581–608. [DOI] [PubMed] [Google Scholar]
- Staniforth, R.A., Burston, S.G., Atkinson, T., and Clarke, A.R. 1994. Affinity of chaperonin-60 for a protein substrate and its modulation by nucleotides and chaperonin-10. Biochem. J. 300 651–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, H.E., Chen, S., Roseman, A.M., Yifrach, O., Horovitz, A., and Saibil, H.R. 1997. Structural basis of allosteric changes in the GroEL mutant Arg197→Ala. Nat. Struct. Biol. 4 690–694. [DOI] [PubMed] [Google Scholar]
- Yaffe, M.B., Farr, G.W., Miklos, D., Horwich, A.L., Sternlicht, M.L., and Sternlicht, H. 1992. TCP1 complex is a molecular chaperone in tubulin biogenesis. Nature 358 245–248. [DOI] [PubMed] [Google Scholar]
- Yan, Z., Fujiwara, S., Kohda, K., Takagai, M., and Imanaka, T. 1997. In vitro stabilization and in vivo solubilization of foreign proteins by the β subunit of a chaperonin from the hyperthermophilic archaeon Pyrococcus sp. strain KOD1. Appl. Environ. Microbiol. 63 785–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yifrach, O. and Horovitz, A. 1994. Two lines of allosteric communication in the oligomeric chaperonin GroEL are revealed by the single mutation Arg196→Ala. J. Mol. Biol. 243 397–401. [DOI] [PubMed] [Google Scholar]
- Yifrach, O. and Horovitz, A. 1995. Nested cooperativity in the ATPase activity of the oligomeric chaperonin GroEL. Biochemistry 34 5303–5308. [DOI] [PubMed] [Google Scholar]
- Yifrach, O. and Horovitz, A. 1996. Allosteric control by ATP of non-folded protein binding to GroEL. J. Mol. Biol. 255 356–361. [DOI] [PubMed] [Google Scholar]
- Yifrach, O. and Horovitz, A. 1998. Mapping the transition state of the allosteric pathway of GroEL by protein engineering. J. Amer. Chem. Soc. 120 13262–13263. [Google Scholar]
- Yifrach, O. and Horovitz, A. 2000. Coupling between protein folding and allostery in the GroE chaperonin system. Proc. Natl. Acad. Sci. 97 1521–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]