A chondroitin sulfate A-binding DBL important in placental malaria has been overproduced, purified and crystallized. Diffraction data were collected to 1.9 Å resolution.
Keywords: Plasmodium falciparum, PfEMP1 protein, chondroitin sulfate A
Abstract
The PfEMP1 proteins of the malaria parasite Plasmodium falciparum are inserted into the membrane of infected red blood cells, where they mediate adhesion to a variety of human receptors. The DBL domains of the var2csa-encoded PfEMP1 protein play a critical role in malaria of pregnancy, tethering infected cells to the surface of the placenta through interactions with the glycosaminoglycan carbohydrate chondroitin sulfate A (CSA). A CSA-binding DBL domain has been overproduced in a bacterial expression system, purified and crystallized. Native data sets extending to 1.9 Å resolution have been collected and phasing is under way.
1. Introduction
Malaria is the most deadly parasitic infection affecting humanity, causing 500 million serious cases and 2 million deaths each year. Plasmodium falciparum is the causative agent of the most deadly forms of malaria. The parasite has a complex life cycle, involving stages in both the mosquito vector and the human host. The deadly symptoms of the disease occur during the blood phase of the life cycle, during which the parasites live and divide within human erythrocytes.
The surface of a Plasmodium-infected erythrocyte is decorated with parasite-encoded proteins of the PfEMP1 family. The genome of the 3D7 strain of P. falciparum contains 59 var genes that encode PfEMP1s (Gardner et al., 2002 ▶). The PfEMP1 proteins consist of an intracellular acidic domain at the C-terminus and a single putative transmembrane helix. The majority of the protein is extracellular and consists of two to ten discrete domains (Gardner et al., 2002 ▶). These mostly fall into two families: the cysteine-rich interdomain region (CIDR) and Duffy binding-like (DBL) domains.
The CIDR and DBL domains have important adhesive functions, tethering infected erythrocytes to the surface of blood vessels, thereby allowing the parasite to avoid detection by the spleen. Many human cell-surface proteins act as binding partners for PfEMP1 domains, with individual PfEMP1s able to interact with multiple receptors, including CD36, complement receptor 1 and ICAM-1 (Baruch et al., 1996 ▶; Rowe et al., 1997 ▶). Also implicated in severe malaria are interactions between PfEMP1s and carbohydrates of the glycosaminoglycan family, including chondroitin sulfate A (CSA) and heparan sulfates (Fried & Duffy, 1996 ▶; Chen et al., 1998 ▶; Vogt et al., 2003 ▶).
PfEMP1 proteins play an important role in the development of malaria during pregnancy. The formation of the placenta provides a new adhesive target for the infected erythrocytes, with accumulation of erythrocytes causing placental inflammation and blocking blood flow to the developing child. This leads to the death or underweight birth of many children and kills an estimated 75 000–100 000 foetuses each year (Rowe & Kyes, 2004 ▶).
The major placental receptor for the binding of infected erythrocytes is the carbohydrate chondroitin sulfate A (Fried & Duffy, 1996 ▶). Placental parasite strains uniformly bind to CSA and soluble CSA disrupts these interactions (Fried et al., 2006 ▶). In addition, antibodies that develop in women who have been exposed to malaria during pregnancy block CSA binding of infected cells, while similar antibodies are not found in adult men or in women who have not been pregnant (Fried et al., 1998 ▶; Ricke et al., 2000 ▶; O’Neil-Dunne et al., 2001 ▶). These antibodies are associated with protection against placental infection and with high birth weight in subsequent pregnancies (Duffy & Fried, 2003 ▶).
The parasite receptor that binds to CSA is the PfEMP1 protein encoded by the var2csa gene. This gene is expressed in placental isolates and shows a transcription pattern that correlates with ability to bind CSA (Rowe & Kyes, 2004 ▶). The var2csa gene is highly conserved in different strains of P. falciparum and its expression is upregulated in strains selected to bind to CSA. It is also highly expressed in isolates taken from placental samples, but is not found in isolates from children (Salanti et al., 2003 ▶; Tuikue Ndam et al., 2005 ▶), and the protein is only detected on the surface of parasites after selection for CSA binding (Salanti et al., 2004 ▶; Barfod et al., 2006 ▶). Disruption of the var2csa gene in several different parasite strains leads to a loss of CSA binding that cannot be fully recovered (Viebig et al., 2005 ▶; Duffy et al., 2006 ▶) and var2CSA antibodies are found in plasma from women who have been pregnant but not in that from men or children. These antibodies are abundant in pregnant woman from East and West Africa and are associated with successful outcome of pregnancy (Salanti et al., 2004 ▶). Indeed, antibodies from memory B cells of multigravid women bind to domains from var2CSA and interact with blood cells infected with a CSA-binding parasite strain (Barfod et al., 2007 ▶). The var2CSA protein therefore provides an important potential target for the development of drugs and vaccines for the treatment of placental malaria.
The var2CSA proteins contain several domains that bind specifically to CSA in vitro, including DBL2X and DBL6∊ from the 3D7 strain and DBL2X and DBL3X from strain A4 (Gamain et al., 2005 ▶). In this study, the DBL3X domain from the A4 strain has been cloned, expressed, purified and crystallized. The structure of this domain, alone and in complex with CSA, will provide stereochemical details about the mode of carbohydrate binding and may contribute to the development of treatments for placental malaria.
2. Experimental procedures
2.1. Cloning, expression and purification
The DBL3X domain of var2CSA (accession codes AY372123 and AAQ73926) was cloned from FCR3 strain genomic DNA. Comparison with the sequences of the DBL domains of EBA-175 (Tolia et al., 2005 ▶) and the Duffy antigen receptor from P. knowlesi (Singh et al., 2006 ▶) suggested the DBL3X domain to consist of residues 1218–1577.
The pEt15b vector (Novagen) was modified by the insertion of DNA encoding an N-terminal hexahistidine tag and a cleavage site for the TEV protease. Primers 1 (CATGGGCCACCATCACCATCACCATGAGAACCTGTATTTTCAGGGCGGATCCGCTAGCTCGAGGTACCATATGC) and 2 (GATCGCATATGGTACCTCGAGCTAGCGGATCCGCCCTGAAAATACAGGTTCTCATGGTGATGGTGATGGTGGCC) were mixed, heated to 373 K and cooled at 1 K min−1 to form a double-stranded product. This was ligated into NcoI–BamHI-cleaved pEt15b to generate a modified vector. Primers 3 (AAGGATCCTCATGTGACCTTAACGCAACC) and 4 (AAGCTAGCTTATTCGCACGAACATATACTGCT) were used to amplify the DBL3X domain by the polymerase chain reaction from genomic DNA and the product was ligated into the BamHI–NheI site of the modified pEt15b vector in frame with the TEV cleavage site.
Origami B (Novagen) Escherichia coli were transformed both with the pRIG plasmid (Baca & Hol, 2000 ▶) and the plasmid encoding the DBL3X domain. Cells were grown to an optical density at 600 nm of 1.0 and were induced with 1 mM IPTG. Expression took place overnight at 298 K.
Cells were pelleted, resuspended in solubilization buffer (20 mM Tris pH 8.0, 0.3 M NaCl, 10 mM imidazole, 0.5% Triton X-100) and lysed by sonication. The cell lysate was centrifuged for 30 min at 45 000g and purified by affinity chromatography using Ni–NTA Sepharose (Qiagen). The protein was loaded onto the Ni–NTA resin, washed with solubilization buffer and eluted with 20 mM Tris pH 8.0, 0.1 M NaCl, 0.2 M imidazole.
The protein was buffer-exchanged into 20 mM phosphate pH 7.0, 150 mM NaCl, 3 mM reduced glutathione, 0.3 mM oxidized glutathione and cleaved by the addition of TEV protease. 1 mg protease was added per 10 mg protein and incubated at room temperature overnight. Cleaved protein was passed through an Ni–NTA affinity column and a Q-Sepharose column (GE Healthcare). The eluant was concentrated using an Amicon Ultra centifugal filter device (10 000 Da molecular-weight cutoff) and further purified using a Superdex 200 16/60 (GE Healthcare) column run using 20 mM Tris pH 8.0, 50 mM NaCl. The protein was concentrated to 10 mg ml−1. Each litre of bacterial culture yielded 5–10 mg purified protein.
2.2. Crystallization
Crystals were grown using the hanging-drop vapour-diffusion technique by mixing 1 µl protein solution with 1 µl reservoir solution and equilibrating against 1 ml reservoir solution. Initial conditions were found using a PEG 4K grid screen (Hampton Research). Crystals grew in 20–30% PEG 4000 at pH 6.5–8.0, appearing after 7–10 d and growing to full size within 14–21 d.
2.3. Data collection and processing
The crystals giving the highest resolution diffraction were grown in 25% PEG 4000, 20 mM Tris pH 8.0. They were cryoprotected by transfer into 25% PEG 4000, 20 mM Tris pH 8.0, 25% glycerol and flash-frozen in liquid nitrogen. Diffraction data were collected at 100 K on beamline ID23.1 at the European Synchrotron Radiation Facility (ESRF, Grenoble, France). Data were processed using MOSFLM (Leslie, 1992 ▶) and SCALA (Evans, 1993 ▶) from the CCP4 suite (Collaborative Computational Project, Number 4, 1994 ▶) and were consistent with a primitive orthorhombic lattice. Systematic absences in the h00, 0l0 and 00k reflections indicated the crystals to belong to space group P212121. A complete data set was collected to a resolution limit of 1.9 Å.
3. Results and discussion
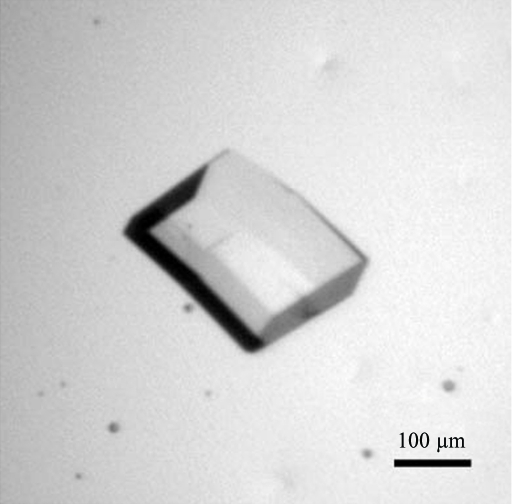
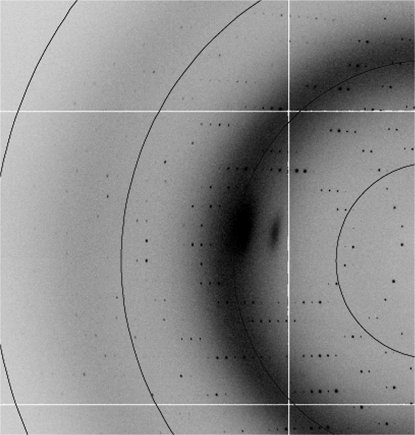
A bacterial expression system was used to produce large quantities of a chondrotin sulfate A-binding DBL domain from the var2CSA cell-surface protein of P. falciparum. The protein was purified and subjected to hanging-drop crystallization trials. Crystals were observed within 7–10 d with 20–30% PEG 4000 at pH 6.5–8.0 in the well. The crystals grew to maximum dimensions of 0.2 × 0.2 × 0.1 mm (Fig. 1 ▶) and diffracted to 1.9 Å resolution (Fig. 2 ▶). A complete data set has been collected (Table 1 ▶). We are currently working towards determination of the phases to solve this structure. The structure could provide valuable information to guide the development of drugs and vaccines to target placental malaria.
Figure 1.
Crystal of a chondroitin sulfate-binding DBL domain from a P. falciparum var2csa-encoded PfEMP1 protein.
Figure 2.
Diffraction pattern collected at 100 K. The resolution circles lie at 7.1, 3.6, 2.4 and 1.8 Å.
Table 1. Data-collection statistics.
Values in parentheses are for the highest resolution shell.
| Space group | P212121 |
| Unit-cell parameters (Å) | a = 42.1, b = 86.9, c = 92.9 |
| Wavelength (Å) | 0.903 |
| I/σ(I) | 17.3 (3.5) |
| Completeness (%) | 99.6 (99.9) |
| Multiplicity | 5.0 (5.2) |
| Rmerge (%) | 6.6 (35.9) |
| No. of reflections | 128548 |
| No. of unique reflections | 25499 |
Acknowledgments
I would like to thank Tom Blundell and Ben Luisi for support and for assistance with data collection and the Royal Society for funding. MKH is a Royal Society University Research Fellow.
References
- Baca, A. M. & Hol, W. G. J. (2000). Int. J. Parasitol.30, 113–118. [DOI] [PubMed]
- Barfod, L., Bernasconi, N. L., Dahlback, M., Jarrossay, D., Anderson, P. H., Salanti, A., Ofori, H. F., Turner, L., Resende, M., Nielsen, M. A., Theander, T. G., Sallusto, F., Lanzavecchia, A. & Hviid, L. (2007). Mol. Microbiol.63, 335–347. [DOI] [PMC free article] [PubMed]
- Barfod, L., Nielsen, M. A., Turner, L., Dahlback, M., Jensen, A. T., Hviid, L., Theander, T. G. & Salanti, A. (2006). Infect. Immun.74, 4357–4360. [DOI] [PMC free article] [PubMed]
- Baruch, D. I., Gormley, J. A., Howard, R. J. & Pasloske, B. L. (1996). Proc. Natl Acad. Sci. USA, 93, 3497–3502. [DOI] [PMC free article] [PubMed]
- Chen, Q., Barragan, A., Fernandez, V., Sundstrom, A., Schlichtherle, M., Sahlen, A., Carlson, J., Datta, S. & Wahlgren, M. (1998). J. Exp. Med.187, 15–23. [DOI] [PMC free article] [PubMed]
- Collaborative Computational Project, Number 4 (1994). Acta Cryst. D50, 760–763.
- Duffy, M. F., Maier, A. G., Byrne, T. J., Marty, A. J., Alliott, S. R., O’Neill, M. T., Payne, P. D., Rogerson, S. J., Cowman, A. F., Crabb, B. S. & Brown, G. V. (2006). Mol. Biochem. Parasitol.148, 117–124. [DOI] [PubMed]
- Duffy, P. E. & Fried, M. (2003). Infect. Immun.71, 6620–6623. [DOI] [PMC free article] [PubMed]
- Evans, P. R. (1993). Proceedings of the CCP4 Study Weekend. Data Collection and Processing, edited by L. Sawyer, N. Isaacs & S. Bailey, pp. 114–133. Warrington: Daresbury Laboratory.
- Fried, M., Domingo, G. J., Gowda, C. D., Mutabingwa, T. K. & Duffy, P. E. (2006). Exp. Parasitol.113, 36–42. [DOI] [PubMed]
- Fried, M. & Duffy, P. E. (1996). Science, 272, 1502–1504. [DOI] [PubMed]
- Fried, M., Nosten, F., Brockman, A., Brabin, B. J. & Duffy, P. E. (1998). Nature (London), 395, 851–852. [DOI] [PubMed]
- Gamain, B., Trimnell, A. R., Scheidig, C., Scherf, A., Miller, L. H. & Smith, J. D. (2005). J. Infect. Dis.191, 1010–1013. [DOI] [PubMed]
- Gardner, M. J. et al. (2002). Nature (London), 419, 498–511.
- Leslie, A. G. W. (1992). Jnt CCP4/ESF–EACBM Newsl. Protein Crystallogr.26
- O’Neil-Dunne, I., Achur, R. N., Agbor-Enoh, S. T., Valiyaveettil, M., Naik, R. S., Ockenhouse, C. F., Zhou, A., Megnekou, R., Leke, R., Taylor, D. W. & Gowda, D. C. (2001). Infect. Immun.69, 7487–7492. [DOI] [PMC free article] [PubMed]
- Ricke, C. H., Staalsoe, T., Koram, K., Akanmori, B. D., Riley, E. M., Theander, T. G. & Hviid, L. (2000). J. Immunol.165. 3309–3316. [DOI] [PubMed]
- Rowe, J. A. & Kyes, S. (2004). Mol. Microbiol.53, 1011–1019. [DOI] [PMC free article] [PubMed]
- Rowe, J. A., Moulds, J. M., Newbold, C. I. & Miller, L. H. (1997). Nature (London), 388, 292–295. [DOI] [PubMed]
- Salanti, A., Dahlback, M., Turner, L., Nielsen, M. A., Barfod, L., Magistrado, P., Jensen, A. T., Lavstsen, T., Ofori, M. F., Marsh, K., Hviid, L. & Theander, T. G. (2004). J. Exp. Med.200, 1197–1203. [DOI] [PMC free article] [PubMed]
- Salanti, A., Staalsoe, T., Lavstsen, T., Jenses, A. T. R., Sowa, M. P. K., Arnot, D. E., Hviid, L. & Theander, T. G. (2003). Mol. Microbiol.49, 179–191. [DOI] [PubMed]
- Singh, S. K., Hora, R., Belrhali, H., Chitnis, C. E. & Sharma, A. (2006). Nature (London), 439, 741–744. [DOI] [PubMed]
- Tolia, N. H., Enemark, E. J., Sim, B. K. & Joshua-Tor, L. (2005). Cell, 122, 183–193. [DOI] [PubMed]
- Tuikue Ndam, N. G., Salanti, A., Bertin, G., Dahlback, M., Fievet, N., Turner, L., Gaye, A., Theander, T. & Deloron, P. (2005). J. Infect. Dis.192, 331–335. [DOI] [PubMed]
- Viebig, N. K., Gamain, B., Scheidig, C., Lepolard, C., Przyborzki, J., Lanzer, M., Gysin, J. & Scherf, A. (2005). EMBO Rep.6, 775–781. [DOI] [PMC free article] [PubMed]
- Vogt, A. M., Barragen, A., Chen, Q., Kironde, F., Spillmann, D. & Wahlgren, M. (2003). Blood, 101, 2405–2411. [DOI] [PubMed]