Abstract
Monellin (MN) is a sweet-tasting plant protein known to form fibrous aggregates in the heat-denatured state. Here the amyloid-type aggregation process of MN is extensively characterized. The amyloidgenesis was initiated in a highly denatured state of MN. A seeding effect of skipping a lag phase of the amyloid formation kinetics established a nucleation-dependent aggregation mechanism. A finely controlled experimental protocol revealed an additional prenucleus stage preceding the maturation of the nucleus, indicating that the initial lag phase is composed of multiple conformational events. The results obtained for the aggregation properties of the separate A and B subunit chains of MN and a recombinant single-chain MN suggest that the B chain exclusively contributed to the amyloid-type aggregation. These findings suggest a scheme for the amyloidgenesis of MN and their subunits, and provide a unique model of amyloidgenesis that is regulated by the subunit composition of protein.
Keywords: Monellin, amyloid, nucleation, seeding effect
Amyloid-type protein aggregation is an important subject of biochemical and biophysical research (Fink 1998; Kelly 1998; Dobson and Karplus 1999). The growth of this research area has been stimulated by the pathological significance of the aggregation events (Sipe 1992; Tan and Pepys 1994; Prusiner et al. 1998; Koo et al. 1999). From a biophysical point of view, amyloid has attracted attention because of its regular fibrous architecture and its relation to the protein-misfolding problem (Dobson and Ellis 1998). Amyloidgenesis is the nucleation-dependent aggregation process mostly initiated in partially denatured states of amyloidgenic proteins (Jarrett and Lansbury 1993; Kelly 1998; Rochet and Lansbury 2000). It was also demonstrated that the amyloid-like fibrous aggregation with a β-sheet-rich structure is a universal phenomenon, not restricted to a special category of proteins (Guijarro et al. 1998; Chiti et al. 1999). However, many unsolved problems remain. For example, subclassification of the amyloid-type aggregation is not yet established. Morphological variations of the fibrous aggregates have often been noted, and may be coupled with diversity in the amyloid formation mechanisms. It is still worth performing comparative kinetic and morphological studies of various amyloid species and thus worth increasing the number of unique model systems.
The results of the present study establish another model system for aggregation analysis, the amyloidgenesis of which can be finely regulated by circumstantial parameters and subunit compositions. The target protein is a heterodimeric, sweet-tasting protein known as monellin (MN; 11.2 kD) composed of A and B subunit chains and isolated from the fruit of the tropical berry Dioscoreophyllum cumminsii (Morris and Cagan 1972). X-ray and NMR methods have revealed the structure of MN as an antiparallel β-sheet composed of four β-strands and one α-helix (Tomic et al. 1992; Somoza et al. 1993; Spadaccini et al. 2001). The A chain provides two β-strands and the B chain forms one α-helix and two β-strands. The total folding pattern is analogous to a pathologically important amyloidgenic protein, cystatin c (Ghiso et al. 1986; Murzin 1993). We previously found a fibrous aggregation in the heat-denatured state of MN (Konno et al. 1999). This was the first report of the amyloid-like aggregation of a plant protein, and supported universality of this type of aggregation. The present results complete a kinetic scheme for the amyloidgenesis of MN including multistep nucleus formation and separate contributions from each subunit chain.
Results
Heat denaturation of monellin
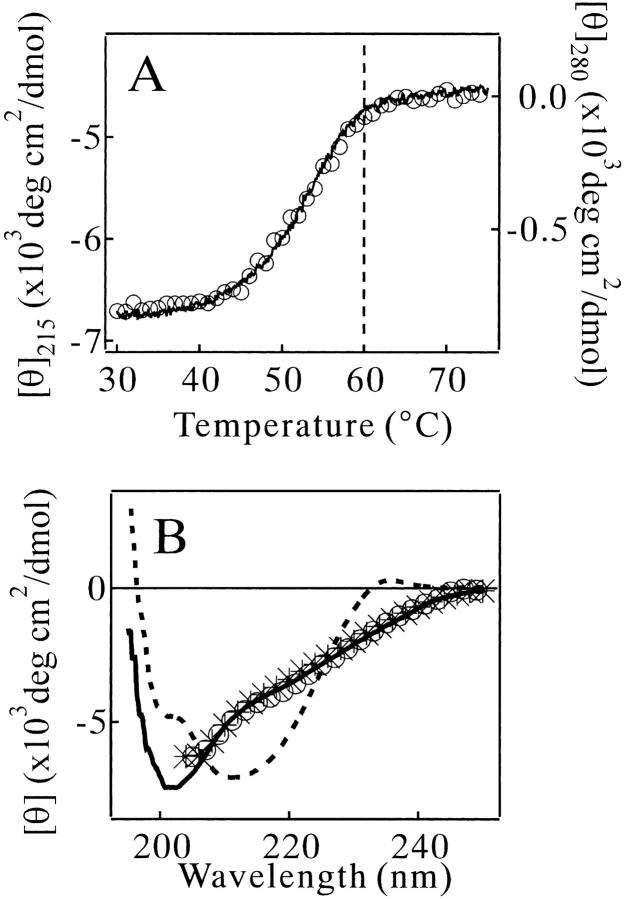
The heat denaturation of MN at pH 2.5 occurred cooperatively in the temperature range between 40 and 60°C (Fig. 1A ▶). After a proper normalization, the transition curve monitored by the far-UV circular dichroism (CD) spectrum was identical to that by the near-UV CD (open circles in Fig. 1A ▶), suggesting that no intermediately denatured state appeared. The denaturation temperature of MN was elevated by increasing the concentration of MN (data not shown), indicating that two chains of MN were dissociated in the heat-denatured state. However, a precise thermodynamic analysis could not be done because of partial irreversibility of the denaturation. The shape of the far-UV CD spectrum of MN in the heat-denatured state was not changed by the addition of 0.3 M NaCl or 0.5 M urea (Fig. 1B ▶), suggesting that the cosolvents did not affect the structure of the heat-denatured MN. The Kratky analysis of solution X-ray scattering (SAXS) of MN presented a typical case of the chain-shape transition from the globular type in the native state to the flexible-chain type in the denatured state (Fig. 2A ▶). The CD and SAXS analyses demonstrated that MN in the heat-denatured states was highly flexible and disordered.
Fig. 1.
(A) Heat denaturation curves of MN in 10 mM glycine (pH 2.5) and 0.3 M NaCl monitored by ellipticity at 215 nm (solid line) or 280 nm (○). The measurements were started at 25°C, and the temperature was increased by 1°C/min. The concentration of MN was 67 μM. (B) Far-UV CD spectra of MN in 10 mM glycine (pH 2.5). The concentration of MN was 45 μM. Broken line: at 25°C. Solid line: at 70°C with no cosolvent. (○): at 70°C in the presence of 0.3 M NaCl. (*): at 70°C in the presence of 0.5 M urea.
Fig. 2.
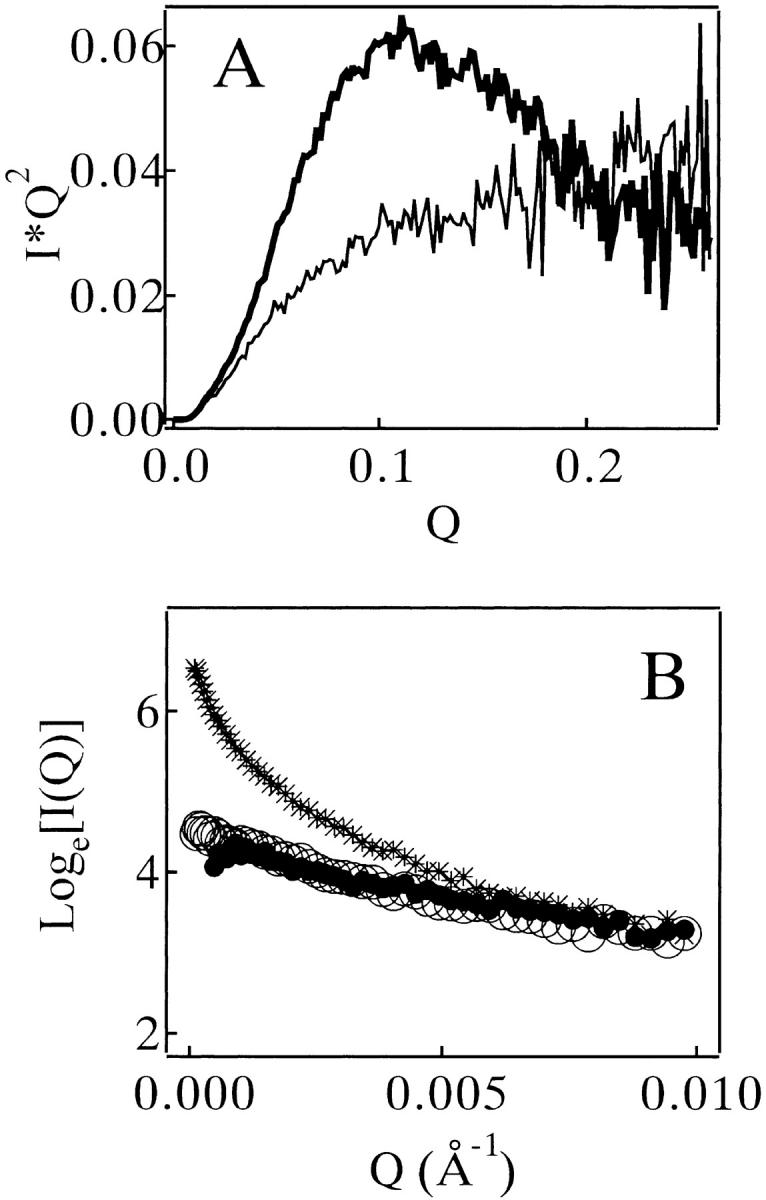
(A) Kratky profiles of SAXS of MN in 10 mM glycine (pH 2.5) at 25°C (bold line) and 75°C (thin line). The concentration of MN was 450 μM. (B) Guinier plots of SAXS of MN in 10 mM glycine (pH 2.5) measured at 25°C. The concentration of MN was 270 μM. (○): no cosolvent, no heating. (•): no cosolvent, heat-treated. (*): 0.1 M NaCl, heat-treated. The heat treatment was done at 75°C for 20 min, and then recovered at 25°C for 6 h.
A Guinier profile of SAXS of MN (270 μM, pH 2.5) without heating showed a clear Guinier region (open circles of Fig. 2B ▶); the radius of gyration (RG ) in the native state of MN was 17.0±0.4 Å. As long as no cosolvent was present, the heat treatment did not alter the profile significantly (filled circles in Fig. 2B ▶). With the addition of 0.1 M NaCl, the same heat treatment induced a change in the profile, demonstrating the aggregates formation (cross symbols in Fig. 2B ▶). The RG value for the heat-denatured state of MN could not be determined properly because of poor quality of its scattering data. The value for a recombinant single-chain monellin (SMN) was 28.6±0.3 Å at 75°C.
Amyloid-type aggregates of heat-denatured monellin
The aggregates formed in the NaCl-rich solution were characterized by various techniques. The MN solution (270 μM, pH 2.5) in the presence of 0.3 M NaCl was incubated at 67.5°C for 2 h and then recovered at 25°C for 1 h. The solution became turbid after the heat treatment. The aggregates had a fibrous appearance with a regular width of about 10 nm (Fig. 3A ▶) and bound strongly with thioflavin T (ThT) and Congo red (CR) (Fig. 4A ▶; see also Fig. 4 ▶ of Konno et al. 1999); these are typical signs for amyloid. Conventional CR staining of the aggregates was also performed, and birefringence was observed (data not shown). The amide I` band of the IR spectrum of the aggregates, located at an extremely low wavenumber, 1617 cm−1 (Fig. 4B ▶), is characteristic of amyloids with a high β-sheet content (Seshadri et al. 1999). Together the results showed that the aggregates of MN formed in the NaCl solution was typical amyloid (Table 1).
Fig. 3.
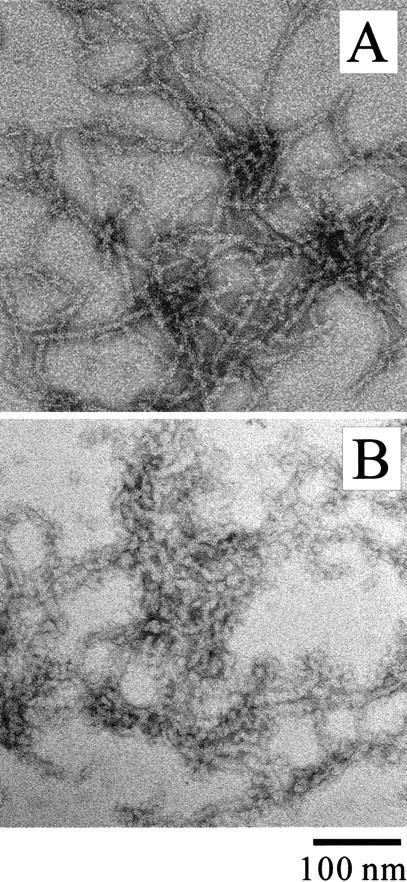
Negatively-stained TEM images of MN aggregates formed in 0.3 M NaCl (A) and 0.5 M urea (B).
Fig. 4.
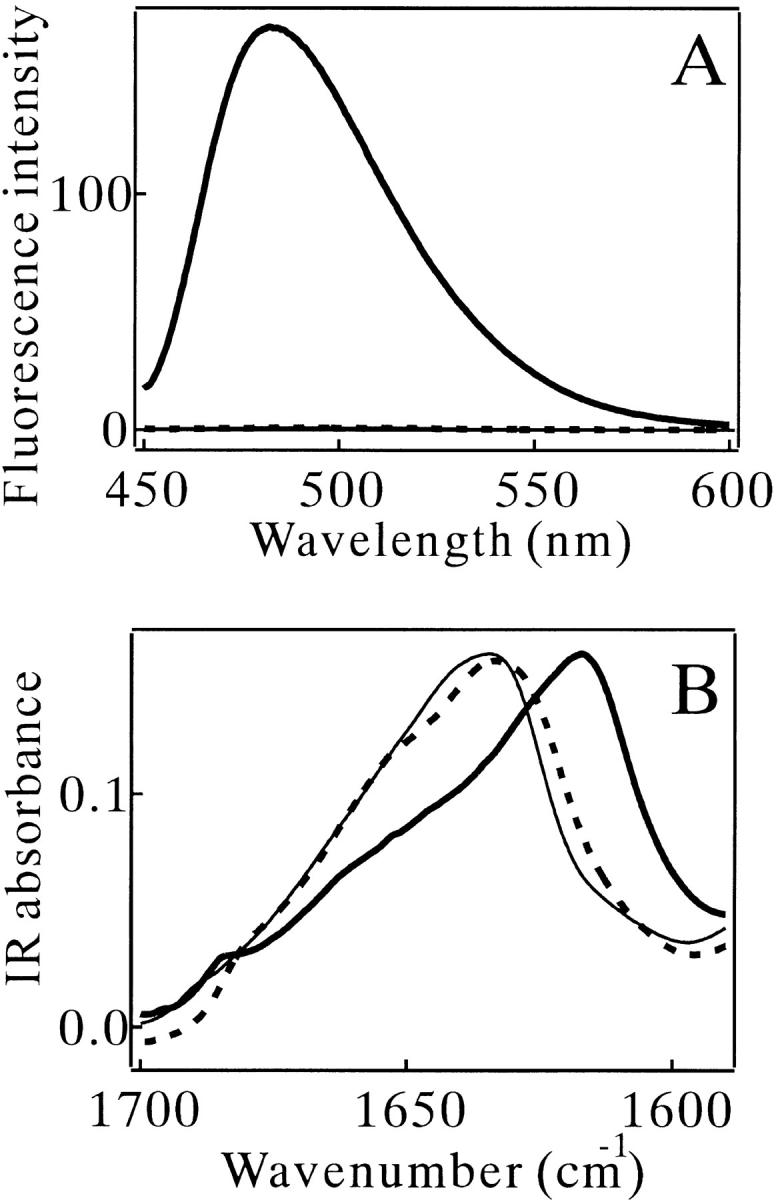
(A) Thioflavin T (ThT) fluorescence and (B) IR spectra of MN. Thin solid lines: no heating. Bold solid lines: heat-treated in 0.3 M NaCl. Broken lines: heat-treated without NaCl. The concentration of MN was 270 μM for (B). The measurements for (A) were done after 40× dilution in 10 mM Tris (pH 7.5) containing 10 μM ThT.
Table 1.
Aggregation properties of monellin (MN) chains at high temperature a
| Aggregation | Dye binding | |||
| Turbidity | Shape | ThT | CR | |
| MN, no heating | − | − | − | − |
| no cosolvent | − | − | − | − |
| 0.3 M NaCl | + | fibrous | + | + |
| 0.5 M urea | + | irregularc | ± | − |
| A1 chain,b 0.3 M NaCl | − | − | − | − |
| A2 chain,b 0.3 M NaCl | − | − | − | − |
| B chain, 0.3 M NaCl | + | fibrous | + | + |
| SMN, 0.3 M NaCl | + | irregular | ± | − |
ThT, thioflavin T; CR, congo red; SMN, a recombinant single-chain monellin.
a 270 μM of MN or SMN and 200 μM of the separate subunit chains were incubated at 67.5°C for 2 h, and then recovered at 25°C for 1 h.
b A1 or A2 chain is the A chain of MN without or with an additional Phe residue in the N-terminal part, respectively.
c The irregular aggregate contained some fibrous and granular components (Fig. 3B ▶).
For comparison, solution containing 0.5 M urea instead of NaCl was also tested. The heat-denatured MN in the urea solution formed a large amount of aggregates, but was not able to bind strongly to ThT or CR (Table 1). The TEM image showed an irregular shape of the aggregates containing granular and fibrous components (Fig. 3B ▶).
Physical factors that alter the amyloid formation kinetics
The kinetic trace of the amyloid formation of MN probed by the ThT binding had an initial lag phase, which was followed by a growth (elongation) phase and a final steady-state phase (Fig. 5A ▶). Both the amyloid growth rate and the length of the initial lag phase depended on the concentrations of MN and NaCl (Fig. 5A ▶). This indicated that molecular association events participated in both the kinetic phases. The temperature of incubation also affected the efficiency of the amyloid formation (Fig. 5B ▶). The most effective temperature in the present experimental condition was about 60°C (Fig. 5C ▶). Increasing and decreasing the temperature from 60°C reduced the efficiency mainly by prolonging the initial lag phase; the temperature change was less effective for the kinetic rate of the growth phase (Fig. 5B ▶). Note that the temperature of 60°C roughly corresponded to a higher border of the transition zone of the heat denaturation of MN (Fig. 1A ▶).
Fig. 5.
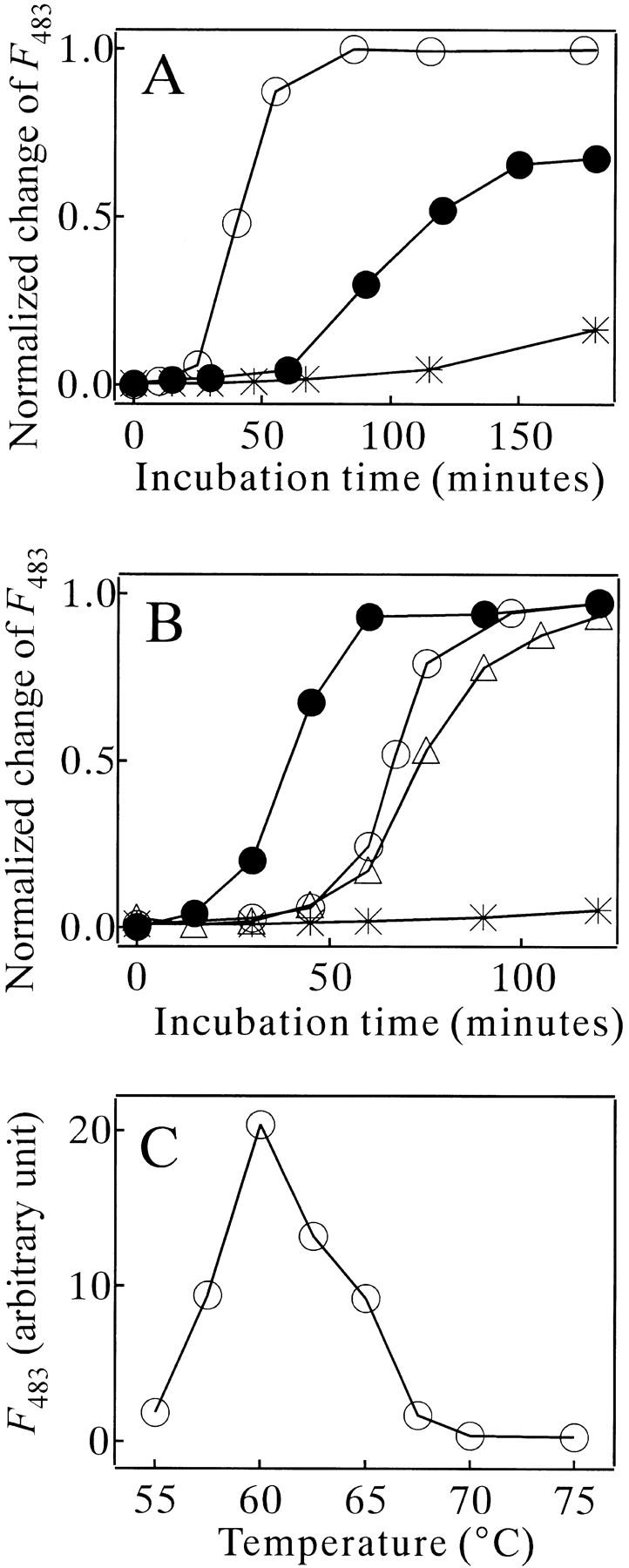
(A) Amyloid formation kinetics of MN monitored by ThT fluorescence at various concentrations of MN and NaCl. The MN samples were incubated at 75°C in 10 mM glycine (pH 2.5). (○) 90 μM MN in 0.3 M NaCl. (|*) 45 μM MN in 0.3 M NaCl. (•) 45 μM MN in 0.35 M NaCl. F483: fluorescence intensity of ThT emitted at 483 nm wavelength. (B) Amyloid formation kinetics of MN monitored by ThT fluorescence at 55°C (○), 60°C (•), 67.5°C (▵) and 75°C (|*). The MN concentration was 67 μM in 10 mM glycine (pH 2.5) and 0.3 M NaCl. Fluorescence changes for (A) and (B) were normalized with their maximum changes (achieved typically at 600 min). (C) ThT binding of MN measured after heat treatment for 45 min in 10 mM glycine (pH 2.5) and 0.3 M NaCl, plotted against the incubation temperature. The concentration of MN was 67 μM.
Amyloid formation kinetics probed by multiple methods
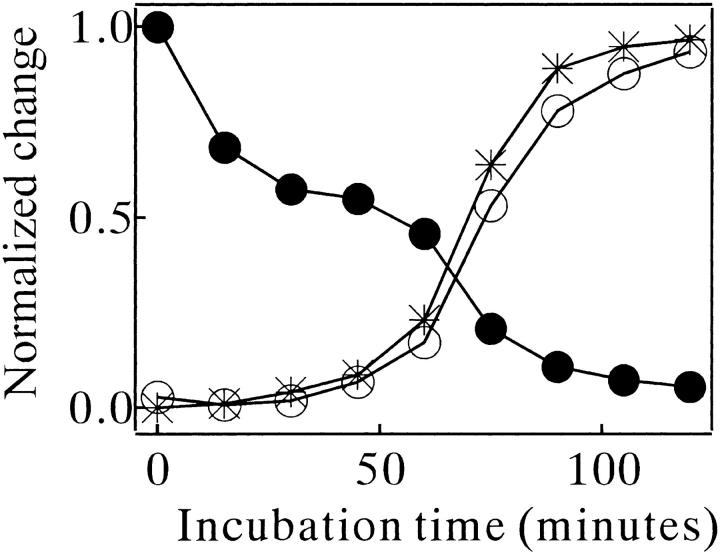
The kinetic data of the ThT binding were compared with those of turbidity and intrinsic tryptophan (Trp) fluorescence measurements. The turbidity changed in concert with the ThT binding (star symbols in Fig. 6 ▶). In contrast, the Trp fluorescence decreased in two discrete steps (filled circles in Fig. 6 ▶). The first step exactly corresponded to the initial lag phase of the ThT-binding trace, and the second step occurred during the period when the ThT binding grew. Essentially the same tendencies of turbidity and Trp fluorescence changes were observed at all concentrations of MN and NaCl used and at all temperatures examined.
Fig. 6.
Amyloid formation kinetics of MN in 10 mM glycine (pH 2.5) and 0.3 M NaCl at 67.5°C monitored by ThT binding (□), intrinsic Trp fluorescence (•) and turbidity (*). The concentration of MN was 67 μM. Each datum was normalized with its maximum change (achieved typically at 600 min).
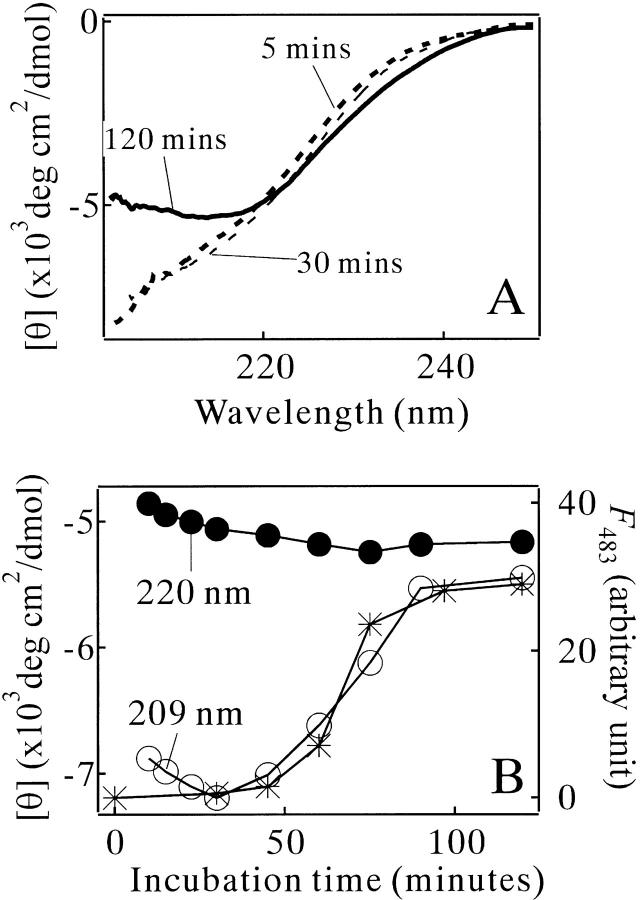
The kinetic process monitored by the far-UV CD spectroscopy gave information about secondary structural change. Within 5 min after the temperature elevation, the shape of the spectra became stable and was typical for a denatured protein (Fig. 7A ▶). In the initial lag phase, the CD spectral shape did not alter significantly. In the subsequent amyloid growth phase, the spectral shape was converted to what indicated a large content of secondary structures (Fig. 7A ▶). The minimum of the broad negative band of the spectrum at 120 min was ∼215 nm (Fig. 7A ▶), typical for a β-sheet conformation (Greenfield and Fasman 1969).
Fig. 7.
Amyloid formation kinetics of MN in 10 mM glycine (pH 2.5) and 0.3 M NaCl at 56°C monitored by the CD spectroscopy. (A) The far-UV CD spectra at the incubation times of 5 (bold broken line), 30 (thin broken line) and 120 min (bold solid line). (B) The kinetic changes of [θ]209 (○), [θ]220 (•) and ThT binding (*). F483: fluorescence intensity of ThT emitted at 483 nm wavelength. The concentration of MN was 67 μM.
A nucleation-dependent process in the amyloid formation of monellin
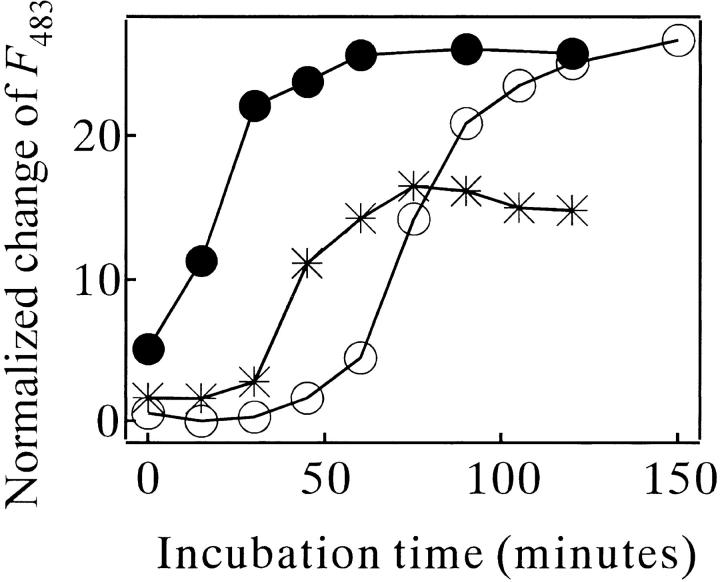
The initial lag phase of the amyloid formation kinetics indicates a nucleation-dependent mechanism (Jarrett and Lansbury 1993). Evidence of this mechanism in the present case was provided by testing the seeding effect (Harper and Lansbury 1997). The ThT fluorescence of the MN solution seeded by a small aliquot of the mature amyloid grew without a lag phase (filled circles in Fig. 8 ▶). The seed content rose abruptly during the stage when the ThT binding appeared marginally (star symbols in Fig. 8 ▶).
Fig. 8.
A seeding effect on the amyloid formation kinetics of MN (67 μM) in 10 mM glycine (pH 2.5) and 0.3 M NaCl at 67.5°C monitored by ThT binding. (○) no seeding. (•) seeded with 10 μM of fully-matured MN amyloid. The plot of (*) was made as follows. The sample at various incubation times at 67.5°C was added as a seed in one-tenth volume to a fresh sample. This seeded sample was then incubated at 67.5°C for 30 min, and its ThT binding was monitored. The ThT fluorescence intensity was plotted against the incubation period of the seed preparation. F483: fluorescence intensity of ThT emitted at 483 nm wavelength.
Interrupting the heat treatment in the initial lag phase provided additional information about the kinetic events. A series of experiments was done using the samples preheated to 67.5°C for various periods (Fig. 9A ▶). This preheating period did not exceed the initial lag period. After the heat treatment, the samples were incubated at 25°C for 24 h, and then the temperature was elevated again to 67.5°C to restart the amyloid formation. The results, monitored by ThT binding, revealed three notable features (Fig. 9A ▶). First, the amyloid growth of the preheated sample in the second heating period occurred rapidly by skipping the rest of the lag phase. This means that the preheated sample evolved to form the nucleus of the amyloid even at 25°C. Second, however, the preheated sample did not exhibit the ThT binding significantly at 25°C, indicating that the growth of the amyloid could not proceed at 25°C even when the nuclei were present. Third, the amyloid growth rate after the second elevation of temperature strongly depended on the length of the first heating period. This implies that the state of the sample after the long interval at 25°C was a function of the length of the first heating period. The kinetic trace monitored by the Trp fluorescence was consistent with the results of the ThT binding with respect to the disappearance of the middle plateau region (Fig. 9B ▶). It should also be noted that the Trp fluorescence intensity was irreversibly changed by the preheating treatment and was unchanged during the interval period at 25°C. A comparison of the data points before and after the interval period at 25°C illustrates this point (arrows in Fig. 9B ▶).
Fig. 9.
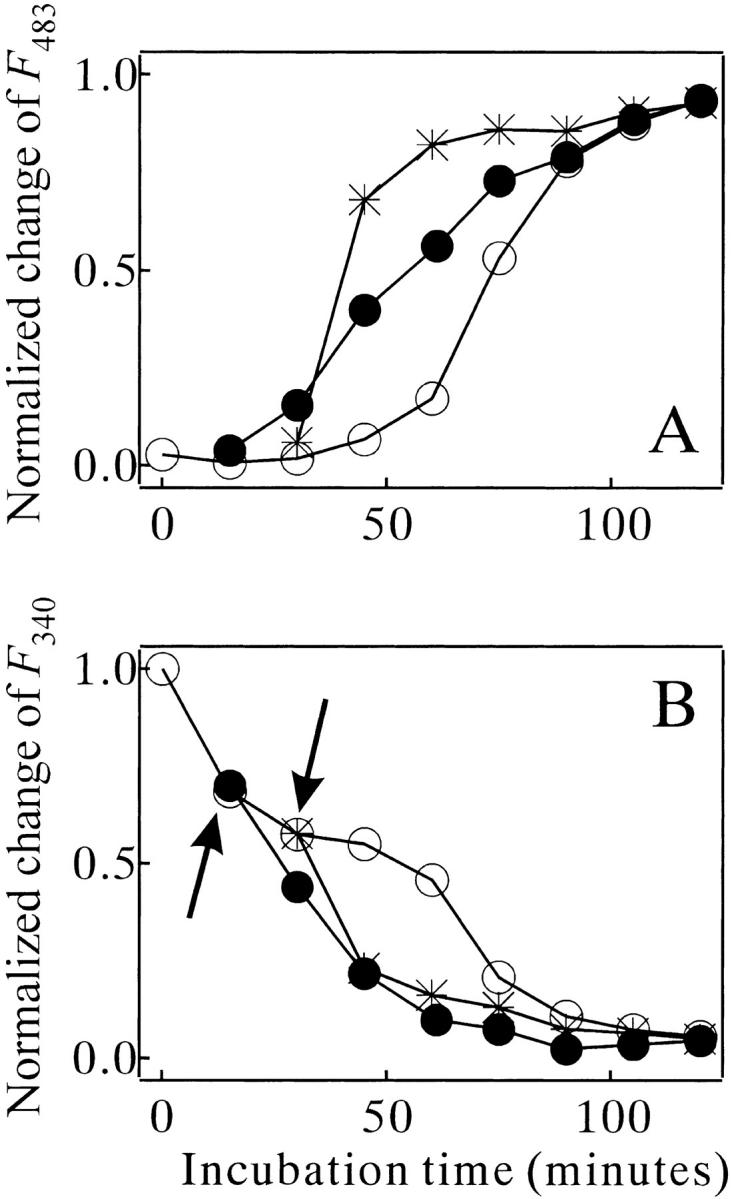
Effects of the preheating treatment on the amyloid formation kinetics of MN (67 μM) in 10 mM glycine (pH 2.5) and 0.3 M NaCl at 67.5°C, monitored by ThT binding (A) and intrinsic Trp fluorescence (B). The sample was preheated for 0 (○), 15 (•), and 30 min (*) at 67.5°C, and left for 24 h at 25°C before the second increase in the temperature. Arrows in (B): see the text. The traces of (•) and (*) start at 15 and 30 min because the preheating time is added to the second heat incubation time. F483: fluorescence intensity of ThT emitted at 483 nm wavelength.
Aggregation properties of separate subunit chains and a recombinant single-chain of monellin
The contributions of each subunit chain of MN to its amyloidgenesis were investigated by examining the aggregation properties of the A and B chains separately in the same solution condition as that used for MN (Table 1). Note that MN from a natural source contains two different subtypes of the A chain, one with an additional Phe residue in the N-terminal portion (Kohmura et al. 1990; designated as A1 and A2 chains in the present study). Table 1 summarizes the results, and the morphology of the aggregates of the B chain is shown in Figure 10A ▶. The results demonstrated that the B chain has the potential to form an amyloid-like aggregate with a fibrous shape and binding ability with ThT and CR. In contrast, the A1 and A2 chains did not form a significant amount of aggregates.
Fig. 10.
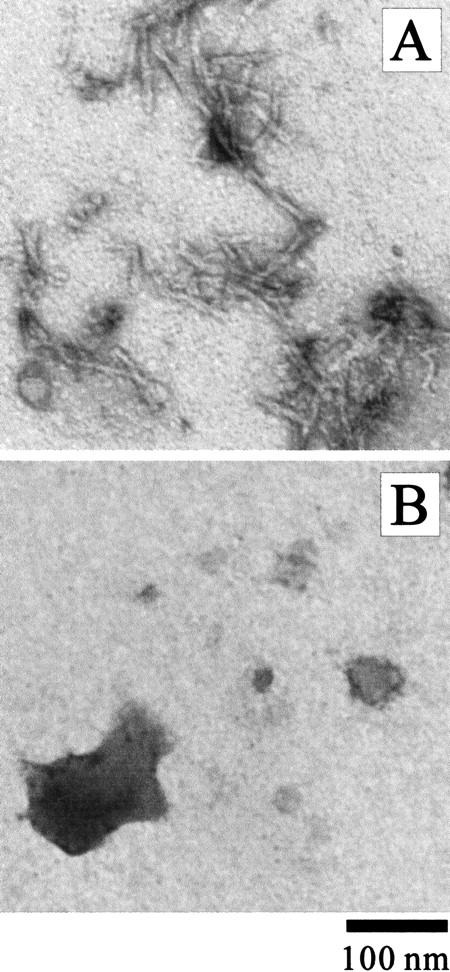
TEM images of the aggregates of the B chain of MN (A) and SMN (B) formed in 10 mM glycine (pH 2.5) and 0.3 M NaCl at 67.5°C.
For further insights into the subunit contribution, a recombinant single-chain monellin (designated SMN) was constructed in which the B chain is covalently connected to the A chain (see the Materials and Methods section). SMN is known to have the same folded structure as that of MN and slightly higher thermodynamic stability (Kim et al. 1989; Somoza et al. 1993; Spadaccini et al. 2001). The far- and near-UV CD spectra and the SAXS profiles of SMN at pH 2.5 were essentially the same as those of MN in both the native and the heat-denatured states (data not shown). Heat-denatured SMN in the presence of NaCl formed aggregates with strong turbidity. However, the aggregates had no amyloid-type feature (Table 1). The aggregates were amorphous in shape and did not bind strongly to ThT or CR (Fig. 10B ▶). The results indicated that the B chain lost its ability to form amyloid when it was connected to the A chain.
Discussion
Amyloidgenesis of MN
The present results confirm that the aggregates of MN in the NaCl solution are typical amyloid, with a solid regular fiber in shape, bind strongly to both ThT and CR, and contain the β-sheet type secondary structures. Amyloidgenesis of many amyloidgenic proteins proceeds via a partially unfolded state of protein (Kelly 1998; Rochet and Lansbury 2000). However, in the present study no partially denatured states of MN in the heat-denaturation transition were observed. The results indicate that the amyloidgenesis of MN was initiated in the highly denatured state (Figs. 1 and 2 ▶ ▶). However, a recent report showed that a partially folded species plays an essential role in amyloid formation of α-synuclein, which has a highly unfolded conformation in its native state (Uversky et al. 2001). At present we cannot exclude the possibility that the contribution of a minor population of partially folded species of MN is essential to the amyloid formation process in the present case. In fact, an unexpected temperature dependency of the amyloid formation rate of MN was observed. The most effective temperature was about 60°C (Fig. 5C ▶), which is at the higher border of the transition zone of the heat denaturation (Fig. 1A ▶). The isolated B-chain of MN, a candidate that forms the amyloid (Table 1), may take a specific partially folded conformation in this temperature range.
The urea-induced nonamyloid-type aggregation of MN was also interesting. The aggregates have a granular and slightly fibrous appearance (Fig. 3B ▶). They may be in some stage intermediate between the amyloid-type and amorphous-type, and may provide clues regarding mechanisms that convert the amorphous aggregation to the amyloid type. The urea-induced aggregation mechanism of MN is unknown at present. Urea usually increases the solubility of hydrophobic and backbone components of protein (Nozaki and Tanford 1963), and will prevent the protein from aggregating. Since the amine groups of urea protonate at acidic pH, the adjustment of the urea solution to pH 2.5 requires an excess amount of hydrochloride. The additional chloride ions may act similarly to those of NaCl. However, the excess did not exceed 2.5 mM in our preparation. Alternatively, the addition of urea may have induced a conformational change of the denatured MN chain, although the CD spectroscopy did not detect such a change (Fig. 1B ▶).
Kinetic properties of the amyloid formation of monellin
The amyloid formation kinetics of MN had an initial lag phase (Fig. 5 ▶), and the mature amyloid provided a seed to skip this phase (Fig. 8 ▶). This indicates that a nucleus formation step must precede the amyloid growth step. This nucleation-dependent mechanism is already well established in many amyloidgenic proteins such as β-amyloid (Harper and Lansbury 1997), paired helical filaments (Friedhoff et al. 1998), α-synuclein (Wood et al. 1999), IAPP (Kayed et al. 1999), and lysozyme (Krebs et al. 2000). A more important finding of the present study is the presence of multiple conformational events in the initial lag phase. These events could be examined because our experimental system made it possible to interrupt the amyloid formation process in the middle of the initial lag phase. As shown in Figure 9 ▶, once the MN molecule was heat-denatured for a significant length of time, the sample could evolve to make the nucleus of the amyloid even at 25°C. However, the amyloid growth rate after the second elevation of temperature was strongly enhanced by prolonging the initial heat-treatment period (Fig. 9A ▶). This implies that the MN molecule must reach a prenucleus state in the initial heating period before the nucleus formation at 25°C. The nucleus formation is composed of at least two distinct steps.
The structural events in the amyloid growth phase were (1) an increase in the β-sheet content, (2) a gain of binding ability of ThT and CR, (3) quenching of the Trp fluorescence, probably originating from an event burying the Trp residue in the fibril interior, and (4) growth of the aggregates up to a visible size. However, in the initial lag phase, only the quenching of the Trp fluorescence was observed (Fig. 6 ▶). This first step of the quenching preceded the growth of nucleus (cf. the filled circles of Fig. 6 ▶ and the star symbols of Fig. 8 ▶). It should also be noted that the quenching did not proceed at 25°C in the preheated sample (Fig. 9B ▶), although the nucleus formation process continued there. These results indicate that the first fluorescence-quenching step is coupled with a conformational transition from the denatured MN to the prenucleus stage, not with the final step of the nucleus formation. The quenching of the Trp fluorescence is probably coupled with a molecular assembly event to bury the Trp residue in the protein interior. It is possible that the B-chains of MN form specific self-aggregates during incubation at high temperature. The prenucleus state does not have any secondary structures or binding ability to the dyes. To form the mature nucleus in the next step, secondary clustering or a conformational transition of the prenucleus state must occur.
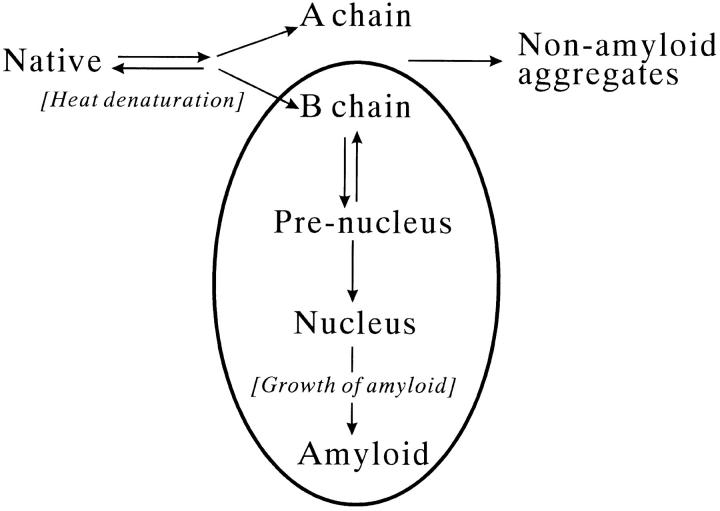
Figure 11 ▶ summarizes an overall kinetic scheme of the amyloid formation of MN. The scheme also includes an amorphous aggregation pathway, because we observed such aggregation in the presence of 0.5 M urea. The present results suggest that the B chain of MN contributes to the amyloidgenesis, and that the A chain does not (Table 1 and Fig. 10 ▶). Predicted pI`s of the A and B subunit chains are 9.46 and 5.33, respectively (using the protocol of Bjellqvist et al. 1993), which can explain the observation that the B chain formed aggregates while the A chain did not at the acidic pH (Table 1). The single Trp residue of MN is located on the B chain, which is consistent with the Trp fluorescence change in the amyloid formation (Fig. 6 ▶). The α-helical part of the B chain in the native state of MN (Somoza et al. 1993) seems to be converted to a β-sheet conformation in the amyloid formation process. The results obtained for SMN also support the exclusive contribution of the B chain to the amyloid formation of MN. The B chain that was covalently connected to the A chain still retained its ability to form aggregates, but the additional part prevented it from participating in the regular assembly.
Fig. 11.
An aggregation scheme of heat-denatured monellin.
The heat-dependent steps of the amyloidgenesis of MN were the prenucleus formation and the amyloid growth steps. The maturation of the nucleus was able to proceed at a lower temperature. This variation in the temperature dependency must be explained in terms of the molecular conformation of MN or its B chain. Another point to be addressed is the possible contribution of the chemical modification of MN at high temperature. Detailed conformational and chemical analyses of the separate chains of MN in the amyloid formation process should be done in the next stage of research. The present study has established a unique model of amyloidgenesis that is regulated by subunit composition of the protein.
Materials and methods
Materials
MN and CR were purchased from Sigma Chemicals. ThT was purchased from Molecular Probe. Other chemicals were of reagent grade, purchased from Nacalai Tesque. MN was purified as described by Fan et al. (1993). All of the sample solutions contained 10 mM glycine (pH 2.5), and their pH was adjusted carefully with small amounts of HCl. The concentration of MN was determined using the extinction coefficient ɛ277 = 1.46 × 104 M−1cm−1 (Morris and Cagan 1980).
Spectroscopic, SAXS, and microscopic measurements
CD spectra were measured with a Jasco J-720 WI spectropolarimeter using quartz cells with a lightpath of 0.1, 1, or 10 mm. The temperature of the solutions was controlled by a Jasco temperature controller within ± 0.1°C. The tryptophan (Trp) fluorescence of the heat-treated samples (typically 67 μM of MN) was measured using a Hitachi F2500 spectrofluorimeter in a quartz cell with a pathlength of 10 mm. The excitation wavelength was 295 nm, and the bandwidths of excitation/emission lights were 5 nm/5 nm. The samples for the infrared spectral measurement (3 mg/mL of MN) were prepared in D2O with 10 mM glycine (pH 2.5) and 0 or 0.3 M NaCl. They were heated at 67.5°C for 1 h and recovered at 25°C for 1 h. The reference MN sample without heating was prepared in D2O with 10 mM glycine (pH 2.5), and kept at 25°C for 24 h. For the IR measurements, the sample solutions were placed in a demountable liquid cell fitted with CaF2 windows, using a 100-μm spacer. The FT-IR spectra were recorded by means of a BioRad FTS575C infrared spectrometer. Two hundred scans at 2 cm−1 resolution were averaged for each sample. During the data acquisition, the spectrometer was purged with dry air. Reference spectra of buffer were acquired under the same scanning conditions. SAXS experiments were conducted at the solution scattering station (SAXS camera) installed at BL-10C, the Photon Factory, Tsukuba, Japan. The SAXS analysis is described elsewhere (Konno et al. 1995). Electron micrographs of aggregates were taken with a Hitachi H-7000 electron microscope operated at 80 kV, at ×20,000 magnification. The heat-treated MN samples were applied to carbon grids and stained with 2% uranyl acetate. The images were recorded on FG electron-microscopic films (Fuji Film) and developed in D-19 developer (Kodak) for 5 min.
ThT and CR binding analysis
For the ThT binding analysis, sample solutions were diluted 40 times with a buffer containing 10 mM Tris (pH 7.5). Immediately after the dilution, ThT was added to a final concentration of 10 μM. ThT binding was monitored by fluorescence measured by a Hitachi F2500 spectrofluorimeter. The excitation wavelength was 440 nm. The bandwidths of excitation/emission lights were 5 nm/5 nm for the spectral measurements and 2.5 nm/10 nm for monitoring the kinetics of aggregate formation. The measurement was started 20 sec after the mixtures were made. The ThT fluorescence after the dilution was stable for at least two min.
For the CR binding analysis, sample solutions were diluted 40 times with a buffer containing 10 mM Tris (pH 7.5) and CR (the final concentration was 10 μM). The CR binding was monitored by absorption spectra obtained with a Hitachi U-3300 spectrophotometer. The CR staining experiments were kindly supported by Dr. H. Naiki (Naiki and Gejyo 1999).
Separation of A- and B-chains of monellin
The isolation of each subunit chain of MN was done by the method of Kohmura et al. (1990) with a minor modification. Briefly, MN was dissolved in 20% acetonitrile containing 0.05% trifluoroacetic acid (TFA), and loaded onto a C18 reverse-phase column. The sample was eluted with 0.05% aqueous TFA and acetonitrile containing 0.05% TFA under linear gradient conditions of 20% to 50% acetonitrile being obtained in 60 min at a flow rate of 0.5 mL/min. The chromatogram was monitored with absorbance at 280 nm, and peaks corresponding to isolated MN chains were collected separately and lyophilized. Stock solutions of the peptides were prepared by dissolving in 20% acetonitrile (the peptide concentrations were 2.5 mM). These were diluted into 10 mM glycine (pH 2.5) and 0.3 M NaCl and heating to 67.5°C for the aggregation experiments.
Preparation of a recombinant single-chain monellin
cDNA encoding a recombinant single-chain monellin (SMN) was totally synthesized using an automatic DNA synthesizer, and cloned into the pET3a expression vector (Stratagene). The DNA sequence of the coding region is: atgggcgaat gggaaatcat cgatatcggc ccgtttaccc agaacctggg caaatttgcg gtggatgaag aaaacaaaat cggccagtat ggccgtctga cctttaacaa agtgatccgt ccgagcatga aaaaaaccat ctatgaaaac gagcgcgaaa tcaaaggcta tgaatatcag ctgtatgtgt atgcgagcga taaactgttt cgtgcggata tcagcgaaga ttataaaacc cgtggccgta aactgctgcg ttttaacggc ccggtgccgc cgccgtaa, which encodes the amino acid sequence M G E W E I I D I G P F T Q N L G K F A V D E E N K I G Q Y G R L T F N K V I R P S M K K T I Y E N EREKGYEYQLYVYASDKLFRADISEDYKTRG RKLLRFNGPVPPP. The DNA construct was transformed into BL21(DE3) pLysS Escherichia coli cells. The methods used for expression and purification of the recombinant SMN were as reported by Kim et al. (1989). The purity and molecular mass of SMN were checked by SDS-PAGE and ESI/MS mass spectroscopic analysis (TSQ7000, Finnigan MAT).
Acknowledgments
I thank Dr. H. Naiki for his help with the CR staining and birefringence experiments, and Dr. S. Takahashi, T. Kimura and Prof. Morishima for their support in the IR spectral measurements. The X-ray scattering measurements were performed with approval from the Program Advisory Committee of the Photon Factory (Proposal No. 97G127).
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked "advertisement" in accordance with 18 USC section 1734 solely to indicate this fact.
Abbreviations
MN, monellin
ThT, thioflavin T
CR, Congo red
CD, circular dichroism
TEM, transmission electron microscopy
SAXS, solution X-ray scattering
Trp, tryptophan
IR, infrared
SMN, a recombinant single-chain monellin
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1101/ps.20201.
References
- Bjellqvist, B., Hughes, G.J., Pasquali, C., Paquet, N., Ravier, F., Sanchez, J.C., Frutiger, S., and Hochstrasser, D. 1993. The focusing positions of polypeptides in immobilized pH gradients can be predicted from their amino acid sequences. Electrophoresis 14 1023–1031. [DOI] [PubMed] [Google Scholar]
- Chiti, F., Webster, P., Taddei, N., Clark, A., Stefani, M., Ramponi, G., and Dobson, C.M. 1999. Designing conditions for in vitro formation of amyloid protofilaments and fibrils. Proc. Natl. Acad. Sci. 96 3590–3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson, C. M. and Ellis, R. J. 1998. Protein folding and misfolding inside and outside the cell. EMBO J. 17 5251–5254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson, C.M. and Karplus, M. 1999. The fundamentals of protein folding: Bringing together theory and experiment. Curr. Opin. Struct. Biol. 9 92–101. [DOI] [PubMed] [Google Scholar]
- Fan, P., Bracken, C., and Baum, J. 1993. Structural characterization of monellin in the alcohol-denatured state by NMR: Evidence for β-sheet to α-helix conversion. Biochemistry 32 1573–1582. [DOI] [PubMed] [Google Scholar]
- Fink, A.L. 1998. Protein aggregation: Folding aggregates, inclusion bodies and amyloid. Fold. Des. 3 R9–23. [DOI] [PubMed] [Google Scholar]
- Friedhoff, P., von Bergen, M., Mandelkow, E.M., Davies, P., and Mandelkow, E. 1998. A nucleated assembly mechanism of Alzheimer paired helical filaments. Proc. Natl. Acad. Sci. 95 15712–15717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiso, J., Jensson, O., and Frangione, B. 1986. Amyloid fibrils in hereditary cerebral hemorrhage with amyloidosis of Icelandic type is a variant of gamma-trace basic protein (cystatin C). Proc. Natl. Acad. Sci. 83 2974–2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield, N. and Fasman, G.D. 1969. Computed circular dichroism spectra for the evaluation of protein conformation. Biochemistry 8 4108–4116. [DOI] [PubMed] [Google Scholar]
- Guijarro, J.I., Sunde, M., Jones, J.A., Campbell, I., and Dobson, C.M. 1998. Amyloid fibril formation by an SH3 domain. Proc. Natl. Acad. Sci. 95 4224–4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper, J.D. and Lansbury, P.T. Jr. 1997. Models of amyloid seeding in Alzheimer's disease and scrapie: Mechanistic truths and physiological consequences of the time-dependent solubility of amyloid proteins. Annu. Rev. Biochem. 66 385–407. [DOI] [PubMed] [Google Scholar]
- Jarrett, J.T. and Lansbury, P.T. Jr. 1993. Seeding "one-dimensional crystallization" of amyloid: A pathogenic mechanism in Alzheimer's disease and scrapie? Cell 73 1055–1058. [DOI] [PubMed] [Google Scholar]
- Kayed, R., Bernhagen, J., Greenfield, N., Sweimeh, K., Brunner, H., Voelter, W., and Kapuriniotu, A. 1999. Conformational transitions of islet amyloid polypeptide (IAPP) in amyloid formation in vitro. J. Mol. Biol. 287 781–796. [DOI] [PubMed] [Google Scholar]
- Kelly, J.W. 1998. The alternative conformations of amyloidogenic proteins and their multi-step assembly pathways. Cur. Opin. Struct. Biol. 8 101–106. [DOI] [PubMed] [Google Scholar]
- Kim, S.-H., Kang, C.-H., Kim, R., Cho, J. M., Lee, Y.-B., and Lee, T.K. 1989. Redesigning a sweet protein: Increased stability and renaturability. Protein Eng. 2 571–575. [DOI] [PubMed] [Google Scholar]
- Kohmura, M., Nio, N., and Ariyoshi, Y. 1990. Complete amino acid sequence of the sweet protein monellin. Agri. Biol. Chem. 54 2219–2224. [PubMed] [Google Scholar]
- Konno, T., Kataoka, M., Kamatari, Y., Kanaori, K., Nosaka, A., and Akasaka, K. 1995. Solution X-ray scattering analysis of cold- heat-, and urea-denatured states in a protein, Streptomyces subtilisin inhibitor. J. Mol. Biol. 251 95–103. [DOI] [PubMed] [Google Scholar]
- Konno, T., Murata, K., and Nagayama, K. 1999. Amyloid-like aggregates of a plant protein: A case of a sweet-tasting protein, monellin. FEBS Lett. 454 122–126. [DOI] [PubMed] [Google Scholar]
- Koo, E.H., Lansbury, P.T. Jr., and Kelly, J.W. 1999. Amyloid diseases: Abnormal protein aggregation in neurodegeneration. Proc. Natl. Acad. Sci. 96 9989–9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs, M.R., Wilkins, D.K., Chung, E.W., Pitkeathly, M.C., Chamberlain, A.K., Zurdo, J., Robinson, C.V., and Dobson, C.M. 2000. Formation and seeding of amyloid fibrils from wild-type hen lysozyme and a peptide fragment from the beta-domain. J. Mol. Biol. 300 541–549. [DOI] [PubMed] [Google Scholar]
- Morris, J.A. and Cagan, R.H. 1972. Purification of monellin, the sweet principle of Dioscoreophyllum cumminsii. Biochim. Biophys. Acta 261 114–122. [DOI] [PubMed] [Google Scholar]
- Morris, J.A. and Cagan, R.H. 1980. Formation of oligomeric monellin in protein denaturants. Proc. Soc. Exp. Biol. Med. 164 351–354. [DOI] [PubMed] [Google Scholar]
- Murzin, A.G. 1993. Sweet-tasting protein monellin is related to the cystatin family of thiol proteinase inhibitors. J. Mol. Biol. 230 689–694. [DOI] [PubMed] [Google Scholar]
- Naiki, H. and Gejyo, F. 1999. Kinetic analysis of amyloid fibril formation. Methods Enzymol. 309 305–318. [DOI] [PubMed] [Google Scholar]
- Nozaki, Y. and Tanford, C. 1963. The solubility of amino acids and related compounds in aqueous urea solutions. J. Biol. Chem. 238 4074–4081. [PubMed] [Google Scholar]
- Prusiner, S.B., Scott, M.R., DeArmond, S.J., and Cohen, F.E. 1998. Prion protein biology. Cell 93 337–348. [DOI] [PubMed] [Google Scholar]
- Rochet, J.-C. and Lansbury, P.T. Jr. 2000. Amyloid fibrillogenesis: Themes and variations. Curr. Opin. Struct. Biol. 10 60–68. [DOI] [PubMed] [Google Scholar]
- Seshadri, S., Khurana, R., and Fink, A.L. 1999. Fourier transform infrared spectroscopy in analysis of protein deposits. Methods Enzymol. 309 559–576. [DOI] [PubMed] [Google Scholar]
- Sipe, J.D. 1992. Amyloidosis. Annu. Rev. Biochem. 61 947–975. [DOI] [PubMed] [Google Scholar]
- Somoza, J. R., Jiang, F., Tong, L., Kang, C.-H., Cho, J. M., and Kim, S.-H. 1993. Two crystal structures of a potently sweet protein. Natural monellin at 2.75 A resolution and single-chain monellin at 1.7 A resolution. J. Mol. Biol. 234 390–404. [DOI] [PubMed] [Google Scholar]
- Spadaccini, R., Crescenzi, O., Tancredi, T., De Casamassimi, N., Saviano, G., Scognamiglio, R., Di Donato, A., and Temussi, P.A. 2001. Solution structure of a sweet protein: NMR study of MNEI, a single chain monellin. J. Mol. Biol. 305 505–514. [DOI] [PubMed] [Google Scholar]
- Tan, S.Y. and Pepys, M.B. 1994. Amyloidosis. Histopathology 25 403–414. [DOI] [PubMed] [Google Scholar]
- Tomic, M.T., Somoza, J.R., Wemmer, D.E., Park, Y.W., Cho, J.M., and Kim, S.H. 1992. 1H resonance assignments, secondary structure and general topology of single-chain monellin in solution as determined by 1H 2D-NMR. J. Biomol. NMR 2 557–572. [DOI] [PubMed] [Google Scholar]
- Uversky, V. N., Li, J., and Fink, A. L. 2001. Evidence for a partially folded intermediate in α-synuclein fibril formation. J. Biol. Chem. 276 10737–10744. [DOI] [PubMed] [Google Scholar]
- Wood, S.J., Wypych, J., Steavenson, S., Louis, J.-C., Citron, M., and Biere, A.L. 1999. α-Synuclein fibrillogenesis is nucleation-dependent. Implications for the pathogenesis of Parkinson's disease. J. Biol. Chem. 274 19509–19512. [DOI] [PubMed] [Google Scholar]