Abstract
Sorcin is a 22 kD calcium-binding protein that is found in a wide variety of cell types, such as heart, muscle, brain and adrenal medulla. It belongs to the penta-EF-hand (PEF) protein family, which contains five EF-hand motifs that associate with membranes in a calcium-dependent manner. Prototypic members of this family are the calcium-binding domains of calpain, such as calpain dVI. Full-length human sorcin has been crystallized in the absence of calcium and the structure determined at 2.2 Å resolution. Apart from an extended N-terminal portion, the sorcin molecule has a globular shape. The C-terminal domain is predominantly α-helical, containing eight α-helices and connecting loops incorporating five EF hands. Sorcin forms dimers through the association of the unpaired EF5, confirming this as the mode of association in the dimerization of PEF proteins. Comparison with calpain dVI reveals that the general folds of the individual EF-hand motifs are conserved, especially that of EF1, the novel EF-hand motif characteristic of the family. Detailed structural comparisons of sorcin with other members of PEF indicate that the EF-hand pair EF1–EF2 is likely to correspond to the two physiologically relevant calcium-binding sites and that the calcium-induced conformational change may be modest and localized within this pair of EF-hands. Overall, the results derived from the structural observations support the view that, in sorcin, calcium signaling takes place through the first pair of EF-hands.
Keywords: Calcium-binding proteins, crystal structure, penta-EF-hand, PEF, sorcin, X-ray
Sorcin (soluble resistance-related calcium-binding protein) is a 22 kDa member of the penta-EF-hand family (PEF) of calcium-binding proteins (Maki et al. 1997). The best-characterized function of sorcin is its association with the cardiac ryanodine receptor (RyR; Meyers et al. 1995a). In response to the small calcium release triggered by voltage-controlled L-type calcium channels, RyRs mediate release of larger amounts of calcium from the sarcoplasmic reticulum ("calcium-induced calcium release") and trigger muscle contraction (Valdivia 1998). In the presence of micromolar concentrations of calcium, sorcin binds reversibly to RyRs and inhibits calcium release (Lokuta et al. 1997). This provides rapid attenuation of the RyR response and prevents excessive depletion of calcium stores. Other targets of sorcin have been identified as L-type calcium channels in cardiac and skeletal muscle (Meyers et al., 1998), annexin VII in adrenal medulla (Brownawell and Creutz 1997), and presenilin 2 in brain (Pack-Chung et al. 2000). As sorcin is found in a wide variety of cell types, it is likely to have other functions in addition to its role in cardiac muscle contraction.
Sorcin can bind two calcium ions per monomer (Zamparelli et al. 1997) with high affinity (apparent Kd ∼1 μM; Meyers et al. 1995b). Sedimentation equilibrium analysis shows that sorcin forms dimers in the absence of calcium (Zamparelli et al. 2000). Addition of calcium induces aggregation (Zamparelli et al. 1997) and quenches tryptophan fluorescence (Meyers et al. 1995b), indicating that calcium binding induces a conformational change that results in the exposure of buried hydrophobic residues. This allows sorcin to translocate reversibly to membranes, including the sarcoplasmic reticulum, and induces binding to RyRs.
To investigate how calcium-induced conformational changes in sorcin regulate its function, we have determined the structure of full-length human sorcin in the absence of calcium. This is the fourth structure determined for a PEF protein. Together with the structures of calpain dVI (Blanchard et al. 1997; Lin et al. 1997), calpain dIV (Strobl et al. 2000), and grancalcin (Jia et al., 2000), it provides opportunities to compare structures across this family, both in the presence and absence of calcium, and improves our understanding of structure-function relationships in PEF proteins.
Results
The structure of calcium-free human sorcin is illustrated in Figure 1A ▶. As predicted, it forms a homodimer in which each monomer adopts the penta EF-hand motif, which was first revealed by the crystal structures of calpain dVI.
Fig. 1.
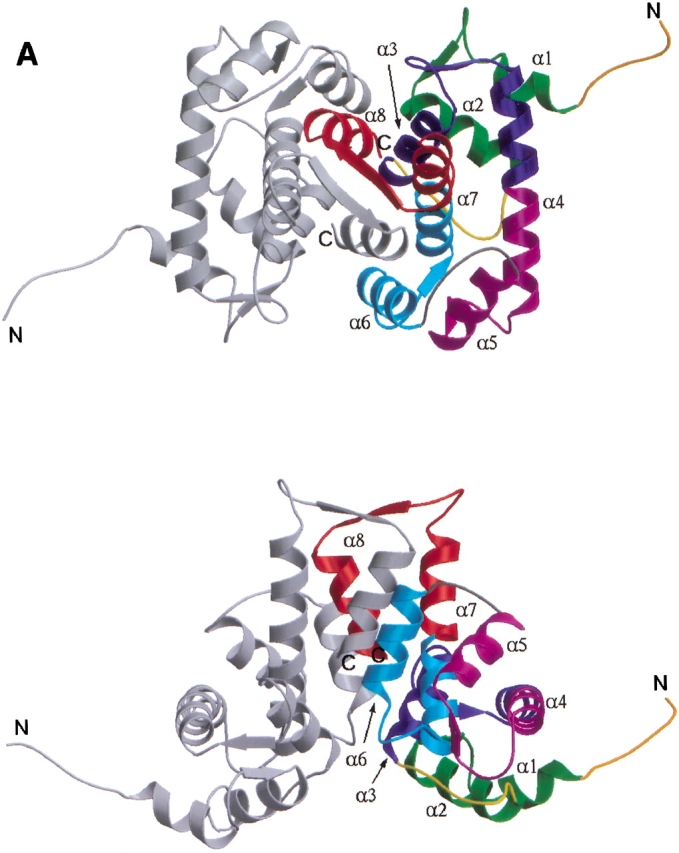
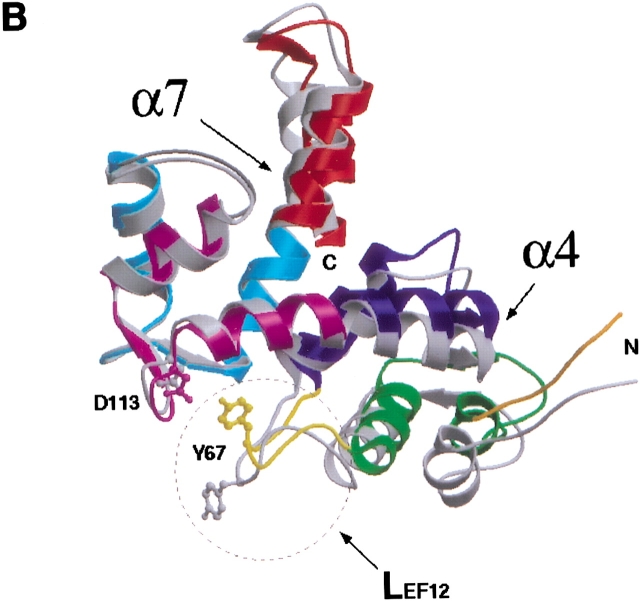
(A) Ribbon representation of dimeric sorcin shown in two views. (Top) along the symmetry axis of the dimer with the EF5 near the viewer; (bottom) viewing perpendicular to the symmetry axis. Each monomer contains helices and loops that form five EF-hands. These EF-hands associate into pairs; EF1 pairs with EF2 whereas EF3 pairs with EF4, and EF5 pairs with its counterpart from the other monomer to form part of the dimer interface. One of the monomers is shown in monchrome (gray) whereas the other is color-coded to show the sub-domain structures: EF1 (green); EF2 (blue); EF3 (magenta); EF4 (cyan); EF5 (red); LEF12, the linker connecting the adjacent EF-hands within the first pair EF1–EF2 (yellow); LEF34, the linker connecting the adjacent EF-hands within the second pair EF3–EF4 (gray); and the short N-terminal fragment (dark orange). For each EF hand, the individual helices are denoted as E (N-terminal helix) and F (C-terminal helix) with an appropriate number indicating to which EF hand it belongs, whereas the loops flanked by the E and F helices are denoted as L with the same corresponding number. A sorcin monomer contains eight helices that are numbered from α1 to α8. Correspondence between this numbering scheme and the E/F notation: α1–E1, α2–F1, α3–E2, α4–fused F2 and E3, α5–F3, α6–E4, α7–fused F4 and E5, and α8–F5. (B) Superposition of the individual monomers of sorcin after the alignment of their EF3–EF4 sub-domains. The view is related by ∼90° rotation about the vertical axis to the bottom panel of Figure 1A ▶. The molecules are color-coded as in A. A circle is shown around LEF12 to highlight the structural difference between two individual monomers in this region. The side-chains of residues Tyr 67 and Asp 113 are shown in ball-and-stick representations. This figure was produced using the programs MOLSCRIPT (Kraulis 1991) and Raster3D (Merrit and Bacon 1997).
Overall fold of the monomer
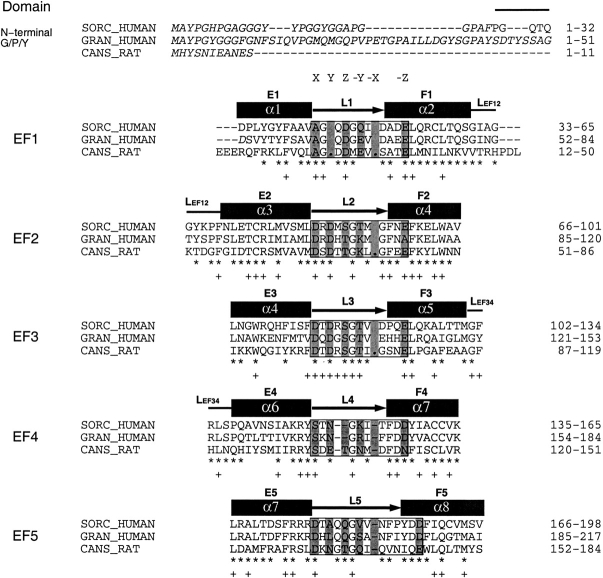
Sorcin is 30% identical in amino acid sequence to calpain dVI and 60% to grancalcin (Fig. 2 ▶). As expected, the overall fold of the sorcin monomer resembles closely that of calpain dVI and grancalcin. Apart from the extended N-terminal portion (residues 28–32), the sorcin monomer has a globular shape. The C-terminal domain (residues 33–198) contains the helices and connecting loops that form five EF hands. Of the eight helices (α1–α8) in each monomer, two of the six-turn helices, α4 and α7, contribute to two consecutive EF-hands. In a typical manner, the EF-hands associate into pairs through a short two-stranded β-sheet arrangement such that EF1 pairs with EF2 and EF3 pairs with EF4. The fifth motif, EF5, is unpaired in the monomer, but pairs with EF5 of the second monomer forming part of the dimer interface.
Fig. 2.
Structure-based sequence alignment of human sorcin (SORC_HUMAN, SWISS-PROT accession code P30626; Wang et al. 1995), human grancalcin (HUMAN_GRAN, SWISS-PROT accession code P28676; Boyhan et al. 1992), and domain VI of rat calpain (CANS_RAT, SWISS-PROT accession code Q64537; Graham-Siegenthaler et al. 1994). Noted are the sorcin residues that are identical to grancalcin (asterisk), those identical to calpain dVI (plus), and those omitted from the final model in the crystal structures (italics). The alignment was obtained initially using ClustalW (Thompson et al. 1994) and was revised based on the superposition of the crystal structures of sorcin and calpain dVI; the gaps are noted (dash). Helices (dark rectangles) are labeled α1–α8; loops are also represented (dark arrows). The EF-hand motifs are supersecondary helix–loop–helix structural elements characterized by a canonical 12-residue loop that exhibits pentagonal bipyramidal coordination of the calcium ion (Strynadka and James 1989). The five equatorial calcium ion ligands are contributed by the side chains of two acidic residues (positions Y and −Z, the latter providing two ligands), one polar group (Ser or Thr, position Z), and the main chain carbonyl oxygen (position −Y). The two apical ligands are contributed by the side-chain of an acidic group (position X) and a water molecule (position −X). In many instances the EF-hands differ from the canonical sequence resulting in variants of calcium coordination or loss of calcium binding capability. In the novel EF-hand motif discovered in calpain dVI, EF1, the loop contains only 11 amino acids and lacks the canonical carboxylate side chain at positions X and Y. The ligand in position X is a carbonyl oxygen from Ala 25, whereas a water molecule occupies position Y. Sequences corresponding to the 12-residue consensus sequence of the canonical EF-hand are boxed. The residues that form the calcium coordination sphere are labeled as X, Y, Z, −Y, −X, and −Z based on the structure of calcium-bound calpain dVI and comparison with the canonical EF-hand motif is shown (gray shading). The solvent molecules that coordinate the calcium ion are shown (dots). All three proteins contain G/P/Y-rich N-terminal extensions of various lengths shown in the first line of the alignment. The C-terminal calcium-binding domains are grouped by their EF-hand motifs, in which the nomenclatures for all secondary structure elements are the same as indicated in Figure 1A ▶.
Dimeric sorcin is structurally asymmetrical. The structural differences can be described by dividing the sorcin monomer into three subdomains: EF1–EF2, EF3–EF4, and EF5. The corresponding subdomains from each monomer superimpose well, but the relative orientations of each subdomain differ between the individual monomers (Fig. 1B ▶). The six-turn helices, α4 and α7, from both monomers exhibit a small degree of bending which is larger in one monomer than the other (Table 1), resulting in differences in the relative orientations of the domains. Another difference lies in the conformation of LEF12; in one monomer the phenol of Tyr 67 from L EF12 interacts with the carboxylate of Asp 113 from L3, whereas L EF12 of the other monomer takes a different path and the phenol of Tyr 67 points towards the solvent. The former conformation is more compact and corresponds to the monomer that contains the straighter α4 and α7 helices. This structure is also more comparable to that of calpain dVI and grancalcin, in which α4 and α7 are relatively straight and the individual monomers are superimposable. It is possible that this structural difference between the two monomers is induced by crystal contacts. However, it does illustrate the flexible nature of the sorcin molecule.
Table 1.
Inter-helical anglesa
| Structures | E1/F1 | E2/F2 | E3/F3 | E4/F4 | E5/F5 | α4(1)/α4(2)b | α7(1)/α7(2)b |
| Sorcin (A)c | 142 | 115 | 138 | 137 | 152 | −15 | −10 |
| Sorcin (B)c | 143 | 114 | 136 | 136 | 151 | −22 | −15 |
| Grancalcind | 142 | 116 | 138 | 136 | 157 | 12 | −5 |
| Calpain (calcium-free)d | 143 | 121 | 150 | 135 | 153 | 9 | −0.5 |
| Calpain (calcium-bond)d | 124 | 118 | 142 | 134 | 158 | 9 | 3.3 |
a Inter-helical angles were calculated using the program "interhlx," kindly provided by K. Yap and M. Ikura of the Ontario Cancer Institute, Toronto, Canada.
b α4(1) and α7(1) are the N-terminal half (ten residues, three helical turns) of α4 and α7, while α4(2) and α7(2) are the C-terminal half of the corresponding helices. The inter-helical angles, α4(1)/α4(2) and α7(1)/α7(2), indicate the degree of bending of the six-turn helices.
c Sorcin (A) and sorcin (B) represent the individual monomers of sorcin.
d Since the individual monomers are superimposable in grancalcin and calpain dVI (both calcium-free and calcium-bond forms), the calculations are done for only one of the monomers. Coordinates are taken from RCSB Protein Data Bank: grancalcin, entry code 1FO4; calcium-free calpain dVI, entry code 1AJ5; and calcium-bound calpain dVI, entry code 1DVI.
Calcium-binding and EF-hands
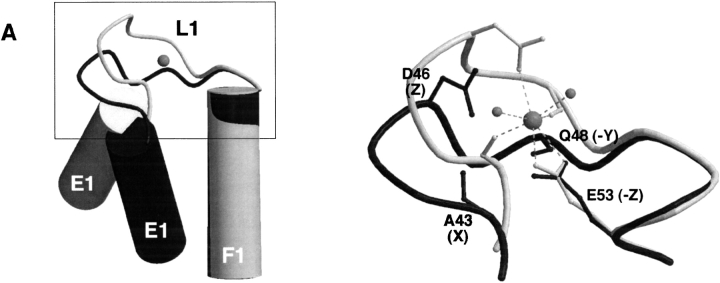
As in calpain dVI, the calcium-binding loop of EF1 (L1) in sorcin comprises 11 amino acids. Although it is one residue shorter than that of the canonical EF-hand (Kawasaki and Kretsinger 1995), its sequence is similar to that of calpain dVI EF1 (Fig. 2 ▶). As revealed by the crystal structure, EF1 of calpain dVI binds calcium in a slightly modified arrangement compared to the canonical EF-hand (Fig. 2 ▶; Blanchard et al. 1997; Lin et al. 1997), which involves two backbone carbonyl oxygens in addition to the carboxylates from Asp 18 and Glu 25. These calcium-coordinating residues are also present in sorcin EF1, which thus is expected to bind calcium with high affinity. The conformation of EF1 in calcium-free sorcin differs from that of the calpain dVI EF1 in the calcium-bound state (Fig. 3A ▶). Specifically, the residues corresponding to those coordinating the calcium in calpain dVI are not in positions suitable for calcium coordination. The E1/F1 inter-helical angle is about 140° (Table 1), which differs from the "open" conformation observed in calcium-bound calpain dVI but similar to the "closed" form observed in calcium-free calpain dVI. These structural observations indicate that a conformational rearrangement would be required for a calcium ion to bind to sorcin EF1.
Fig. 3.
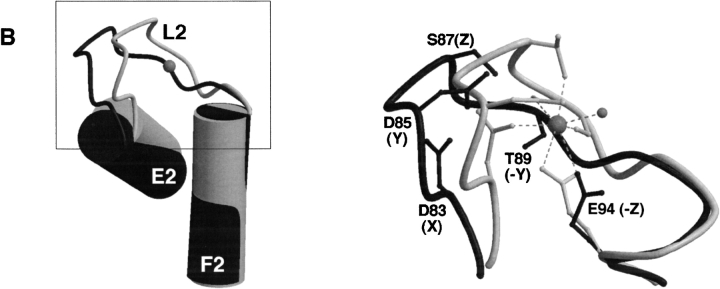
Schematic representation of EF-hands of sorcin compared with that of calpain dVI. The F-helix was used for alignment. Shown are sorcin (dark gray) and calpain dVI (light gray; RCSB Protein Data Bank entry code 1DVI; Blanchard et al. 1997). (Left) Helices E and F (cylinders), loop L (coil), and the calcium ion (gray sphere). (Right) The loop region (boxed in the left panel) is enlarged to show the detailed conformation and some of the residues (backbone carbonyl or side-chain) that are responsible for calcium coordination, in which the calcium ion (gray sphere) is shown connected (dashed lines) to its ligands and the water (small gray sphere). This figure was produced using the programs MOLSCRIPT (Kraulis 1991) and Raster3D (Merrit and Bacon 1997). (A) Superposition of EF1 of sorcin and calcium-bound EF1 of calpain dVI. The helices E1 and F1 in calpain are nearly perpendicular to each other, with F1 coplanar with the page and E1 pointing toward the viewer (left). In contrast, helices E1 and F1 in sorcin are closer to an anti-parallel orientation with respect to each other. (B) Superposition of EF2 of sorcin and calcium-bound EF2 of calpain dVI. The helices E2 and F2 in both calpain and sorcin are nearly perpendicular to each other, with F2 coplanar with the page and E2 pointing toward the viewer (left).
As in calpain dVI, both EF2 and EF3 in sorcin contain the consensus sequence of a canonical EF-hand (Fig. 2 ▶), indicating a high affinity for calcium. In the calcium-free state, the conformations of EF2 and EF3 are such that the canonical liganding oxygens are not in ideal positions for calcium coordination. However, the E2/F2 inter-helical angle is ∼115°, corresponding to an "open" conformation of a typical calcium-bound EF-hand (Fig. 3B ▶), whereas that of EF3 is in the "closed" conformation of a typical calcium-free EF-hand. In calpain dVI, calcium binding to EF2 and EF3 changes merely their loop conformations to bring all the liganding residues into position to coordinate calcium, whereas their inter-helical angles remain essentially unchanged (Table 1, "open" for EF2 and "closed" for EF3; Blanchard et al. 1997; Lin et al. 1997). Similar features were observed with grancalcin (Jia et al. 2000), although EF2 in grancalcin does not bind calcium because of a Glu → Ala mutation at position −Z. Therefore, the calcium-independent "open" conformation of EF2 and "closed" conformation of EF3 may be typical for PEF proteins.
Sorcin EF4 is atypical in terms of the consensus EF-hand sequence. As in grancalcin, the EF4 in sorcin is two residues shorter than that of the canonical form (Fig. 2 ▶). This deletion occurs in L4; the amino acid side-chains in several canonical positions are not suitable for coordinating calcium. These findings indicate that sorcin EF4 is unlikely to bind calcium at its usual location. Therefore, the main function of EF4 is probably providing a stabilizing partner for EF3 rather than binding calcium.
Like calpain dVI, sorcin EF5 has a two-residue insertion in L5 that extends the intermolecular β-sheet and moves the conserved Glu at position −Z away from a position suitable for calcium coordination (Fig. 2 ▶). In calpain dVI, EF5 is free of calcium even at a calcium ion concentration of 200 mM. The sorcin EF5 is likely to have even lower calcium-binding affinity because the residue at position Y is Ala instead of the conserved Asp. Therefore, we conclude that the role of EF5 is to stabilize the dimer.
Dimerization
Sedimentation equilibrium analysis showed that calcium-free sorcin forms dimers in solution under physiological conditions (Zamparelli et al. 2000). Sorcin crystallized in the absence of calcium was observed as a dimer in the asymmetric unit, in which the individual monomers of the dimer were related by pseudo-two-fold symmetry. As in calpain dVI and grancalcin, the sorcin dimer is formed by interaction of the EF5 motifs from each monomer (Fig. 1A ▶), in which they associate in a way similar to a conventional EF-hand pair, with L5 from both monomers forming an anti-parallel two-stranded β-sheet. The E5/F5 inter-helical angle is such that a four-helix bundle is formed at the dimer interface consisting of E5 and F5 from both molecules. Additional secondary structural elements involved in dimerization are E4 from both monomers. In addition to the hydrogen-bond network formed by L5 of individual monomers, the interactions in dimerization are mainly hydrophobic along with some salt bridges. The high degree of conservation in the residues forming the dimer interface and especially their hydrophobic character indicates that all PEF proteins exist as dimers.
Discussion
It has been proposed that EF-hands, which have a calcium-dependent regulatory function, undergo major calcium-induced conformational changes, most importantly a change in the inter-helical angle between two helices of each EF-hand (Strynadka and James 1989). Using the change in inter-helical angle as a metric, four of the five EF-hand motifs, EF2–EF5, appear to be insensitive to changes in calcium concentration (Table 1). Thus, the EF2–EF5 portion of sorcin would appear to play a structural role rather than a regulatory one. On the other hand, these comparisons also show clearly that a conformational change in EF1 has to be accompanied by calcium binding. Such a rearrangement is likely to involve a relative movement between helices E1 and F1, including a change in their inter-helical angle. The differences, which are confined to the same EF1 region in sorcin and calpain dVI, point to a functional role for this region in calcium signaling. Taking into account that EF2 is structurally coupled to EF1, we propose that the EF-hand pair EF1–EF2 corresponds to the two physiologically relevant calcium-binding sites in sorcin (Zamparelli et al. 1997). Furthermore, calcium binding to this EF-hand pair would introduce a rather small conformational change localized to EF1–EF2. This conformational change may be associated with exposure of buried hydrophobic residues acting as a switch to promote sorcin binding to the membrane-bound targets. In support of this model, solution studies of sorcin and its fragments have shown that the EF1–EF2 pair corresponds to the high-affinity calcium binding sites (Zamparelli et al. 2000). Moreover, calcium binding to EF1–EF2 triggers the activation of sorcin, which uses the C-terminal calcium-binding domain to interact with RyR. The small calcium-induced structural changes may be typical for proteins that monitor intracellular calcium concentrations. However, the mechanism by which conformational changes localized in EF1–EF2 trigger the recognition of RyR by sorcin remains to be determined.
Calcium-dependent interaction of sorcin with annexin VII requires the N-terminal domains of both proteins (Brownawell and Creutz 1997; Verzili et al. 2000). As the N-terminal G/P/Y-rich domain in sorcin is connected directly to EF1, it is likely that calcium binding to EF1 would also induce conformational changes in the N-terminal domain and in turn stimulate a new set of interactions between sorcin and other proteins. In sorcin, calcium-induced quenching of intrinsic fluorescence, which is characteristic of full-length sorcin, does not take place in the C-terminal calcium-binding domain (Zamparelli et al. 2000). This implies that the calcium-dependent conformational changes alter the relative positions of the N- and C-terminal domains rather than affecting the structure of the calcium-binding domain itself. All PEF proteins contain such N-terminal domains. However, they vary greatly in length and amino acid composition (Maki et al. 1997). It is plausible that these N-terminal domains play an important role in target recognition by the PEF proteins. This segment is flexible in the absence of calcium and is often disordered, as observed in the sorcin crystal.
In conclusion, the 2.2Å-resolution structure of calcium-free sorcin provides the first atomic-level details of this protein. The results from this structure, together with other structures of other PEF proteins, provide insight into the structural bases for the biological function of sorcin and should aid the design of experiments addressing how sorcin interacts with its membrane target proteins in response to changes in cellular calcium concentration.
Materials and methods
Protein preparation, evaluation, and crystallization
Full-length human sorcin was expressed in E.coli, yielding 30 mg of protein per liter of cells. Bacteria were resuspended in 20 mM HEPES (pH 7.4), 10 mM NaCl, 1 mM EGTA, and EDTA-free Complete Protease Inhibitor (Roche Molecular Biochemicals, Indianapolis, IN) tablets; passed through a Microfluidizer (Microfluidics Corp., Newton, MA); and centrifuged at 50,000 g for 30 min at 4°C. The soluble fraction was treated with PEI to precipitate nucleic acids and centrifuged as above. The resulting supernantant was purified on a Q-Sepharose column using a gradient of 10–250 mM NaCl, followed by a S100 (Pharmacia) column in 20 mM HEPES (pH 7.4), 100 mM NaCl, and 1 mM DTT.
Sorcin (4 μM) was mixed with 10 μM fluo-4 pentapotassium salt (Molecular Probes, Eugene, OR) and diluted into urea (treated with Chelex-100 resin) at a final urea concentration of either 6.4 M or 7.8 M. After 1 h, fluorescence (485 nm excitation, 520 nm emission) at ambient temperature was recorded and compared to a calibration curve obtained with calcium chloride solutions. No calcium was detected in the denatured sorcin.
Crystallization of full-length wild-type human sorcin was carried out using the hanging-drop vapor diffusion technique. A reservoir solution containing 0.5M NaCl, 0.1 HEPES (pH 7.0), and 2.5mM EGTA yielded crystals in tetragonal space group P43212 (a = b = 118.25 Å and c = 85.49Å) that diffracted to beyond 2.2 Å resolution. There was one dimer of sorcin molecule per asymmetric unit; the solvent content of the crystal was ∼64%.
Data collection and structure determination
Before data collection, crystals of sorcin were equilibrated in a cryo-solution containing 20% (v/v) ethylene glycol and 5% (v/v) polyethylene glycol (average Mr = 400) made from their reservoir solution before being flash-frozen in nitrogen gas for X-ray data collection at −170°C. Two heavy atom derivatives were prepared by soaking the native crystal in either ethyl mercury thiosalycylate or Di-u-iodobis(ethylenediamine)diplatinum(II). Diffraction data were measured on a Rigaku R-AXIS IV Image plate mounted on a Rigaku RU200 rotating-anode generator (Rigaku/MSC). Measured intensities were integrated, scaled, and merged using HKL software package (Otwinowski and Minor 1997).
The sorcin structure was determined at 2.4Å resolution by a combination of multiple isomorphous replacement and anomalous scattering (MIRAS), followed by refinement to 2.2Å; all procedures were carried out using CNX software package (Brunger et al. 1998). The final model consists of protein residues 27–198 for both monomers (the N-terminal 27-residue fragment was omitted because it was not visible in the final electron density maps) and 303 water molecules, giving an R-factor of 21.2% with R-free of 25.6%. The final RMS deviations from the target bond and angle values are 0.007Å and 1.199°. 91.7% and 8.3% of all main chain angles fall into the favored and additionally allowed regions, respectively PROCHECK; Laskowski et al. 1996).
The present coordinates have been deposited to the RCSB Protein Data Bank under entry code IJUO.
Acknowledgments
We thank Drs. Ajay and M.D. Sintchak for their valuable discussions and suggestions; Drs. T. Ernst and M. Jacobs for their critical reading of the manuscript; Drs. B. Hare and Y. Wu for their comments on the figures; and Dr. K. Wilson for his support, advice, and helpful comments on the manuscript.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked "advertisement" in accordance with 18 USC section 1734 solely to indicate this fact.
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1101/ps.36701.
References
- Blanchard, H., P. Grochulski, Y. Li, J.S. Arthur, P.L. Davies, J.S. Elce, and M. Cygler. 1997. Structure of a calpain Ca(2+)-binding domain reveals a novel EF-hand and Ca(2+)-induced conformational changes. Nat. Struct. Biol. 4 532–538. [DOI] [PubMed] [Google Scholar]
- Boyhan, A., C.M. Casimir, J.K. French, C.G. Teahan, and A.W. Segal. 1992. Molecular cloning and characterization of grancalcin, a novel EF-hand calcium-binding protein abundant in neutrophils and monocytes. J. Biol. Chem.. 267 2928–2933. [PubMed] [Google Scholar]
- Brownawell, A.M., and C.E. Creutz. 1997. Calcium-dependent binding of sorcin to the N-terminal domain of synexin (annexin VII). J. Biol. Chem.. 272 22182–22190. [DOI] [PubMed] [Google Scholar]
- Brunger, A.T., P.D. Adams, G.M. Clore, W.L. DeLano, P. Gros, R.W. Grosse-Kunstleve, J.S. Jiang, J. Kuszewski, M. Nilges, N.S. Pannu, R.J. Read, L.M. Rice, T. Simonson, and G.L. Warren. 1998. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr D Biol Crystallogr. 54 905–921. [DOI] [PubMed] [Google Scholar]
- Graham-Siegenthaler, K., S. Gauthier, P.L. Davies, and J.S. Elce. 1994. Active recombinant rat calpain II. Bacterially produced large and small subunits associate both in vivo and in vitro. J Biol Chem. 269 30457–30460. [PubMed] [Google Scholar]
- Jia, J., Q. Han, N. Borregaard, K. Lollike, and M. Cygler. 2000. Crystal structure of human grancalcin, a member of the penta-EF-hand protein family. J Mol Biol. 300 1271–1281. [DOI] [PubMed] [Google Scholar]
- Kawasaki, H., and R.H. Kretsinger. 1995. Calcium-binding proteins 1: EF-hands. Protein Profile. 2 297–490. [PubMed] [Google Scholar]
- Kraulis, P.J. 1991. MOLSCRPT: A program to produce both detailed and schematic plots of protein structures. J. appl. Crystallog. 24 946–950. [Google Scholar]
- Laskowski, R.A., J.A. Rullmannn, M.W. MacArthur, R. Kaptein, and J.M. Thornton. 1996. AQUA and PROCHECK-NMR: programs for checking the quality of protein structures solved by NMR. J Biomol NMR. 8 477–486. [DOI] [PubMed] [Google Scholar]
- Lin, G.D., D. Chattopadhyay, M. Maki, K.K. Wang, M. Carson, L. Jin, P.W. Yuen, E. Takano, M. Hatanaka, L.J. DeLucas, and S.V. Narayana. 1997. Crystal structure of calcium bound domain VI of calpain at 1.9 A resolution and its role in enzyme assembly, regulation, and inhibitor binding. Nat Struct Biol. 4 539–547. [DOI] [PubMed] [Google Scholar]
- Lokuta, A.J., M.B. Meyers, P.R. Sander, G.I. Fishman, and H.H. Valdivia. 1997. Modulation of cardiac ryanodine receptors by sorcin. J Biol Chem. 272 25333–25338. [DOI] [PubMed] [Google Scholar]
- Maki, M., S.V. Narayana, and K. Hitomi. 1997. A growing family of the Ca2+-binding proteins with five EF-hand motifs. Biochem J. 328 718–720. [PMC free article] [PubMed] [Google Scholar]
- Merrit, E.A., and D.J. Bacon. 1997. Raster3D: photo-realistic molecular graphics. In Methods Enzymol. Vol. 277. 505–524. [DOI] [PubMed]
- Meyers, M.B., V.M. Pickel, S.S. Sheu, V.K. Sharma, K.W. Scotto, and G.I. Fishman. 1995a. Association of sorcin with the cardiac ryanodine receptor. J Biol Chem. 270 26411–26418. [DOI] [PubMed] [Google Scholar]
- Meyers, M.B., T.S. Puri, A.J. Chien, T. Gao, P.H. Hsu, M.M. Hosey, and G.I. Fishman. 1998. Sorcin associates with the pore-forming subunit of voltage-dependent L-type Ca2+ channels. J Biol Chem. 273 18930–18935. [DOI] [PubMed] [Google Scholar]
- Meyers, M.B., C. Zamparelli, D. Verzili, A.P. Dicker, T.J. Blanck, and E. Chiancone. 1995b. Calcium-dependent translocation of sorcin to membranes: functional relevance in contractile tissue. FEBS Lett. 357 230–234. [DOI] [PubMed] [Google Scholar]
- Otwinowski, Z., and W. Minor. 1997. Processing of X-ray diffraction data collected in oscillation mode. In Methods Enzymol. Vol. 276. 307–326. [DOI] [PubMed]
- Pack-Chung, E., M.B. Meyers, W.P. Pettingell, R.D. Moir, A.M. Brownawell, I. Cheng, R.E. Tanzi, and T.W. Kim. 2000. Presenilin 2 interacts with sorcin, a modulator of the ryanodine receptor. J Biol Chem. 275 14440–14445. [DOI] [PubMed] [Google Scholar]
- Strobl, S., C. Fernandez-Catalan, M. Braun, R. Huber, H. Masumoto, K. Nakagawa, A. Irie, H. Sorimachi, G. Bourenkow, H. Bartunik, K. Suzuki, and W. Bode. 2000. The crystal structure of calcium-free human m-calpain suggests an electrostatic switch mechanism for activation by calcium. Proc. Natl. Acad. Sci. 97 588–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strynadka, N.C., and M.N. James. 1989. Crystal structures of the helix-loop-helix calcium-binding proteins. Annu Rev Biochem. 58 951–998. [DOI] [PubMed] [Google Scholar]
- Thompson, J.D., D.G. Higgins, and T.J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res.. 22 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdivia, H.H. 1998. Modulation of intracellular Ca2+ levels in the heart by sorcin and FKBP12, two accessory proteins of ryanodine receptors. Trends Pharmacol Sci. 19 479–482. [DOI] [PubMed] [Google Scholar]
- Verzili, D., C. Zamparelli, B. Mattei, A.A. Noegel, and E. Chiancone. 2000. The sorcin-annexin VII calcium-dependent interaction requires the sorcin N-terminal domain. FEBS Lett. 471 197–200. [DOI] [PubMed] [Google Scholar]
- Wang, S.L., M.F. Tam, Y.S. Ho, S.H. Pai, and M.C. Kao. 1995. Isolation and molecular cloning of human sorcin a calcium-binding protein in vincristine-resistant HOB1 lymphoma cells. Biochim Biophys Acta. 1260 285–293. [DOI] [PubMed] [Google Scholar]
- Zamparelli, C., A. Ilari, D. Verzili, L. Giangiacomo, G. Colotti, S. Pascarella, and E. Chiancone. 2000. Structure-function relationships in sorcin, a member of the penta EF-hand family. Interaction of sorcin fragments with the ryanodine receptor and an Escherichia coli model system. Biochemistry. 39 658–666. [DOI] [PubMed] [Google Scholar]
- Zamparelli, C., A. Ilari, D. Verzili, P. Vecchini, and E. Chiancone. 1997. Calcium- and pH-linked oligomerization of sorcin causing translocation from cytosol to membranes. FEBS Lett. 409 1–6. [DOI] [PubMed] [Google Scholar]