Abstract
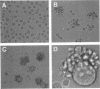
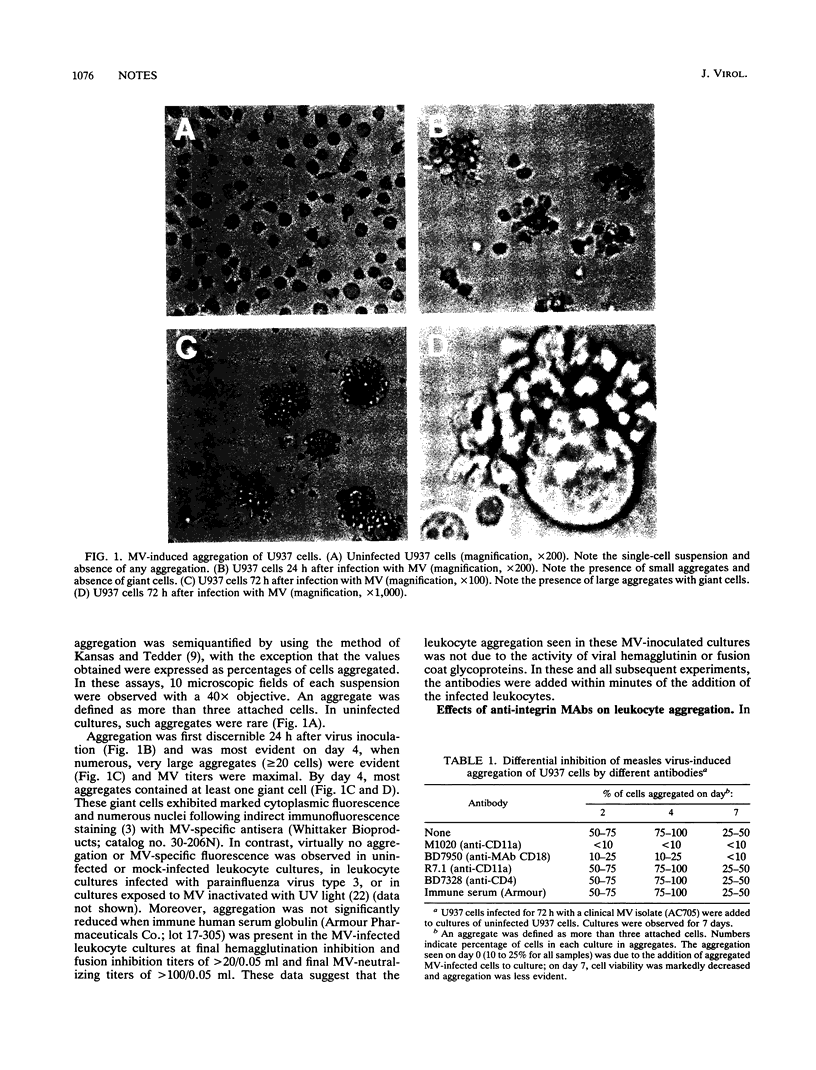
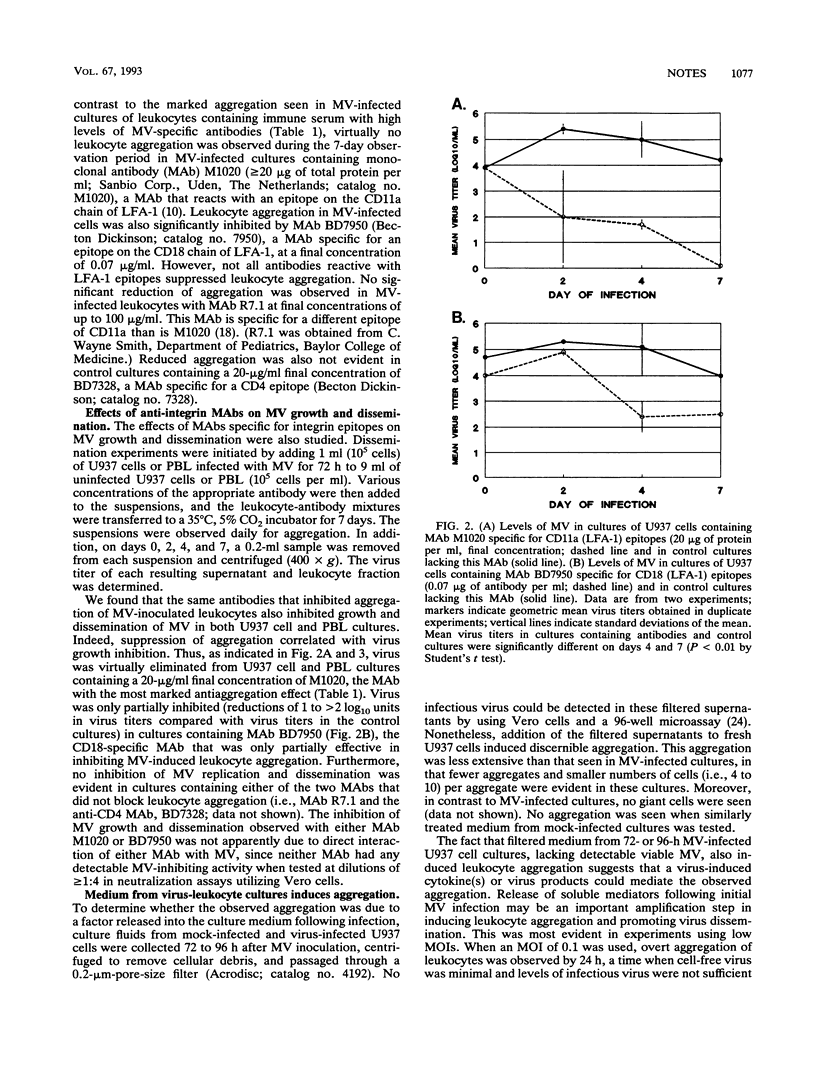
Measles virus (MV) infection of U937 cell or peripheral blood leukocyte cultures was shown to induce changes in the expression of leukocyte function antigen 1 (LFA-1) and cause marked aggregation of these cells. Addition of selected monoclonal antibodies specific for LFA-1 epitopes that did not neutralize MV in standard neutralization assays were found to block both virus-induced leukocyte aggregation and virus dissemination. These data suggest that MV modulation of LFA-1 expression on leukocytes may be an important step in MV pathogenesis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- COONS A. H. Fluorescent antibody methods. Gen Cytochem Methods. 1958;1:399–422. [PubMed] [Google Scholar]
- GRESSER I., CHANY C. Isolation of measles virus from the washed leucocytic fraction of blood. Proc Soc Exp Biol Med. 1963 Jul;113:695–698. doi: 10.3181/00379727-113-28465. [DOI] [PubMed] [Google Scholar]
- Hildreth J. E., Orentas R. J. Involvement of a leukocyte adhesion receptor (LFA-1) in HIV-induced syncytium formation. Science. 1989 Jun 2;244(4908):1075–1078. doi: 10.1126/science.2543075. [DOI] [PubMed] [Google Scholar]
- Johnson R. T. The pathogenesis of acute viral encephalitis and postinfectious encephalomyelitis. J Infect Dis. 1987 Mar;155(3):359–364. doi: 10.1093/infdis/155.3.359. [DOI] [PubMed] [Google Scholar]
- Joseph B. S., Lampert P. W., Oldstone M. B. Replication and persistence of measles virus in defined subpopulations of human leukocytes. J Virol. 1975 Dec;16(6):1638–1649. doi: 10.1128/jvi.16.6.1638-1649.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kansas G. S., Tedder T. F. Transmembrane signals generated through MHC class II, CD19, CD20, CD39, and CD40 antigens induce LFA-1-dependent and independent adhesion in human B cells through a tyrosine kinase-dependent pathway. J Immunol. 1991 Dec 15;147(12):4094–4102. [PubMed] [Google Scholar]
- Keizer G. D., Borst J., Figdor C. G., Spits H., Miedema F., Terhorst C., De Vries J. E. Biochemical and functional characteristics of the human leukocyte membrane antigen family LFA-1, Mo-1 and p150,95. Eur J Immunol. 1985 Nov;15(11):1142–1148. doi: 10.1002/eji.1830151114. [DOI] [PubMed] [Google Scholar]
- Keizer G. D., Visser W., Vliem M., Figdor C. G. A monoclonal antibody (NKI-L16) directed against a unique epitope on the alpha-chain of human leukocyte function-associated antigen 1 induces homotypic cell-cell interactions. J Immunol. 1988 Mar 1;140(5):1393–1400. [PubMed] [Google Scholar]
- Kipps A., Dick G., Moodie J. W. Measles and the central nervous system. Lancet. 1983 Dec 17;2(8364):1406–1410. doi: 10.1016/s0140-6736(83)90932-7. [DOI] [PubMed] [Google Scholar]
- McChesney M. B., Fujinami R. S., Lerche N. W., Marx P. A., Oldstone M. B. Virus-induced immunosuppression: infection of peripheral blood mononuclear cells and suppression of immunoglobulin synthesis during natural measles virus infection of rhesus monkeys. J Infect Dis. 1989 Apr;159(4):757–760. doi: 10.1093/infdis/159.4.757. [DOI] [PubMed] [Google Scholar]
- Modai D., Pik A., Marmor Z., Weissgarten J., Cohen N., Averbukh Z., Golik A., Rosenmann E. Liver dysfunction in measles, liver biopsy findings. Dig Dis Sci. 1986 Mar;31(3):333–333. doi: 10.1007/BF01318127. [DOI] [PubMed] [Google Scholar]
- Moench T. R., Griffin D. E., Obriecht C. R., Vaisberg A. J., Johnson R. T. Acute measles in patients with and without neurological involvement: distribution of measles virus antigen and RNA. J Infect Dis. 1988 Aug;158(2):433–442. doi: 10.1093/infdis/158.2.433. [DOI] [PubMed] [Google Scholar]
- Patarroyo M. Leukocyte adhesion in host defense and tissue injury. Clin Immunol Immunopathol. 1991 Sep;60(3):333–348. doi: 10.1016/0090-1229(91)90091-n. [DOI] [PubMed] [Google Scholar]
- Patarroyo M., Prieto J., Ernberg I., Gahmberg C. G. Absence, or low expression, of leukocyte adhesion molecules CD11 and CD18 on Burkitt lymphoma cells. Int J Cancer. 1988 Jun 15;41(6):901–907. doi: 10.1002/ijc.2910410623. [DOI] [PubMed] [Google Scholar]
- Rossen R. D., Smith C. W., Laughter A. H., Noonan C. A., Anderson D. C., McShan W. M., Hurvitz M. Y., Orson F. M. HIV-1-stimulated expression of CD11/CD18 integrins and ICAM-1: a possible mechanism for extravascular dissemination of HIV-1-infected cells. Trans Assoc Am Physicians. 1989;102:117–130. [PubMed] [Google Scholar]
- Salonen R., Ilonen J., Salmi A. Measles virus infection of unstimulated blood mononuclear cells in vitro: antigen expression and virus production preferentially in monocytes. Clin Exp Immunol. 1988 Feb;71(2):224–228. [PMC free article] [PubMed] [Google Scholar]
- Smith C. W., Rothlein R., Hughes B. J., Mariscalco M. M., Rudloff H. E., Schmalstieg F. C., Anderson D. C. Recognition of an endothelial determinant for CD 18-dependent human neutrophil adherence and transendothelial migration. J Clin Invest. 1988 Nov;82(5):1746–1756. doi: 10.1172/JCI113788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan J. L., Barry D. W., Lucas S. J., Albrecht P. Measles infection of human mononuclear cells. I. Acute infection of peripheral blood lymphocytes and monocytes. J Exp Med. 1975 Sep 1;142(3):773–784. doi: 10.1084/jem.142.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C. S., Wyde P. R., Wilson S. Z., Knight V. Efficacy of aerosolized recombinant interferons against vesicular stomatitis virus-induced lung infection in cotton rats. J Interferon Res. 1984 Fall;4(4):449–459. doi: 10.1089/jir.1984.4.449. [DOI] [PubMed] [Google Scholar]
- White R. G., Boyd J. F. The effect of measles on the thymus and other lymphoid tissues. Clin Exp Immunol. 1973 Mar;13(3):343–357. [PMC free article] [PubMed] [Google Scholar]
- Wyde P. R., Ambrose M. W., Voss T. G., Meyer H. L., Gilbert B. E. Measles virus replication in lungs of hispid cotton rats after intranasal inoculation. Proc Soc Exp Biol Med. 1992 Oct;201(1):80–87. doi: 10.3181/00379727-201-43483. [DOI] [PubMed] [Google Scholar]