Abstract
An increase in the incidence of aneuploidy is well documented with increasing maternal age, in particular in human females. Remarkably, little is known regarding the underlying molecular basis for the age-associated increase in aneuploidy, which is a major source of decreased fertility in humans. Using mouse as a model system we find that eggs obtained from old mice (60–70 weeks of age) display a six-fold increase in the incidence of hyperploidy as assessed by chromosome spreads. Expression profiling of transcripts in oocytes and eggs obtained from young and old mice reveals that ~5% of the transcripts are differentially expressed in oocytes obtained from old females when compared to oocytes obtained from young females (6–12 weeks of age) and that this fraction increases to ~33% in eggs. The latter finding indicates that the normal pattern of degradation of maternal mRNAs that occurs during oocyte maturation is dramatically altered in eggs obtained from old mice and could therefore be a contributing source to the decline in fertility. Analysis of the differentially expressed transcripts also indicated that the strength of the spindle assembly checkpoint is weakened and that higher errors of microtubule-kinetochore interactions constitute part of molecular basis for the ageassociated increase in aneuploidy in females. Last, BRCA1 expression is reduced in oocytes obtained from old females and RNAi-mediated reduction of BRCA1 in oocytes obtained from young females results in perturbing spindle formation and chromosome congression following maturation.
Keywords: aneuploidy, expression profiling, gene expression, mouse oocyte, aging, mRNA degradation
Introduction
A hallmark of animal development is an age-related decrease in fertility. In mammals, this is largely attributed to females producing eggs of reduced developmental competence. In humans, egg donation studies (Navot et al., 1994; Sauer, 1998) indicate that the egg, rather than the reproductive tract, is the major cause of infertility, with a marked increase in infertility commencing around 35 years-of-age. An increase in aneuploidy is likely the major underlying factor responsible for the increase in infertility with advancing age in human females (Hassold and Chiu, 1985; Hassold and Hunt, 2001; Pellestor et al., 2002). Aneuploidy is a leading cause of pregnancy loss, and when development goes to term, aneuploidy is an aggravating source of developmental disabilities and mental retardation, e.g., trisomy 21.
The incidence of aneuploidy in eggs from women in their 20s’ is ~2%, but dramatically increases to 35% around 40 years-of-age. It is likely to be even higher because the connection between a spontaneous abortion and the underlying aneuploidy is frequently not recognized; it is estimated that by the fifth decade of life as many as 50% of the ovulated eggs are aneuploid (Hassold, 1986; Hunt and Hassold, 2002). Most aneuplodies associated with increased maternal age are due to non-disjunction and meiotic errors that occur during meiosis I (Lamb et al., 2005; Pellestor et al., 2002). Spindle abnormalities and faulty chromosome congression on the metaphase plate are associated with advanced maternal age and likely contribute to the observed increased incidence of aneuploidy (Battaglia et al., 1996). Remarkably, little is known regarding the underlying molecular basis for the age-associated increase in aneuploidy.
In every eukaryotic cell division, it is critical that chromosomes form correct attachments to spindle microtubules before chromosome separation in anaphase (Musacchio and Hardwick, 2002; Musacchio and Salmon, 2007). If anaphase begins before correct attachments are established, chromosomes will not segregate equally, leading to aneuploid daughter cells. A feedback control mechanism, the spindle assembly checkpoint (SAC), monitors kinetochore-MT interactions and delays the onset of anaphase until all chromosomes are correctly attached (Musacchio and Salmon, 2007). Unattached kinetochores activate the checkpoint, whereas attachment of all kinetochores silences the checkpoint so that anaphase can proceed
A priori oocytes should possess a robust SAC to minimize production of aneuploid eggs that would severely compromise female reproductive fitness. In contrast to mitosis or MII, it takes several hours to form a bipolar MI spindle, typically 6–8 h following germinal vesicle breakdown (GVBD) in mice (Brunet et al., 1999). In order to minimize aneuploidy the presence of a robust SAC would provide sufficient time for the bivalents to attach correctly after MI spindle formation.
In fact, oocytes do mount a robust SAC. MT poisons, such as nocodazole, activate the SAC by preventing MT attachment to kinetochores. For example, treating mouse oocytes with nocodazole under conditions that totally disrupt spindle assembly stabilizes both securin and cyclin B (and hence maintains high levels of CDK1 activity) and prevents homolog disjunction (Homer et al., 2005a; Homer et al., 2005b). Ablating MAD2 with a Mad2 antisense morpholino in the presence of nocodazole results in degradation of both securin and cyclin B1. In the absence of nocodazole, MAD2-depleted oocytes enter MI precociously and an increased incidence of aneuploidy is observed (Homer et al., 2005b). Consistent with this finding is that maturation of oocytes heterozygous for Mad2 results in chromosomes improperly segregating during MI with the consequent formation of an aneuploid MII egg (Niault et al., 2007). These findings demonstrate that the SAC is responsible for delaying anaphase and contributes to preventing aneuploidy in oocytes.
Reduced strength of the SAC in old oocytes could be a contributing factor to the increased incidence of aneuploidy associated with increasing maternal age, because chromosomes will separate at anaphase before correct MT attachments have been established. Based on our current understanding of checkpoint signaling, potential causes of a weakened checkpoint include (1) changes in expression levels of key components, (2) failure to localize checkpoint signaling proteins to unattached kinetochores, (3) reduced turnover of these proteins at kinetochores, which would prevent the generation of a diffusible signal. The observation that old mouse oocytes enter MI earlier than their younger counterparts is consistent with reduced SAC strength (Eichenlaub-Ritter and Boll, 1989). In addition, an age-associated decrease in chromosome cohesion could contribute to the observed increase in aneuploidy (Hodges et al., 2005).
Female mice also display an age-associated decline in fertility. For example, older mice have fewer implantation sites and a higher incidence of embryo resorption (Holinka et al., 1979). Mice exhibit an age-associated increase in aneuploidy (Eichenlaub-Ritter and Boll, 1989; Zuccotti et al., 1998b; Liu and Keefe, 2002; Cukurcam et al., 2007) that has been attributed, at least in part, to a faster progression through the first meiotic division in oocytes from aged females, which would compromise the time available for proper chromosome congression prior to chromosome segregation (Eichenlaub-Ritter and Boll, 1989). Thus, mouse may be an appropriate model to study the effect of age on egg quality, including the molecular basis for the age-associated increase in aneuploidy.
Senescence-accelerated mice (SAM) become prematurely infertile relative to most strains of laboratory mice, e.g., SAM fail to breed around 8–9 months-of-age (Liu and Keefe, 2002). An increased incidence of misaligned chromosomes at both MI and MII, as well as gross perturbations in spindle morphology are observed in oocytes obtained from old, but not young, SAM. We elected not to use these mice for our studies because the molecular basis for senescence acceleration is not known. Thus, the observed phenotype in SAM may not accurately reflect molecular mechanisms that underlie the age-associated increase in aneuploidy, i.e., it is best to use old mice to study this association.
We report here further evidence of an age-associated increase in aneuploidy in mice as assessed by cytogenetic analysis of chromosome numbers from (6–12 weeks-ofage) and old (66 weeks-of-age) eggs. Expression profiling global patterns of gene expression of oocytes obtained from young and old mice revealed mis-expression of many genes, including genes involved in the SAC, chromosome congression and attachment to kinetocore microtubules, and spindle assembly. In addition, the normal pattern of degradation of maternal mRNAs was not observed following maturation of old oocytes. Expression of BRCA1 is reduced in old oocytes and RNAi-mediated reduction of BRCA1 in young oocytes perturbs spindle formation, chromosome alignment on the spindle, and formation of multiple spindle poles, leading to unequal chromosome segregation and aneuploidy. Taken together, these results provide further support that the mouse is a suitable model to study age-related effects on aneuploidy.
Materials and Methods
Oocyte and egg collection
B6SJLF1 female mice were purchased from Jackson Laboratory, old B6D2F1 mice were from National Institutes of Aging and CF1 mice from Harlan Sprague–Dawley (Indianapolis, IN). Groups of mice ranging in age from 6–8 weeks, 12 months and 15–16 months were given an ip injection of 5 IU equine chorionic gonadotropin (eCG) 44 h prior to harvest of germinal vesicle (GV)-intact oocytes and cumulus cell-free oocytes were isolated as previously described (Schultz et al., 1983). Metaphase II-arrested eggs were obtained by first injecting mice with 5 IU eCG and then with 5 IU hCG 44 h later; MII-arrested eggs were harvested from the oviducts 16 h later. Cumulus cells were removed by a brief treatment with hyaluronidase (3 mg/ml) in M2 medium at room temperature. For maturation in vitro, oocytes were collected milrinone-containing (2.5 µM) MEM and then transferred to CZB medium (Chatot et al., 1989) containing 2.5 µM milrinone to inhibit spontaneous maturation; milrinone is a specific PDE3 inhibitor (Wiersma et al., 1998). Maturation was initiated by first washing the oocytes through three drops of MEM and then transferring them to milrinone-free CZB and the oocytes were cultured at 37° C in an atmosphere of 5% CO2 in air. All animal experiments were approved by the Institutional Animal Use and Care Committee and were consistent with National Institutes of Health guidelines.
RNA isolation, amplification, and microarray analysis
Four sets of oocytes (25 oocytes/sample) and eggs (35 eggs/sample) were collected for microarray analysis from mice 6–8 weeks and 15 months old. Oocytes or eggs were washed in MEM medium and transferred to 20 µl of extraction buffer (Acturus, Mountain View, CA). The cells were then immediately used or stored at −80° C until use. RNA was extracted, amplified and converted to biotinylated cRNA as previously described (Pan et al., 2005); for each replicate 30–70 µg of cRNA were generated. The cRNA was submitted to the Penn Microarray Facility and hybridized to MOE430 v2 GeneChips, ,which covers 39,000 transcripts and variants. Statistical analysis for microarray data and real-time RT-PCR using gene-specific primers were performed as described previously (Pan et al., 2005). Data concerning quality controls parameters, e.g., % present, 3’–5’ ratios for actin and Gapdh are found in Table S1.
dsRNA preparation and microinjection
The dsRNA used to target mouse Brca1 mRNA spans 480 nucleotides (nucleotide 3480–4229), and has no homology with other mRNA by BLAST analysis. The DNA fragment was amplified by RT-PCR, cloned in the PCR II vector, and used as the template for SP6 and T7 RNA polymerases (Promega, Madison, WI) to obtain sense and antisense RNAs. The primers were: 5’-aactagagcacagcgtatgccagagaaa-3’ and 5’- taactcgagcacagcgtatgcca-3’. In vitro transcription and annealing were performed as described previously (Svoboda et al., 2000).
GV-oocytes were microinjected in bicarbonate-free Whitten’s medium (Whitten, 1971) under mineral oil (Sigma) with 10 pl of 1 µg/µl long dsRNA for Brca1, or long dsRNA for GFP as previously described (Svoboda et al., 2000). The injected oocytes were then incubated for 24 h in CZB medium containing 2.5 µM milrinone in an atmosphere of 5% CO2 in air at 37°C. The oocytes were washed and then transferred to milrinone-free medium to initiate maturation and scored for germinal vesicle breakdown (GVBD) 2 h later. Only oocytes that underwent GVBD within the first 2 h of culture were included in the study. To obtain oocytes at metaphase I and MII, oocytes were cultured for 6–8 h or 16 h, respectively, following transfer to milrinone-free medium. At the end of the culture period, oocytes were fixed, stained, and analysed as described below.
Immunocytochemistry
The following primary antibodies were diluted for immunocytochemistry: anti-BRCA1 (1:100; R&D Systems), anti-β-tubulin (1:400; Sigma, St. Louis, MO). Anti-ATRX antibody (1:20) was a gift from Dr. Rabindranath De La Fuente. The following secondary antibodies were used: Alexa Fluor 488-conjugated goat anti-mouse IgG (1:500; Molecular Probes, Carlsbad, California), and Cy 5 conjugated donkey anti-rabbit IgG (1:100; Jackson Immunoresearch).
Oocytes/eggswere fixed in 3.7% paraformaldehyde for 1 h at room temperature or overnight at 4°C, permeabilized for 15 min in PBS containing 0.1% Triton X-100, and then incubated with the primary antibody for 1 h or overnight at 4°C. The cells were then incubated in the appropriate secondary antibodies for 1 h, and mounted in Vectashield (Vector Laboratories, Burlingame, CA) to visualize chromosomes.
Oocytes/eggs were analyzed on a Leica TCS NT confocal microscope. The meiotic stage of individual oocytes was classified on the basis of chromosome configuration and spindle morphology as described previously (LeMaire-Adkins et al., 1997).
Metaphase II chromosome preparations
Chromosome preparations of MII arrested oocytes were made as previously described (Hodges and Hunt, 2002). Briefly, oocytes were placed in 1% citrate (hypotonic conditions), transferred to a small drop of acidified water on a microscope slide, and fixed in situ with several drops of 3 parts methanol:1 part acetic acid. After air-drying, slides were stained with 5 mg/ml of Giemsa.
Results
Age-associated decrease in ovulation, increase in aneuploidy, and altered spindle morphology
Prior to assessing the effect of maternal age on gene expression, we determined that the average life expectancy of our mice is ~132 weeks. Mice used in our studies were ~60–70 weeks-of-age. Using an average life expectancy of human females of 84 years, our mice would correspond to women 38–45 years old, assuming a linear relationship.
We first confirmed that increasing age is associated with a decrease in ovulation and an increase in aneuploidy (Zuccotti et al., 1998). Ovulated eggs arrested at MII were collected from oviducts of superovulated B6D2F1 mice 6–8 weeks old (n=25 mice), 6 (n=15), 12 (n=20), 16 (n=25), and 18 (n=15) months old. The number of eggs collected per mouse was 39.5 ± 7.4, 31±4.3, 16.8±6.2, 4.4±3.6, and 2.7±3.1, respectively. A similar decline was observed using B6SJLF1 mice, i.e., the numbers of ovulated eggs per mouse was 42±7.3, 18.6±7.6, and 5.3±3.2 when collected from mice 6 weeks of age (n=25), 12 months (n=10) and 16 months (n=18) of age, respectively. For both strains, ~20% of mice 16–18 months of age didn’t ovulate.
We next ascertained whether an increase in maternal age is associated with an increase incidence in aneuploidy. Our analysis was restricted to hyperploidy because it is less influenced by spreading conditions (Cukurcam et al., 2003; Eichenlaub-Ritter and Boll, 1989). Chromosome spreads of MII-arrested eggs revealed a significant increase in the incidence of hyperploidy in eggs obtained from mice 69–72 weeks of age months old when compared to mice 6–10 weeks-of-age (Table 1); a representative chromosome spread is shown in Fig. 1. If the incidence of hypoploidy is the same as that of hyperploidy, the overall incidence of aneuploidy in these old eggs could reach 50%. A study that used CD-1 mice observed an incidence of ~3% and ~12% hyperploidy in eggs obtained from mice 8–10 and 32–35 weeks-of-age, respectively (Zuccotti et al., 1998).
Table 1.
Incidence of aneuploidy in young and old eggs
| Source | Incidence of Aneuploidy (%) |
|---|---|
| Young (8 w) | 3/71 (4.2%) |
| Old (70 w) | 11/44 (25%) |
The fraction represents the number of aneuploid eggs over the total number of eggs examined that yielded chromosome spreads that were countable. The number in parentheses is the % aneuploid eggs. The difference in the incidence of aneuploidy is significant (p<0.01, Chisquare).
Figure 1.

Chromosome spread derived from old oocyte. Shown is an example of a hyperploid metaphase II egg that matured in vivo.
Although the morphology of MII spindles present in ovulated eggs obtained from mice ~64 weeks of age appeared normal, closer examination revealed that the pole-topole distance of spindles was shorter (25.3 ± 3.1 µm vs 28.5 ± 4.2 µm, p <0.05) and spindle thickness was greater (7.4± 0.6 µm vs 6.7 ± 0.5 µm, p <0.05) in old eggs when compared to young eggs (Fig. 3). Moreover, only 4% of young MII eggs displayed an apparent abnormality in chromosome alignment on the spindle, an incidence similar to that observed for the incidence of aneuploidy assayed by chromosome spreads. A sharp increased incidence of displaced/misaligned chromosomes to 18% was observed in old eggs.
Figure 3.
Hierarchical cluster analysis of transcripts expressed in oocytes and eggs obtained from young and old mice. GV, oocyte; MII, metaphase II-arrested egg. 6w and 66w refer to age of females from which oocytes and eggs were collected. In pseudocolor scheme, red represents high level of expression and blue low level.
Age-associated changes in global gene expression
We conducted expression-profiling experiments using young (6 weeks-of-age) and old (66 weeks-of-age) oocytes and eggs to identify changes in gene expression that may contribute to the age-associated decrease in aneuploidy, as well as the known decline in egg quality. Using the mRNA labeling method previously reported (Zeng et al., 2004); (Pan et al., 2005), we generated transcript profiles of old and young oocytes and eggs using the Affymetrix Murine Genome Array MOE430 v2 chip. Somatic cell contamination was minimal, because we did not detect the presence of Fst (follistatin), Bmp2, 4, or 7 transcripts in the oocyte and egg preparations (Table S2).
To minimize noise, we only analyzed the detected probe-sets that were called “Present” in three out of four replicates for each group. An unsupervised hierarchical cluster analysis using all genes detected revealed that all replicates independently clustered as anticipated according to their age (Fig. 3), with branch distances, in most instances, fairly minimal between replicates.
Similar numbers of transcripts were detected in young and old oocytes and young and old eggs (Table S3) and as previously found (Zeng et al., 2004), fewer transcripts were detected in eggs than in oocytes. We analyzed 17,225 probe-sets detected in oocytes and determined that 5.2% of the transcriptome significantly differed between young and old oocytes. Of these 904 transcripts, 47 changed more than 2-fold and only a single transcript (Ceacam2, carcinoembryonic antigen-related cell adhesion molecule 2) changed more than 5-fold (Table 2). In contrast, 4,992 of 14,994 transcripts detected in young and old eggs (33.3%) displayed a significant change in abundance, with 253 of them changing more than 2-fold. Thus, the effect of maternal age appears to have a much more pronounced effect on the transcript profile in eggs than in oocytes. Gene lists for these differentially expressed transcripts are found in Table S4 and Table S5. Note that only transcripts that displayed at least a 40% change are found in Table S5.
Table 2.
Number of differentially expressed transcripts in young and old oocytes and eggs
| 6w Vs 66w | Probesets | Unigenes | 1X | 1.4X | 2X | 5X | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| up | down | up | down | up | down | up | down | |||
| GV(4.8%FDR) | 17225 | 10398 | 357 | 579 | 133 | 165 | 11 | 36 | 1 | 0 |
| MII(4.5%FDR) | 14994 | 9114 | 2368 | 2624 | 1018 | 973 | 128 | 125 | 0 | 4 |
The data were generated from the non-redundant list of significantly different transcripts in young and old oocytes and eggs. 1X refers to being significantly different, whereas 1.4X, 2X, and 5X refer to being different by either a 1.4-fold, 2-fold, or 5-fold increase or decrease in relative expression, respectively. FDR, false discovery rate.
Fully-grown oocytes are transcriptionally quiescent (Bouniol-Baly et al., 1999); (De La Fuente et al., 2004a) and oocyte maturation is accompanied by extensive degradation of maternal transcripts, with >50% of the polyadenylated RNA being deadenylated or degraded (Paynton et al., 1988). Messenger RNA degradation, however, is not indiscriminate, but rather mRNAs are selectively degraded during maturation (Su et al., 2007). To ascertain whether inappropriate mRNA degradation during maturation of old oocytes is a major source for the differences in transcript profile present in young and old eggs, we compared our microarray to that of Su et al. (2007).
The major difference between these microarray experiments is that we used oligo dT for the reverse transcription reaction whereas Su et al. used a mixture of primers. Our method labeled essentially just polyA-containing RNA and the efficiency of reverse transcription would depend on the length of the poly A tail. In contrast Su et al. labeled all mRNAs resulting in the efficiency of reverse transcription being essentially independent of the length of the poly A tail. Of the 3,002 transcripts Su et al. noted were degraded during maturation, we found that 2,716 (90%) were degraded during maturation of young eggs, i.e., there was excellent concordance between the two microarray data sets. Among these 2,716 transcripts, the relative abundance of 751 (28%) was significantly different in old eggs. Noteworthy is that the relative abundance of most of these transcripts was higher in old eggs, when compared to their younger brethren, implying that degradation of these transcripts was not complete. These results suggest that mRNA degradation during maturation of old oocytes is perturbed and could lead to production of eggs of reduced developmental competence. Biological processes that are perturbed in old eggs and gene lists are found in Table S6. The large number of perturbed biological processes is consistent with the large number of aberrantly expressed transcripts in old eggs (Table 2).
Expression Analysis Systematic Explorer (EASE) analysis (Hosack et al., 2003) of our previous experiments profiling gene expression during oocyte growth and preimplantation development revealed a plethora of biological processes that were affected (Zeng et al., 2004; Pan et al., 2005). In contrast, only a small number of biological processes were affected in old oocytes: protein folding/response to unfolded proteins, protein metabolism/metabolism, intracellular transport, and mitotic cell cycle (Table S7, which includes gene lists). The small number of affected biological processes is consistent with only a small fraction (~5%) of the transcripts being misregulated (Table 2). Strikingly, transcripts involved in cell cycle/mitotic cell cycle are overrepresented. Examining the lists of genes present in these categories, as well as perusing the lists of differentially expressed genes, revealed mis-expression of transcripts central for the SAC, chromosome congression and kinetochore-MT attachment, and spindle formation (Table 3). For example, INCENP is required for AURKB/C function and hence reduced Incenp expression would perturb regulation of kinetochore MT attachments. Reduced BUBR1 function would compromise the SAC, and reduced function of KIFC1, a minus-end directed kinesin, would perturb spindle assembly (Mountain et al., 1999).
Table 3.
Differentially expressed transcripts in oocytes involved in SAC and chromosome segregation
| Transcript | Relative Expression |
|---|---|
| SAC | |
| Bub1 | 1.28 |
| Bub1b | 0.64 |
| Cdc20 | 0.81 |
| Cdc27 | 1.21 |
| Chek1 | 0.67 |
| Cnb1 | 0.76 |
| Congression/Kinetochore MT | |
| Aurkb | 1.47 |
| Incenp | 0.77 |
| Kif2c (MCAK) | 0.54 |
| Plk1 | 0.51 |
| Nudc | 0.56 |
| Cenpe | 0.70 |
| Spindle Assembly | |
| Kifc1 (HSET) | 0.55 |
| Numa1 | 0.67 |
| Ran | 1.44 |
| Tpx2 | 1.17 |
| Dynein light chains | 1.50 |
Although differences in fold-expression for several of the transcripts appear small, two points need to be emphasized. First, a modest reduction in expression of several components that cooperate in a particular process can lead to a more pronounced effect due to the cooperative nature of interactions among the players. Second, the microarray studies were performed on pools of oocytes/eggs. Because variability between individual oocytes/eggs likely exists, the average values obtained in the microarray experiments would not represent individual cells. For example, a value of 0.80 may reflect a complete loss in 1 out of 5 cells and no change in the other four. Consistent with this viewpoint is that only a fraction of the old eggs are aneuploid
Finding perturbations in expression of genes involved in cell cycle regulation was reassuring. What was unanticipated was discovering that expression of genes involved in protein folding was affected. Given the central role of protein folding in generating a cellular milieu requisite for the execution of basic cell functions and processes, decreased expression of these transcripts could negatively impact oocyte development.
Validation of expression patterns
A recent study using the NIA 22k gene microarray reported that of the ~11,000 transcripts detected, the relative abundance of 530 transcripts present in MII eggs obtained from mice 42–45 weeks old significantly differed when compared to eggs obtained from mice 5–6 weeks old (Hamatani et al., 2004). Although the mouse strains and age, as well as the microarray platforms are different, 265 genes (48%) are common between our data set and that of Hamatani et al. (2004). This high degree of concordance implies that changes in relative abundance of these transcripts is indeed linked with aging. Nevertheless, the data sets cannot be compared meaningfully with respect to analyzing biological processes that are perturbed due to the dramatic differences in the age of mice used to obtain the oocytes for expression profiling.
We also used real-time quantitative RT-PCR to confirm the microarray data. Because we are interested in the molecular basis for the age-related increase in aneuploidy, we selected four transcripts whose expression was decreased in old oocytes/eggs and could lead to aneuploidy. ATRX, a member of the SNF2 family of helicase/ATPases, is required for chromosome alignment and meiotic spindle organization in metaphase II stage mouse oocytes (De La Fuente et al., 2004b). BRCA1 is essential for proper mitotic spindle assembly (Joukov et al., 2006), and a hallmark of Brca1 deficiency in somatic cells is an increase in aneuploidy. CENPE is a kinesin required for chromosome attachment to spindle microtubules and chromosome movement (Wood et al., 1997). SMC2 is involved in chromosome condensation (Losada and Hirano, 2005).
Results of the qRT-PCR analysis revealed excellent agreement with the microarray data (Table 4). The concordance of these two independent masurements strongly suggests that the expression profiles derived from the GeneChip analysis accurately represent the qualitative changes in gene expression that are a result of increased maternal age on gene expression in oocytes. Last, the amount of ATRX and BRCA1, as detected by immunocytochemistry, was reduced in old eggs, confirming that the decrease in transcript abundance is reflected at the protein level (Fig. 4). In young oocytes ATRX was detected within the heterochromatic region and BRCA1 on spindle and spindle pole regions in eggs. In old oocytes/eggs, the signal was either markedly reduced or absent.
Table 4.
qRT-PCR confirmation of microarray data
| Gene Symbol | Ratio by microarray | Ratio by real time PCR |
|---|---|---|
| Atrx | 0.43 | 0.48 |
| Brca1 | 0.44 | 0.48 |
| Cenpe | 0.60 | 0.73 |
| Smc2l1 | 0.49 | 0.59 |
Figure 4.

ATRX and BRCA1 expression in young and old oocytes/eggs. ATRX expression was visualized in GV-intact oocytes in young (A) and old (B) oocytes. The staining is nuclear and clearly reduced in old oocytes. (C) BRCA1 expression (red) was assessed in young (C) and old (D) MII eggs , where it associates with the spindle poles. BRCA1 staining on the spindle poles is reduced; DNA is in green. The bar in panel A represents 10 µm and the magnification in the other panels is similar.
Role of BRCA1 in oocyte maturation
Results described above indicated reduced amounts of BRCA1 are present in old eggs. We elected to focus functional studies on BRCA1 in oocyte maturation because among the host of functions that BRCA1 serves, Brca1 deficiency is associated with an increase in aneuploidy (Wang et al., 2004). Injection of long Brca1 RNA into oocytes resulted in reducing the relative abundance of Brca1 mRNA by 50% by MI and 70% by MII relative to control oocytes injected with long Egfp dsRNA (Fig. 5A).
Figure 5.

(A) RNAi-mediated reduction of Brca1 mRNA. Oocytes were injected with either Egfp dsRNA or Brca1 dsRNA and the amount of Brca1 mRNA relative to that present in the Egfp-injected oocytes was determined by qRT-PCR 7 h and 16 h following injection. The experiment was performed three times and the data are presented as mean ± SEM. Immunocytochemical detection of BRCA1 in Egfp dsRNA- (B) and Brca1 dsRNA-injected (C) oocyte that was then matured to MII. A total of 410 oocytes were injected and shown is a representative example. The arrow points to the region where the MII spindle is present.
RNAi-mediated reduction of Brca1 mRNA led to a decrease in BRCA1 protein associated with the spindle poles and spindle, as determined by immunocytochemistry (Fig. 5B,C), but more importantly to defects in spindle formation. In control Egfp dsRNA injected oocytes, microtubules became organized into a bipolar spindle and chromosomes were aligned on a metaphase plate by 7 h for MI eggs and 16 h for MII eggs. In contrast, oocytes injected with Brca1 dsRNA a spectrum of abnormal chromosome configurations and spindle morphologies were observed (Fig. 6, Table 5). For example, in many Brca1 dsRNA-injected oocytes, condensed chromosomes formed a ring-like structure after 7 h or up to 16 h of maturation, and no spindle formed. This structure was reminiscent of that observed following depletion of CDC6 in mouse oocytes (Anger et al., 2005). The incidence of misaligned chromosomes on the metaphase plate was also increased in Brca1 dsRNA-injected oocytes. In such cases, chromosomes were located towards the spindle poles rather than on a metaphase plate. In other instances, some chromosomes were not associated with the spindle. Also of interest is that some (~8%) of these injected oocytes formed multiple bipolar spindles (Table 5). A significant fraction of oocytes injected with Brca1 dsRNA did not undergo GVBD (Table 5); the basis for this interesting observation is not known.
Figure 6.
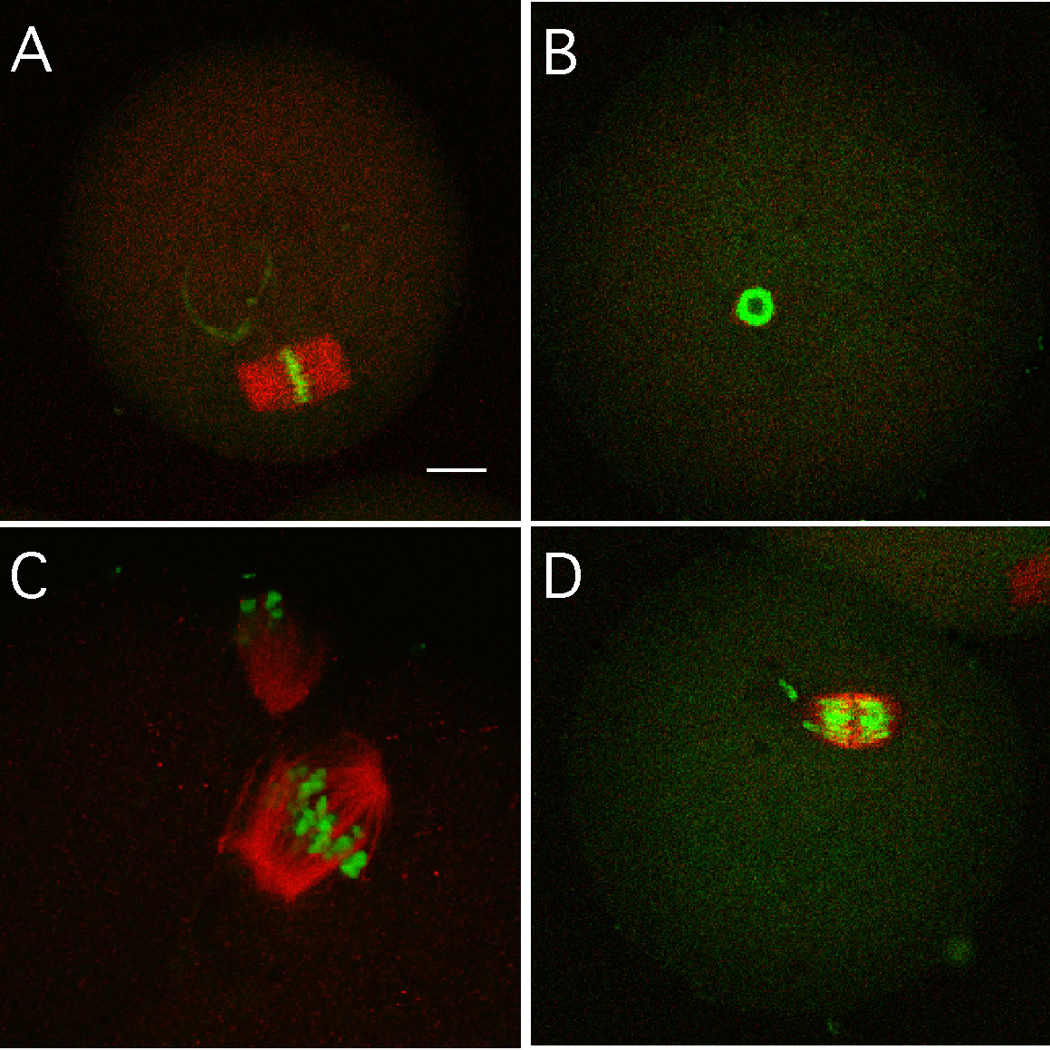
Immunocytochemical analysis of spindle morphology and chromosome congression in BRCA1-depleted oocytes matured to MII. (A) Control oocyte injected with Egfp dsRNA. (B–D) Oocytes injected with Brca1 dsRNA. In B, a spindle didn't form but the chromosomes condensed into a hollow ball-like structure. In C, a disorganized spindle formed with chromosomes poorly congressed on the metaphase plate. In D, an unattched chromosome is present. The bar in panel A represents 10 µm and the magnification in the other panels is similar.
Table 5.
Spectrum of abnormal chromosome configurations in BRCA1-deficient eggs
| Meiotic arrest | >2 polar spindles | DNA misalignment | Total injected | |
|---|---|---|---|---|
| 7 h IVM (Egfp) | 0 | 0 | 3 (4.1%) | 72 |
| 7 h IVM (Brca1) | 18 (31.6%) | 0 | 9 (15.8%) | 57 |
| 14 h IVM (Egfp) | 4 (2.5%)\ | 0 | 3 (1.9%) | 157 |
| 14 h IVM (Brca1) | 30 (24.2%) | 10 (8.1%) | 17 (13.7%) | 124 |
7 h IVM and 14 h IVM refer to the time that the oocytes were cultured following injection of either Egfp or Brca1 dsRNA prior to initiation of in vitro maturation (IVM).
Consistent with a role for BRCA1 in proper chromosome segregation, RNAi-mediated depletion of BRCA1 in young oocytes led to an increase in hyperploidy. Analysis of air-dried chromosome spreads of metaphase II eggs that had been injected with Egfp dsRNA at the GV-stage revealed that the majority of control oocytes had a normal chromosome complement of 20 monovalents, with only 2 of 61 (3.2%) being hyperploid. In contrast, following BRCA1 depletion, hyperploidy was observed in 8 of 73 (10.9%) oocytes. A single extra metaphase II chromosome was observed in 5 of 8 oocytes, whereas the remaining three hyperploid oocytes contained a total of 22, 24 and 32 chromosomes. Our method of analysis did not resolve whether the extra chromosome(s) was a dyad, consisting of two chromatids, or a single chromatid. Assuming an equal incidence of hypoploidy, the total incidence of aneuploidy in BRCA1-depleted oocytes could reach ~22%.
Discussion
Although several models have been proposed to explain how the age-associated increase in aneuploidy could occur, little is known about how it does occur. The “limited oocyte pool” model posits that suboptimal follicles are selected for ovulation due to the reduced number of antral follicles present in older women (Warburton, 2005). Data collected to date, however, do not provide any evidence that women with reduced ovarian reserve are more prone to aneuploid pregnancies. Another model is the “two-hit” model in which the first hit occurs during fetal development when the oocytes are entering meiosis and undergoing recombination (Lamb et al., 1996). This “hit” would generate a bivalent that is at risk for giving rise to an aneuploidy and would be age-independent. The second hit would occur in the aged ovary, likely during oocyte development and/or maturation, and result in an oocyte that improperly processes the susceptible bivalent. This hit would be age-dependent and could be a consequence of hormonal imbalances, peri-follicular microcirculation, reduced oxygen supply or reduced chromosome cohesion (Pellestor et al., 2005). In addition, formation of normal spindles in oocytes derived from old females appears compromised (Battaglia et al., 1996; Volarcik et al., 1998). Such spindles may favor mis-segregation of chromosomes and premature separation of sister chromatids (PSSC). In fact, an increased incidence in failure of chromosomes to congress on an MI plate and PSSC are observed in oocytes obtained from old females (Volarcik et al., 1998). A more general view of the two-hit model is that the quality of the cytoplasm, which includes the transcriptome, in old eggs is compromised. Perturbations in the transcriptome could lead to defects in processes that ensure accurate segregation of all bivalents, independent of events that occurred during fetal development. Results of experiments reported here provide further evidence that mouse is a suitable model system to assess the molecular basis for the age-associated increase in aneuploidy that occurs in women.
Our expression profiling of young and old oocytes reveals changes in expression of several kinetochore components of the SAC, including protein kinases (e.g., BubR1, Bub1, Aurora and Polo-like kinases), and Cdc20, a critical activator of the Anaphase Promoting Complex. Interestingly, the relative abundance of MAD2 and BUB1 transcripts is reduced in oocytes obtained from older women (>35 years-of-age)(Steuerwald et al., 2005), a finding that further strengthens the validity of mouse as a model system to study age-associated increases in aneuploidy. A second process prevents errors by regulating connections between kinetochores and spindle microtubules and results in a spindle with chromosomes correctly bioreinted. Our expression profiling also reveals changes in expression of kinetochore proteins involved in chromosome congression (e.g., the kinesin Cenpe (Yen et al., 1992; Abrieu et al., 2000; Yao et al., 2000)) and regulating kinetochore/spindle microtubule interactions (e.g., a kinesin, Kif2C and Incenp, an inner centromere protein that complexes with AURKB (Ruchaud and Earnshaw, 2007)).
Taken together these results suggest that the SAC in old oocytes is less robust than in their younger counterparts and could be a major source of the observed increase in aneuploidy. It should be noted that the observation that XO oocytes, which will have an unpaired chromosome at MI, exhibit no apparent delay in onset of anaphase. Diffusibility of the APC/C-inhibitory signal is likely to be particularly important in oocytes because of the large volume of cytosol; this large volume may account for oocytes derived from XO mice, which have an unpaired chromosome, entering MI anaphase with little or no delay (LeMaire-Adkins et al., 1997).
The observation that kinetochore-MT attachments form only at the end of MI in female meiosis (Brunet et al., 1999), in marked contrast to mitotic divisions, suggests that the timing of this process is critical for MI. If kinetochores bind MTs too early, before formation of a bipolar spindle and chromosome congression, incorrect attachments are more likely to form. Because capture of MTs by kinetochores is a stochastic process, incorrect attachments will inevitably occur with some frequency and must be corrected to prevent aneuploidy (Ault and Rieder, 1992). Errors include syntelic attachments, in which both homologous chromosomes of a bivalent are connected to the same spindle pole, and merotelic attachments, in which a centromere is physically attached to both poles. Based on studies in mitotic cells, the kinase Aurora B (AURKB), in concert with the KIF2C/MCAK depolymerase (Zhang et al., 2007), plays a critical role in correcting these errors (Tanaka et al., 2002; Hauf et al., 2003; Lampson et al., 2004). AURKB is part of the chromosomal passenger complex with INCENP and Survivin, which localizes to kinetochores until anaphase. AURKB directly regulates kinetochore-MT interactions through phosphorylation of other kinetochore proteins that bind MTs (Biggins et al., 1999; Cheeseman et al., 2002; Cheeseman et al., 2006; DeLuca et al., 2006). Phosphorylation by AURKB also regulates localization and activity of a kinesin-13 family member, KIF2C or MCAK (mitotic centromere-associated kinesin) (Andrews et al., 2004); (Ohi et al., 2004; Lan et al., 2004). Rather than using MTs as a track, kinesin-13 proteins depolymerize MT fibers from their ends (Desai et al., 1999). Although its role at the centromere is not completely understood, KIF2C is thought to destabilize incorrect MT fibers at the kinetochore (Kline-Smith et al., 2004). Another kinase at the centromere, PLK1, also regulates kinetochore-MT interactions, at least in part through phosphorylation of the kinetochore protein NUDC (Nishino et al., 2006). Changes in expression levels of INCENP, which is essential for AURKB function, as well as KIF2C, PLK1, and NudC are observed in old mouse oocytes and may contribute to increased aneuploidy.
There is a growing consensus that degradation of maternal mRNA is essential to undergo successfully the maternal-to-embryonic transition (DeRenzo and Seydoux, 2004; Stitzel and Seydoux, 2007). This transition entails transforming a highly differentiated oocyte to totipotent blastomeres by the 2-cell stage. In contrast to the marked stability of mRNA during oocyte growth, oocyte maturation triggers a substantial decrease in mRNA (Schultz, 1993). Although the molecular basis underlying this transition is not known, our expression profiling studies reveals that many maternal mRNAs in old oocytes are not properly degraded during maturation. This could result in producing an egg whose developmental competence is reduced, thereby contributing to the age-associated decline in fertility.
Brca1 was originally isolated as a gene linked to inherited breast and ovarian cancer. It’s widely thought that BRCA1 maintains genomic integrity via participation in homologous-recombination-mediated double-strand-break repair, the regulation of cell-cycle checkpoint responses, and centrosome amplification control (Venkitaraman, 2002). It was initially believed that BRCA1 functions largely in S phase (Scully et al., 1997); Venkitaraman, 2002), mounting evidence suggests that it is essential for proper chromosome segregation. BRCA1 binds to γ-tubulin and localizes in centrosomes and spindle microtubules (Hsu et al., 2001; Hsu and White, 1998). Moreover, BRCA1-deficient or mutated cells exhibit mitotic defects and abnormal chromosome segregation (Joukov et al., 2001; Wang et al., 2004; Xu et al., 1999a; Xu et al., 1999b). In our study, reducing the amount of BRCA1 resulted in multiple spindles and chromosome misalignment following oocyte maturation; these phenotypes are similar to that observed in somatic cells, indicating a conserved function for BRCA1 in the meiotic and mitotic cell cycle. BRCA1 may also be critical for spindle assembly because reducing Brca1 by RNAi also resulted in oocytes that didn’t form a spindle following maturation. Moreover, we found Brca1 plays a role in spindle assembly, because many Brca1 dsRNA injected cells couldn’t form a spindle. This phenotype may reflect a function for BRCA1 linked to its associating with the microtubule organizing centers (MTOCs) that drive spindle formation during oocyte maturation (Schuh and Ellenberg, 2007); oocytes lack centrioles and centrosomes. Reduced amounts of BRCA1 protein in old oocytes could be a contributing factor underlying the age-associated increase in the incidence of aneuploidy that we observe.
Numerous studies of model systems including yeast, C. elegans, Drosophila, and mice, as well as in humans, have identified several processes that likely contribute to the aging phenotype (Johnson et al., 1999). These include the effects of oxidative damage associated with cellular metabolism and genomic instability, e.g., mitochondrial mutations, telomere shortening, and chromosomal pathologies. Results presented here provide new information that perturbations in degradation of maternal mRNAs and reduced strength of the SAC (and possibly higher errors of microtubule-kinetochore interactions) constitute part of the molecular basis for the age-associated increase in aneuploidy in females.
Supplementary Material
Figure 2.

Morphology of MII spindle in young and old eggs. Eggs from young (A) and old (B) mice were processed for immunocytochemical detection of DNA (green) and tubulin (green). Shown are examples that represent more extreme cases. Also note in B the presence of an unattached chromosome, a condition that would inevitably lead to production of an aneuploid egg. A total of 50 and 40 spindles in young and old eggs was analyzed, respectively. The bar in panel A represents 10 µm and the magnification in panel B is similar.
Acknowledgments
This research was supported by a grant from the NIH (U01 HD 44575 to R.M.S. as part of the NICHD Cooperative Program on Female Health and Egg Quality). The authors thank John Eppig and Dick Tasca for critically reading the manuscript and their comments, and Mike Lampson for numerous discussions regarding the spindle assembly checkpoint and spindle microtubule-kinetochore attachments. The authors also want to thank Reviewer 2 for many helpful comments and suggestions that were incorporated into the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abrieu A, Kahana JA, Wood KW, Cleveland DW. CENP-E as an essential component of the mitotic checkpoint in vitro. Cell. 2000;102:817–826. doi: 10.1016/s0092-8674(00)00070-2. [DOI] [PubMed] [Google Scholar]
- Andrews PD, Ovechkina Y, Morrice N, Wagenbach M, Duncan K, Wordeman L, Swedlow JR. Aurora B regulates MCAK at the mitotic centromere. Dev. Cell. 2004;6:253–268. doi: 10.1016/s1534-5807(04)00025-5. [DOI] [PubMed] [Google Scholar]
- Anger M, Stein P, Schultz RM. CDC6 requirement for spindle formation during maturation of mouse oocytes. Biol. Reprod. 2005;72:188–194. doi: 10.1095/biolreprod.104.035451. [DOI] [PubMed] [Google Scholar]
- Ault JG, Rieder CL. Chromosome mal-orientation and reorientation during mitosis. Cell Motil. Cytoskeleton. 1992;22:155–159. doi: 10.1002/cm.970220302. [DOI] [PubMed] [Google Scholar]
- Battaglia DE, Goodwin P, Klein NA, Soules MR. Influence of maternal age on meiotic spindle assembly in oocytes from naturally cycling women. Hum. Reprod. 1996;11:2217–2222. doi: 10.1093/oxfordjournals.humrep.a019080. [DOI] [PubMed] [Google Scholar]
- Biggins S, Severin FF, Bhalla N, Sassoon I, Hyman AA, Murray AW. The conserved protein kinase Ipl1 regulates microtubule binding to kinetochores in budding yeast. Genes Dev. 1999;13:532–544. doi: 10.1101/gad.13.5.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouniol-Baly C, Hamraoui L, Guibert J, Beaujean N, Szöllösi MS, Debey P. Differential transcriptional activity associated with chromatin configuration in fully grown mouse germinal vesicle oocytes. Biol. Reprod. 1999;60:580–587. doi: 10.1095/biolreprod60.3.580. [DOI] [PubMed] [Google Scholar]
- Brunet S, Maria AS, Guillaud P, Dujardin D, Kubiak JZ, Maro B. Kinetochore fibers are not involved in the formation of the first meiotic spindle in mouse oocytes, but control the exit from the first meiotic M phase. J. Cell Biol. 1999;146:1–12. doi: 10.1083/jcb.146.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatot CL, Ziomek CA, Bavister BD, Lewis JL, Torres I. An improved culture medium supports development of random-bred 1-cell mouse embryos in vitro. J. Reprod. Fertil. 1989;86:679–688. doi: 10.1530/jrf.0.0860679. [DOI] [PubMed] [Google Scholar]
- Cheeseman IM, Anderson S, Jwa M, Green EM, Kang J, Yates JR, 3rd, Chan CS, Drubin DG, Barnes G. Phospho-regulation of kinetochore-microtubule attachments by the Aurora kinase Ipl1p. Cell. 2002;111:163–172. doi: 10.1016/s0092-8674(02)00973-x. [DOI] [PubMed] [Google Scholar]
- Cheeseman IM, Chappie JS, Wilson-Kubalek EM, Desai A. The conserved KMN network constitutes the core microtubule-binding site of the kinetochore. Cell. 2006;127:983–997. doi: 10.1016/j.cell.2006.09.039. [DOI] [PubMed] [Google Scholar]
- Cuurcam S, Hegele-Hartung C, Eichenlaub-Ritter U. Meiosis-activating sterol protects oocytes from precocious chromosome segregation. Hum. Reprod. 2003;18:1908–1917. doi: 10.1093/humrep/deg378. [DOI] [PubMed] [Google Scholar]
- De La Fuente R, Viveiros MM, Burns KH, Adashi EY, Matzuk MM, Eppig JJ. Major chromatin remodeling in the germinal vesicle (GV) of mammalian oocytes is dispensable for global transcriptional silencing but required for centromeric heterochromatin function. Dev. Biol. 2004a;275:447–458. doi: 10.1016/j.ydbio.2004.08.028. [DOI] [PubMed] [Google Scholar]
- De La Fuente R, Viveiros MM, Wigglesworth K, Eppig JJ. ATRX, a member of the SNF2 family of helicase/ATPases, is required for chromosome alignment and meiotic spindle organization in metaphase II stage mouse oocytes. Dev. Biol. 2004b;272:1–14. doi: 10.1016/j.ydbio.2003.12.012. [DOI] [PubMed] [Google Scholar]
- DeLuca JG, Gall WE, Ciferri C, Cimini D, Musacchio A, Salmon ED. Kinetochore microtubule dynamics and attachment stability are regulated by Hec1. Cell. 2006;127:969–982. doi: 10.1016/j.cell.2006.09.047. [DOI] [PubMed] [Google Scholar]
- DeRenzo C, Seydoux G. A clean start: degradation of maternal proteins at the oocyte-to-embryo transition. Trends Cell Biol. 2004;14:420–426. doi: 10.1016/j.tcb.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Desai A, Verma S, Mitchison TJ, Walczak CE. Kin I kinesins are microtubule-destabilizing enzymes. Cell. 1999;96:69–78. doi: 10.1016/s0092-8674(00)80960-5. [DOI] [PubMed] [Google Scholar]
- Eichenlaub-Ritter U, Boll I. Nocodazole sensitivity, age-related aneuploidy, and alterations in the cell cycle during maturation of mouse oocytes. Cytogenet. Cell Genet. 1989;52:170–176. doi: 10.1159/000132871. [DOI] [PubMed] [Google Scholar]
- Hamatani T, Falco G, Carter MG, Akutsu H, Stagg CA, Sharov AA, Dudekula DB, VanBuren V, Ko MS. Age-associated alteration of gene expression patterns in mouse oocytes. Hum. Mol. Genet. 2004;13:2263–2278. doi: 10.1093/hmg/ddh241. [DOI] [PubMed] [Google Scholar]
- Hassold T. Chromosome abnormalities in human reproduction. Trends in Genetics. 1986;2:105–110. [Google Scholar]
- Hassold T, Chiu D. Maternal age-specific rates of numerical chromosome abnormalities with special reference to trisomy. Hum. Genet. 1985;70:11–17. doi: 10.1007/BF00389450. [DOI] [PubMed] [Google Scholar]
- Hassold T, Hunt P. To err (meiotically) is human: the genesis of human aneuploidy. Nat. Rev. Genet. 2001;2:280–291. doi: 10.1038/35066065. [DOI] [PubMed] [Google Scholar]
- Hauf S, Cole RW, LaTerra S, Zimmer C, Schnapp G, Walter R, Heckel A, van Meel J, Rieder CL, Peters JM. The small molecule Hesperadin reveals a role for Aurora B in correcting kinetochore-microtubule attachment and in maintaining the spindle assembly checkpoint. J. Cell Biol. 2003;161:281–294. doi: 10.1083/jcb.200208092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges CA, Hunt PA. Simultaneous analysis of chromosomes and chromosome-associated proteins in mammalian oocytes and embryos. Chromosoma. 2002;111:165–169. doi: 10.1007/s00412-002-0195-3. [DOI] [PubMed] [Google Scholar]
- Hodges CA, Revenkova E, Jessberger R, Hassold TJ, Hunt PA. SMC1beta-deficient female mice provide evidence that cohesins are a missing link in age-related nondisjunction. Nat. Genet. 2005;37:1351–1355. doi: 10.1038/ng1672. [DOI] [PubMed] [Google Scholar]
- Holinka CF, Tseng YC, Finch CE. Reproductive aging in C57BL/6J mice: plasma progesterone, viable embryos and resorption frequency throughout pregnancy. Biol. Reprod. 1979;20:1201–1211. doi: 10.1095/biolreprod20.5.1201. [DOI] [PubMed] [Google Scholar]
- Homer HA, McDougall A, Levasseur M, Murdoch AP, Herbert M. Mad2 is required for inhibiting securin and cyclin B degradation following spindle depolymerisation in meiosis I mouse oocytes. Reproduction. 2005a;130:829–843. doi: 10.1530/rep.1.00856. [DOI] [PubMed] [Google Scholar]
- Homer HA, McDougall A, Levasseur M, Yallop K, Murdoch AP, Herbert M. Mad2 prevents aneuploidy and premature proteolysis of cyclin B and securin during meiosis I in mouse oocytes. Genes Dev. 2005b;19:202–207. doi: 10.1101/gad.328105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosack DA, Dennis G, Jr, Sherman BT, Lane HC, Lempicki RA. Identifying biological themes within lists of genes with EASE. Genome Biol. 2003;4:R70. doi: 10.1186/gb-2003-4-10-r70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu LC, Doan TP, White RL. Identification of a gamma-tubulin-binding domain in BRCA1. Cancer Res. 2001;61:7713–7718. [PubMed] [Google Scholar]
- Hsu LC, White RL. BRCA1 is associated with the centrosome during mitosis. Proc. Natl. Acad. Sci. U S A. 1998;95:12983–12988. doi: 10.1073/pnas.95.22.12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt PA, Hassold TJ. Sex matters in meiosis. Science. 2002;296:2181–2183. doi: 10.1126/science.1071907. [DOI] [PubMed] [Google Scholar]
- Johnson FB, Sinclair DA, Guarente L. Molecular biology of aging. Cell. 1999;96:291–302. doi: 10.1016/s0092-8674(00)80567-x. [DOI] [PubMed] [Google Scholar]
- Joukov V, Chen J, Fox EA, Green JB, Livingston DM. Functional communication between endogenous BRCA1 and its partner, BARD1, during Xenopus laevis development. Proc. Natl. Acad. Sci. U S A. 2001;98:12078–12083. doi: 10.1073/pnas.211427098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joukov V, Groen AC, Prokhorova T, Gerson R, White E, Rodriguez A, Walter JC, Livingston DM. The BRCA1/BARD1 heterodimer modulates ran-dependent mitotic spindle assembly. Cell. 2006;127:539–552. doi: 10.1016/j.cell.2006.08.053. [DOI] [PubMed] [Google Scholar]
- Kline-Smith SL, Khodjakov A, Hergert P, Walczak CE. Depletion of centromeric MCAK leads to chromosome congression and segregation defects due to improper kinetochore attachments. Mol. Biol. Cell. 2004;15:1146–1159. doi: 10.1091/mbc.E03-08-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb NE, Freeman SB, Savage-Austin A, Pettay DC, Taft L, Hersey J, Gu Y, Shen J, Saker D, May KM, Avramopoulos D, Petersen MB, Hallberg A, Mikkelsen M, Hassold TJ, Sherman SL. Susceptible chiasmate configurations of chromosome 21 predispose to non-disjunction in both maternal meiosis I and meiosis II. Nat. Genet. 1996;14:400–445. doi: 10.1038/ng1296-400. [DOI] [PubMed] [Google Scholar]
- Lamb NE, Yu K, Shaffer J, Feingold E, Sherman SL. Association between maternal age and meiotic recombination for trisomy 21. Am. J. Hum. Genet. 2005;76:91–p9. doi: 10.1086/427266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampson MA, Renduchitala K, Khodjakov A, Kapoor TM. Correcting improper chromosome-spindle attachments during cell division. Nat. Cell Biol. 2004;6:232–237. doi: 10.1038/ncb1102. [DOI] [PubMed] [Google Scholar]
- Lan W, Zhang X, Kline-Smith SL, Rosasco SE, Barrett-Wilt GA, Shabanowitz J, Hunt DF, Walczak CE, Stukenberg PT. Aurora B phosphorylates centromeric MCAK and regulates its localization and microtubule depolymerization activity. Curr. Biol. 2004;14:273–286. doi: 10.1016/j.cub.2004.01.055. [DOI] [PubMed] [Google Scholar]
- LeMaire-Adkins R, Radke K, Hunt PA. Lack of checkpoint control at the metahase/anaphase transition: A mechanism of meiotic nondisjunction in mammalian females. J. Cell Biol. 1997;139:1611–1619. doi: 10.1083/jcb.139.7.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Keefe DL. Ageing-associated aberration in meiosis of oocytes from senescence-accelerated mice. Hum. Reprod. 2002;17:2678–2685. doi: 10.1093/humrep/17.10.2678. [DOI] [PubMed] [Google Scholar]
- Losada A, Hirano T. Dynamic molecular linkers of the genome: the first decade of SMC proteins. Genes Dev. 2005;19:1269–1287. doi: 10.1101/gad.1320505. [DOI] [PubMed] [Google Scholar]
- McCarthy EE, Celebi JT, Baer R, Ludwig T. Loss of Bard1, the heterodimeric partner of the Brca1 tumor suppressor, results in early embryonic lethality and chromosomal instability. Mol. Cell. Biol. 2003;23:5056–5063. doi: 10.1128/MCB.23.14.5056-5063.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountain V, Simerly C, Howard L, Ando A, Schatten G, Compton DA. The kinesin-related protein, HSET, opposes the activity of Eg5 and cross-links microtubules in the mammalian mitotic spindle. J. Cell Biol. 1999;147:351–366. doi: 10.1083/jcb.147.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musacchio A, Hardwick KG. The spindle checkpoint: structural insights into dynamic signalling. Nat. Rev. Mol. Cell Biol. 2002;3:731–741. doi: 10.1038/nrm929. [DOI] [PubMed] [Google Scholar]
- Musacchio A, Salmon ED. The spindle-assembly checkpoint in space and time. Nat. Rev. Mol. Cell Biol. 2007;8:379–393. doi: 10.1038/nrm2163. [DOI] [PubMed] [Google Scholar]
- Navot D, Drews MR, Bergh PA, Guzman I, Karstaedt A, Scott RT, Jr, Garrisi GJ, Hofmann GE. Age-related decline in female fertility is not due to diminished capacity of the uterus to sustain embryo implantation. Fertil. Steril. 1994;61:97–101. doi: 10.1016/s0015-0282(16)56459-0. [DOI] [PubMed] [Google Scholar]
- Niaut T, Hached K, Sotillo R, Sorger PK, Maro B, Benezra R, Wassmann K. Changing mad2 levels affects chromosome segregation and spindle assembly checkpoint control in female mouse meiosis I. PLoS ONE. 2007;2:e1165. doi: 10.1371/journal.pone.0001165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino M, Kurasawa Y, Evans R, Lin SH, Brinkley BR, Yu-Lee LY. NudC is required for Plk1 targeting to the kinetochore and chromosome congression. Curr. Biol. 2006;16:1414–1421. doi: 10.1016/j.cub.2006.05.052. [DOI] [PubMed] [Google Scholar]
- Ohi R, Sapra T, Howard J, Mitchison TJ. Differentiation of cytoplasmic and meiotic spindle assembly MCAK functions by Aurora B-dependent phosphorylation. Mol. Biol. Cell. 2004;15:2895–2906. doi: 10.1091/mbc.E04-02-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan H, O'Brien MJ, Wigglesworth K, Eppig JJ, Schultz RM. Transcript profiling during mouse oocyte development and the effect of gonadotropin priming and development in vitro. Dev. Biol. 2005;286:493–506. doi: 10.1016/j.ydbio.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Paynton BV, Rempel R, Bachvarova R. Changes in state of adenylation and time course of degradation of maternal mRNAs during oocyte maturation and early embryonic development in the mouse. Dev.Biol. 1988;129:304–314. doi: 10.1016/0012-1606(88)90377-6. [DOI] [PubMed] [Google Scholar]
- Pellestor F, Anahory T, Hamamah S. Effect of maternal age on the frequency of cytogenetic abnormalities in human oocytes. Cytogenet. Genome Res. 2005;111:206–212. doi: 10.1159/000086891. [DOI] [PubMed] [Google Scholar]
- Pellestor F, Andreo B, Arnal F, Humeau C, Demaille J. Mechanisms of non-disjunction in human female meiosis: the co-existence of two modes of malsegregation evidenced by the karyotyping of 1397 in-vitro unfertilized oocytes. Hum. Reprod. 2002;17:2134–2145. doi: 10.1093/humrep/17.8.2134. [DOI] [PubMed] [Google Scholar]
- Ruchaud S, Carmena M, Earnshaw WC. Chromosomal passengers: conducting cell division. Nat. Rev. Mol. Cell Biol. 2007;8:798–812. doi: 10.1038/nrm2257. [DOI] [PubMed] [Google Scholar]
- Sauer MV. The impact of age on reproductive potential: lessons learned from oocyte donation. Maturitas. 1998;30:221–225. doi: 10.1016/s0378-5122(98)00077-2. [DOI] [PubMed] [Google Scholar]
- Schuh M, Ellenberg J. Self-organization of MTOCs replaces centrosome function during acentrosomal spindle assembly in live mouse oocytes. Cell. 2007;130:484–498. doi: 10.1016/j.cell.2007.06.025. [DOI] [PubMed] [Google Scholar]
- Schultz RM. Regulation of zygotic gene activation in the mouse. BioEssays. 1993;15:531–538. doi: 10.1002/bies.950150806. [DOI] [PubMed] [Google Scholar]
- Schultz RM, Montgomery RR, Belanoff JR. Regulation of mouse oocyte maturation: Implication of a decrease in oocyte cAMP and protein dephosphorylation in commitment to resume meiosis. Dev. Biol. 1983;97:264–273. doi: 10.1016/0012-1606(83)90085-4. [DOI] [PubMed] [Google Scholar]
- Scully R, Chen J, Plug A, Xiao Y, Weaver D, Feunteun J, Ashley T, Livingston DM. Association of BRCA1 with Rad51 in mitotic and meiotic cells. Cell. 1997;88:265–275. doi: 10.1016/s0092-8674(00)81847-4. [DOI] [PubMed] [Google Scholar]
- Steuerwald N, Cohen J, Herrera RJ, Sandalinas M, Brenner CA. Association between spindle assembly checkpoint expression and maternal age in human oocytes. Mol. Hum. Reprod. 2001;7:49–55. doi: 10.1093/molehr/7.1.49. [DOI] [PubMed] [Google Scholar]
- Steuerwald NM, Bermudez MG, Wells D, Munne S, Cohen J. Maternal age-related differential global expression profiles observed in human oocytes. Reprod Biomed Online. 2007;14:700–708. doi: 10.1016/s1472-6483(10)60671-2. [DOI] [PubMed] [Google Scholar]
- Stitzel ML, Seydoux G. Regulation of the oocyte-to-zygote transition. Science. 2007;316:407–408. doi: 10.1126/science.1138236. [DOI] [PubMed] [Google Scholar]
- Su YQ, Sugiura K, Woo Y, Wigglesworth K, Kamdar S, Affourtit J, Eppig JJ. Selective degradation of transcripts during meiotic maturation of mouse oocytes. Dev. Biol. 2007;302:104–117. doi: 10.1016/j.ydbio.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda P, Stein P, Hayashi H, Schultz RM. Selective reduction of dormant maternal mRNAs in mouse oocytes by RNA interference. Development. 2000;127:4147–4156. doi: 10.1242/dev.127.19.4147. [DOI] [PubMed] [Google Scholar]
- Tanaka TU, Rachidi N, Janke C, Pereira G, Galova M, Schiebel E, Stark MJ, Nasmyth K. Evidence that the Ipl1-Sli15 (Aurora kinase-INCENP) complex promotes chromosome bi-orientation by altering kinetochore-spindle pole connections. Cell. 2002;108:317–329. doi: 10.1016/s0092-8674(02)00633-5. [DOI] [PubMed] [Google Scholar]
- Venkitaraman AR. Cancer susceptibility and the functions of BRCA1 and BRCA2. Cell. 2002;108:171–182. doi: 10.1016/s0092-8674(02)00615-3. [DOI] [PubMed] [Google Scholar]
- Volarcik K, Sheean L, Goldfarb J, Woods L, Abdul-Karim FW, Hunt P. The meiotic competence of in-vitro matured human oocytes is influenced by donor age: evidence that folliculogenesis is compromised in the reproductively aged ovary. Hum. Reprod. 1998;13:154–160. doi: 10.1093/humrep/13.1.154. [DOI] [PubMed] [Google Scholar]
- Wang RH, Yu H, Deng CX. A requirement for breast-cancer-associated gene 1 (BRCA1) in the spindle checkpoint. Proc Natl Acad Sci U S A. 2004;101:17108–17113. doi: 10.1073/pnas.0407585101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton D. Biological aging and the etiology of aneuploidy. Cytogenet. Genome Res. 2005;111:266–272. doi: 10.1159/000086899. [DOI] [PubMed] [Google Scholar]
- Wiersma A, Hirsch B, Tsafriri A, Hanssen RG, Van de Kant M, Kloosterboer HJ, Conti M, Hsueh AJ. Phosphodiesterase 3 inhibitors suppress oocyte maturation and consequent pregnancy without affecting ovulation and cyclicity in rodents. J. Clin. Invest. 1998;102:532–537. doi: 10.1172/JCI2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitten WK. Nutrient requirements for the culture of preimplantation mouse embryox. in vitro. Adv. Biosci. 1971;6:129–139. [Google Scholar]
- Wood KW, Sakowicz R, Goldstein LS, Cleveland DW. CENP-E is a plus end-directed kinetochore motor required for metaphase chromosome alignment. Cell. 1997;91:357–366. doi: 10.1016/s0092-8674(00)80419-5. [DOI] [PubMed] [Google Scholar]
- Xu X, Wagner KU, Larson D, Weaver Z, Li C, Ried T, Hennighausen L, Wynshaw-Boris A, Deng CX. Conditional mutation of Brca1 in mammary epithelial cells results in blunted ductal morphogenesis and tumour formation. Nat. Genet. 1999a;22:37–43. doi: 10.1038/8743. [DOI] [PubMed] [Google Scholar]
- Xu X, Weaver Z, Linke SP, Li C, Gotay J, Wang XW, Harris CC, Ried T, Deng CX. Centrosome amplification and a defective G2-M cell cycle checkpoint induce genetic instability in BRCA1 exon 11 isoform-deficient cells. Mol. Cell. 1999b;3:389–395. doi: 10.1016/s1097-2765(00)80466-9. [DOI] [PubMed] [Google Scholar]
- Yao X, Abrieu A, Zheng Y, Sullivan KF, Cleveland DW. CENP-E forms a link between attachment of spindle microtubules to kinetochores and the mitotic checkpoint. Nat. Cell Biol. 2000;2:484–491. doi: 10.1038/35019518. [DOI] [PubMed] [Google Scholar]
- Yen TJ, Li G, Schaar BT, Szilak I, Cleveland DW. CENP-E is a putative kinetochore motor that accumulates just before mitosis. Nature. 1992;359:536–539. doi: 10.1038/359536a0. [DOI] [PubMed] [Google Scholar]
- Zhang X, Lan W, Ems-McClung SC, Stukenberg PT, Walczak CE. Aurora B phosphorylates multiple sites on mitotic centromere-associated kinesin to spatially and temporally regulate its function. Mol. Biol. Cell. 2007;18:3264–3276. doi: 10.1091/mbc.E07-01-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng F, Baldwin DA, Schultz RM. Transcript profiling during preimplantation mouse development. Dev. Biol. 2004;272:483–496. doi: 10.1016/j.ydbio.2004.05.018. [DOI] [PubMed] [Google Scholar]
- Zuccotti M, Boiani M, Garagna S, Redi CA. Analysis of aneuploidy rate in antral and ovulated mouse oocytes during female aging. Mol. Reprod. Dev. 1998;50:305–312. doi: 10.1002/(SICI)1098-2795(199807)50:3<305::AID-MRD6>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



