Abstract
We hypothesized that rates of myofibrillar and patellar tendon collagen synthesis would fall over time during disuse, the changes being accompanied in muscle by decreases in focal adhesion kinase (FAK) phosphorylation and in gene expression for proteolytic enzymes. We studied nine men (22 ± 4 years, BMI 24 ± 3 kg m−2 (means ± s.d.) who underwent unilateral lower leg suspension for 23 days; five were studied between 0 and 10 days and four between 10 and 21 days. Muscle and tendon biopsies were taken in the postabsorptive state at days 0, 10 and 21 for measurement of protein synthesis, gene expression and protein phosphorylation. Muscle cross-sectional area decreased by 5.2% at 14 days and 10.0% (both P < 0.001), at 23 days, i.e. 0.5% day−1, whereas tendon dimensions were constant. Rates of myofibrillar protein synthesis fell (P < 0.01) from 0.047% h−1 at day 0 to 0.022% h−1 at 10 days without further changes. Tendon collagen synthetic rates also fell (P < 0.01), from 0.052 to 0.023% h−1 at 10 days and then to 0.010% h−1 at 21 days. FAK phosphorylation decreased 30% (P < 0.01) at 10 days. No changes occurred in the amounts/phosphorylation of PKB–P70s6k–mTOR pathway components. Expression of mRNA for MuRF-1 increased ∼3-fold at 10 days without changes in MAFbx or tripeptidyl peptidase II mRNA, but all decreased between 10 and 21 days. Thus, both myofibrillar and tendon protein synthetic rates show progressive decreases during 21 days of disuse; in muscle, this is accompanied by decreased phosphorylation of FAK, with no marked increases in genes for proteolytic enzymes.
Human muscle disuse atrophy is a major problem for hospitalized patients, the inactive elderly and astronauts living in microgravity. The detailed mechanisms of the loss of human muscle are incompletely understood; nevertheless, it has been clear for 20 years that muscle protein synthesis measured after 7 weeks of immobilization in a leg cast is lower than in the contralateral uncasted leg (Gibson et al. 1987) and that this can be ameliorated by electrical stimulation to initiate muscular contraction (Gibson et al. 1988). However, the time course of the changes in muscle protein turnover is not well described: we hypothesized that the rates of muscle protein synthesis would fall with time, but with the fall being faster earlier and slower later. A major aim of this work was to test this hypothesis.
Disuse results in a major loss of muscle force which exceeds the relative loss of muscle cross-sectional area and both muscular and tendinous properties have been shown to contribute to this (Narici & Maganaris, 2006), a finding we have recently confirmed (de Boer et al. 2007). Hindlimb immobilization results in decreases in the activities in Achilles tendon of rats of collagen cross-linking enzymes, i.e. prolyl-4-hydroxylase and galactosylhydroxylysyl glucosyltransferase but without alteration of the concentration of hydroxyproline (Savolainen et al. 1988); these data were taken as indicating a fall in collagen synthesis and cross-linking although neither was measured directly. Recently, it has become possible to measure the rate of synthesis of collagen in a variety of tissues, including tendon (Babraj et al. 2005a); using this technique we previously showed that preceding exercise will elevate both muscle and tendon collagen synthesis markedly for up to 72 h (Miller et al. 2005). We naturally conjectured that disuse would decrease collagen synthesis in human tendon and one of the aims of the present work was to test this hypothesis.
We also wished to obtain more information (in the context of likely decreases in muscle protein synthesis) on the effects of disuse on the phosphorylation of cell signalling molecules associated with mechanotransduction (i.e. focal adhesion kinase, FAK, the expression of which is load dependent; Gordon et al. 2001) and with the PKB–P70–mTOR protein anabolic pathway, which is apparently down-regulated in animals during disuse atrophy (Bodine et al. 2001). We hypothesized that phosphorylation of these cell-signalling molecules (and possibly the total amounts of their protein in muscle) would change to a state indicating reduced translational capacity consequent to reduced mechanical activity.
In our initial work (Gibson et al. 1987) protein breakdown was not measured directly but inferred from differences in leg muscle mass and rates of protein synthesis; we noted then that the observed rate of muscle atrophy could have been achieved over 7 weeks with little or no increase in protein breakdown, being mostly attributable to decreased protein synthesis. Later workers, using techniques based upon tracer amino acid dilution in the whole body and tracer exchange across the leg, confirmed this prediction for periods of immobilization between 14 and 28 days (Ferrando et al. 1996; Paddon-Jones et al. 2006). In rodents, in the early phase after immobilization, disuse appears to involve large rapid decreases in protein synthesis accompanied by somewhat smaller increases in protein breakdown (Tucker et al. 1981; Thomason & Booth, 1990). Therefore, to determine whether there were changes in indirect indices of proteolytic activity much earlier than we had previously measured human muscle protein synthesis after immobilization (i.e. 7 weeks), we determined the expression of two components of the ubiquitin–proteasome pathway, namely the ubiquitin ligases MAFbx and MuRF-1 and also that of tripeptidyl peptidase II (TPPII), an enzyme responsible for terminal steps of proteolysis. We hypothesized that rather than a rise in these markers of proteolysis there would be a fall. We also measured the expression of a number of other mRNA coding for a variety of genes of potential functional significance such as collagen I and myostatin.
Methods
Subjects and procedures
The present paper describes changes in the biochemistry and molecular biology of muscle and tendon in nine subjects who took part in an extensive study of muscle and tendon biomechanical properties which has been reported previously (de Boer et al. 2007). The nine participants (19.1 ± 0.6 years, 179.3 ± 4.7 cm, 72.4 ± 8.6 kg, BMI: 22.5 ± 2.5 kg m−2) gave written, informed consent to participate in this study. All procedures conformed to the declaration of Helsinki and were approved by the Ethics Committee of the Institute for Biophysical and Clinical Research into Human Movement at the Manchester Metropolitan University. Details of screening, exclusion criteria and monitoring for development of deep vein thrombosis have been reported in a previous article (de Boer et al. 2007) together with details of all biomechanical, neurophysiological and scanning procedures and results.
All baseline data were collected in the week before starting the suspension. Subsequent biomechanical, neurophysiological and scanning measurements were performed on days 14 and 23 of immobilization. Protocols to measure muscle and tendon protein synthesis and gene expression and cell signalling were carried out on two groups of subjects studied twice: the first group on day 0 (n = 5) and day 10 (n = 5) and the second at day 10 (n = 4) and day 21 (n = 4). All data were collected in the morning after an overnight fast.
Biomechanical and anatomical data were collected over 23 days. Values of myofibrillar and tendon protein synthesis were obtained from the first group at days 0 and 10 and from the second at days 10 and 21; all gene expression and signalling data are presented as changes for four subjects each at days 0–10 and 10–21, because of the local ethics committee requirement to biopsy each subject twice only. We unfortunately lost part of a sample for one subject in the first group so n fell to 4 for the molecular biology assays on days 0 and 10.
Application of tracers
To label myofibrillar protein and tendon collagen protein, a flooding dose of [1-13C]proline or [15N]proline (20 atoms per cent) was given (made up of 0.75 g of 13C- or 15N-labelled proline; 99 atom per cent (Cambridge Isotope Laboratories, MA, USA) and 3 g of unlabelled proline (Sigma-Aldrich, Dorset, UK). The order of administration was always [1-13C]proline then [15N]proline across the studies on days 0 and 10, and 10 and 21. Blood samples were taken before and every 10–20 min after the flooding dose. Biopsies (50–100 mg) of vastus lateralis muscle were taken 2.5 h after the application of the flooding dose by use of the conchotome technique (Dietrichson et al. 1987), with skin and fascia incisions made under local anaesthesia (1% lignocaine). Biopsies of patellar tendon (5–8 mg) were taken (Miller et al. 2005) under ultrasound guidance after local anaesthesia using a 16 G Monopty biopsy instrument (Bard Inc., Covington, GA, USA). Biopsies were taken from the medial side of the tendon on one occasion and the lateral side on the other, randomly. The muscle and tendon biopsies were blotted, frozen in liquid nitrogen, and stored at −80°C until analysis.
Induction of atrophy
We chose to induce atrophy in the dominant leg. To do this the non-dominant leg of each participant was fitted with a platform shoe; the dominant leg was kept in a slightly flexed position using straps suspending the foot above the ground while walking with crutches (de Boer et al. 2007). Participants had to walk on crutches for the whole duration of the suspension period and were asked to refrain from loading the leg in any way, including by driving vehicles.
Muscle cross-sectional area
Anatomical cross-sectional area (ACSA) of the m. quadriceps femoris was assessed using a 0.2 tesla MRI scanner (E-Scan, Esaote Biomedica, Genoa, Italy) as previously described (de Boer et al. 2007).
Tendon dimensions
Resting tendon length was measured along the sagittal plane from the apex of the patellar to the superior aspect of the tibial tuberosity. Tendon cross-sectional area (CSA) was subsequently assessed by ultrasonography at 25, 50 and 75% of the resting length, along the axial plane as previously described (de Boer et al. 2007). The intraclass correlation coefficients were 0.99 for patellar tendon CSA and patellar tendon length. Typical errors were ±1.5 mm2 for tendon CSA and ±0.6 mm for tendon length. In relative terms, this means that we would have detected any change greater than about 1% in either tendon CSA or resting length.
Extraction of tissue proteins for measurement of synthetic rates
Tendon
Tendon (5–8 mg) was ground in liquid nitrogen to a fine powder, re-suspended in extraction buffer (0.02 m Tris/HCl, pH 7.4, 0.15 m NaCl, 0.1% Triton X-100), and then centrifuged at 1600 g at 4°C for 20 min to pellet the collagen. The pellets were washed twice with 70% ethanol to remove free amino acids before further processing.
Myofibrillar protein
Muscle (30 mg) was ground in liquid nitrogen and re-suspended in extraction buffer (0.02 m Tris, 0.15 m NaCl, 0.1 m EDTA, 0.1% Triton X-100). The homogenate was centrifuged at 1600 g for 20 min, the supernatant removed and the myofibrillar/collagen pellet re-suspended in 0.3 m NaOH. The soluble myofibrillar protein and the insoluble collagen were separated by centrifugation. The myofibrillar fraction was precipitated using 1 m perchloric acid and the pellet washed twice with 70% ethanol.
Plasma protein
To estimate background 13C and 15N labelling in body proline, plasma protein isolated from a blood sample taken prior to the administration of the flooding dose of labelled proline was used. Bulk plasma protein was precipitated using ice cold ethanol and pelleted by centrifugation, then washed twice with 70% ethanol.
Protein hydrolysis and gas chromatography–mass spectrometry
Protein from all sources was hydrolysed in a slurry of 0.05 m HCl–Dowex 50WX8-200 (Sigma Ltd, Poole, UK) at 110°C overnight (Balagopal et al. 1997) and the liberated free amino and imino acids purified then eluted in 2 m NH4OH. The amino and imino acids were derivatized as their N-acetyl-n-propyl (NAP) ester (Meier-Augenstein, 1999). NAP amino and imino acids were analysed by capillary GC-C-IRMS (Delta-plus XL, Thermo Fisher Scientific, Hemel Hempstead, UK); separation was achieved on a 25 m × 0.25 mm × 1.0 μm film DB 1701 capillary column (Agilent Technologies, West Lothian, UK). The ratio of proline-to-hydroxyproline concentration in collagen was determined for each chromatogram using the area under the curve for each imino acid.
Analysis of free plasma proline labelling
Plasma was separated from whole blood by spinning at 1600 g for 20 min at 4°C. Plasma was deproteinized with 100% ethanol, dried and re-suspended in 0.5 m HCl. Lipids were removed by ethyl acetate extraction. The 1-13C and 15N labelling of plasma proline was determined after conversion to the tert-butyldimethylsilyl derivative by gas chromatography–mass spectrometry (Babraj et al. 2005a; Meier-Augenstein, 1999) using the Trace DSQ (Thermo Fisher Scientific Hemel Hempstead, UK).
SDS/PAGE and Western blotting
Approximately 30 mg muscle tissue was homogenized with scissors in ice-cold buffer (50 mm Tris-HCl, 1 mm EDTA, 1 mm EGTA, 10 mm β-glycerophosphate, 50 mm NaF, 0.5 mm Na3VO4, 0.1% Triton X-100; pH 7.5) containing one complete mini protease inhibitor tablet (Roche Diagnostics Ltd, Burgess Hill, UK). Proteins from 10 000 g supernatants were quantified with Bradford reagent (Sigma), and 50 μg was combined with 5 × Laemmli sample buffer and separated by SDS/PAGE (Criterion system, Bio-Rad Hemel Hempstead, UK). After electrophoretic separation at 200 V, proteins were transferred to a PVDF membrane at 100 V for 1 h. The membranes were then incubated in 3% low fat milk in TBS with 0.1% tween. Subsequently, membranes were incubated with appropriate antibodies (1 : 1000) overnight at 4°C. Antibodies were purchased from Cell Signalling Technologies (via New England Biolabs (UK) Ltd, Hitchin, UK) unless otherwise stated. Antibodies were applied for the following: phospho-PKB Ser 473, total PKB phospho-p70s6k Thr 389, total p70s6k (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), phospho-FAK Tyr 576/577, total FAK, phospho-eEF2 Thr 56, total eEF2, phospho-4E-BP1 Thr 37/46, total 4E-BP1, phospho-TSC2 Thr 1462 total TSC2, phospho-mTOR Ser 2448, total mTOR phospho-eIF4E Ser 209, total eIF4E. The next morning blots were washed before incubation with anti-rabbit IgG at 1 : 2000 for 1 h at room temperature with shaking. After three washes, membranes were exposed to chemiluminescent reagent for 5 min (Bio-Rad, Hemel Hempstead UK) before exposure to the Chemidoc XRS densitometer (Bio-Rad) for a period short enough to ensure no pixel saturation.
RNA extraction and quantitative RT-PCR
Frozen tissue samples (5–10 mg) were homogenized in TRIZOL® (Sigma) using a Polytron for 60 s on ice. Total RNA was extracted according to the instructions provided by the manufacturer (Sigma). RNA was quantified by spectrophotometry (260 nm) and 0.25 μg were run on a 1% agarose gel to check RNA quality by visualization of 28 s and 18 s rRNA. Reverse transcription (RT) was performed using the iScript synthesis kit (Bio-Rad) with 1 μg of total RNA in a reaction volume of 20 μl (4 μl iScript reaction mix, 1 μl iScript reverse transcriptase, 1 μl RNA template, 14 μl RNase-free water). The final RT product was adjusted to 140 μl using RNase-free water. RT-PCR primers were designed (see Table 1 in Supplemental material) for MAFbx (muscle atrophy F-box), MuRF-1 (muscle specific ring finger-1), TPP2 (tripeptidyl peptidase 2), MHC Iβ (myosin heavy chain type I), MHC IIA (myosin heavy chain type IIA), MHC IIX (myosin heavy chain type IIX), myostatin, collagen (Iα1). Sybr Green® real time RT-PCR analyses were carried out on the iQ5 Real-Time PCR Detection System (Bio-Rad) using the following cycle conditions: 3 min at 95°C, followed by 40 cycles of 1 min at 60°C (63°C for IGF-1ea) and 15 s at 95°C. For each gene, real time RT-PCR was conducted in duplicate with 25 μl reaction volume containing 12.5 μl qPCR SuperMix (Bio-Rad), 0.75 μl of each primer (10 pmol μl−1), 9 μl RNAse-free water and 2 μl of 1 : 10 diluted cDNA. PCR products were checked for amplicon specificity by both melting curve and agarose gel electrophoresis.
Protein synthesis calculations
The rate of tissue protein synthesis was calculated as:
where ΔEp is the change in proline (for myofibrillar protein) or hydroxyproline (for tendon collagen) labelling over time in tissue protein and AUCp is the area under the curve of venous proline labelling with time in hours. We have previously demonstrated that in a number of tissues, values of plasma and tissue-free proline labelling are indistinguishable (Babraj et al. 2002, 2005b) indicating that the flooding dose technique is successful in equilibrating the intracellular and plasma pools. Therefore, venous plasma proline was taken to represent the labelling of prolyl-tRNA in tenocytes and fibroblasts for the calculation of tendon collagen synthetic rates. We have also shown that the flooding proline method is an accurate and precise method for measuring myofibrillar protein FSR, giving values similar to that previously obtained with 13C-labelled leucine and so we used it to measure both myofibrillar protein and collagen synthesis (Babraj et al. 2005a).
Quantification of mRNA, protein amount and degree of phosphorylation
Changes in mRNA expression were quantified using the method of Pfaffl (2001) before logarithmic transformation and statistical analyses, using the values for the same person as the starting point. Protein analyses are reported as both changes in total proteins and as changes in phosphorylated relative to total. This method of analysis was chosen since chronic interventions might alter cellular protein concentrations and by expressing the data in this way, changes in total protein concentrations possibly important in their own right, but also contributory to differences in the extent of phosphorylation, can be seen. Densitometry was performed on the Chemidoc XRS (Bio-Rad) with average densities being reported as arbitrary densitometric units.
Statistics
One-way ANOVA with Tukey–Kramer post hoc test (GraphPad InStat version 3.00 for Windows 95, GraphPad Software, San Diego, CA USA) was used to test significance of differences between values at 0, 10 and 21 days for muscle CSA and muscle and tendon protein synthesis rates. ANOVA for repeated measures was used to analyse data for the muscle CSA and the tendon stiffness. Student's paired t test on log transformed data was used for RT-PCR values and Western analysis of signalling proteins. Significance was assigned at P < 0.05.
Presentation of data
We present here the results for muscle CSA, tendon stiffness and dimensions, protein synthesis in muscle and tendon, plus changes in FAK phosphorylation, gene expression for MAFbx, MuRF-1 and TPPII between 0 and 10 days and 10 and 21 days. All other results are available as online supplemental material.
Results
Muscle and tendon dimensions
Suspension of the lower limb resulted in significant reductions in muscle anatomical CSA, as well as in muscle fascicle length and angle of pennation (see de Boer et al. 2007). The whole quadriceps muscle CSA (determined as the sum of the individual muscles' CSA) decreased by 5.2% (P < 0.001) after 14 days (i.e. at −0.37 ± 0.05% per day) and by 10.0% (P < 0.001) after 23 days (i.e. −0.51 ± 0.21% per day) (Fig. 1). There were no significant differences in the rates of loss of CSA between the two periods.
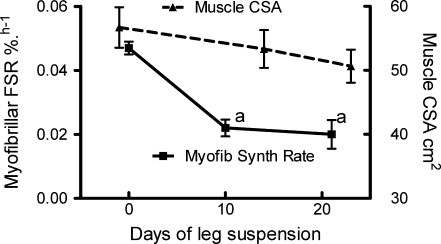
Figure 1.
Effects of unilateral lower limb suspension on human quadriceps cross-sectional area and myofibrillar fractional protein synthesis (FSR) All results are means and standard errors of the mean. Data are for n = 9 for CSA at all time points (see de Boer et al. 2007). Muscle CSA at days 14 and 23 was significantly different (repeated measures ANOVA) from each other and from baseline values (see de Boer et al. 2007). Results for myofibrillar FSR are for n = 9 at 10 days, n = 5 at day 0 and n = 4 at day 21. aP < 0.05 versus day 0-values.
Neither tendon CSA (100.7 ± 12.4 mm2versus 101.1 ± 11.6 mm2, P = 0.347) nor tendon resting length (48.6 ± 3.0 mm versus 48.5 ± 2.8 mm, P = 0.454) was altered between baseline and day 23 of unilateral leg suspension. The values obtained for tendon dimensions are within the range we routinely observe in our studies (unpublished observations). Thus tendon volume was unchanged. Analysis of proline and hydroxyproline concentration of extracted tendon collagen showed no change in the ratio between them (P : OHP, day 0, 2.01 ± 0.19; day 10, 1.85 ± 0.51; day 21 1.80 ± 0.42). Tendon stiffness decreased markedly (Fig. 2) and more rapidly in the latter period more than in the first (de Boer et al. 2007).
Figure 2.
Effects of unilateral lower limb suspension on human patellar tendon stiffness (N mm−1) and tendon collagen synthesis (fractional synthesis rate, FSR per cent h−1) All results are means and standard errors of the mean. Data are for n = 9 for tendon stiffness at all time points (see de Boer et al. 2007). Tendon stiffness values at days 14 and 23 were significantly different (repeated measures ANOVA) from each other and from baseline values (see de Boer et al. 2007). Results for tendon collagen FSR are for n = 9 at 10 days, n = 5 at day 0 and n = 4 at day 21. aP < 0.05 versus day 0 values; bP < 0.05 versus day 10 values of tendon FSR.
Tissue protein synthesis (Figs 1 and 2)
The basal postabsorptive rates of muscle myofibrillar protein synthesis and tendon collagen synthesis at 0.047 ± 0.004% h−1 and 0.052 ± 0.012% h−1 were similar to values previously reported by us in young healthy men. The rates of myofibrillar protein synthesis halved by day 10 (to 0.022 ± 0.007% h−1) (P < 0.01 compared to day 0) and remained depressed at this rate (0.020 ± 0.009) at day 21 (Fig. 1). The rates of myofibrillar protein synthesis decreased faster over days 0–10 than did the rate of loss of muscle CSA over days 0–14, but thereafter the loss of CSA continued at the same rate despite protein synthesis occurring at a lower rate by day 21 than observed at day 10.
The rates of tendon collagen synthesis showed progressive change, with a halving by day 10 (to 0.023 ± 0.018% h−1; P < 0.01 compared to day 0) and a further halving by day 21 (to 0.010 ± 0.004% h−1; P < 0.01 compared to both days 0 and day 10) (Fig. 2). As for the myofibrillar synthetic rate, the rate of decrease of tendon collagen synthesis rate was faster over days 0–10 than the rate of loss of tendon stiffness over days 0–14, whereas the rate of loss of stiffness accelerated thereafter and that of tendon collagen FSR declined.
Muscle gene expression (Figs 3, 4 and 5 and Supplemental material)
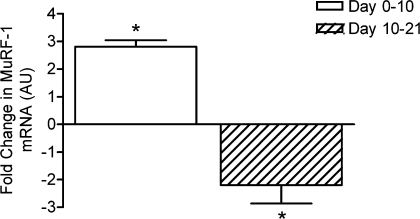
Figure 3.
Changes in MuRF1 mRNA between days 0 and 10 and 10 and 21 in the two groups of four subjects studied twice Values are means and standard errors of the mean. *P < 0.05 for change between times indicated.
Figure 4.
Changes in MAFBx mRNA between days 0 and 10 and 10 and 21 in the two groups of four subjects studied twice Values are means and standard errors of the mean. *P < 0.05 for change between times indicated.
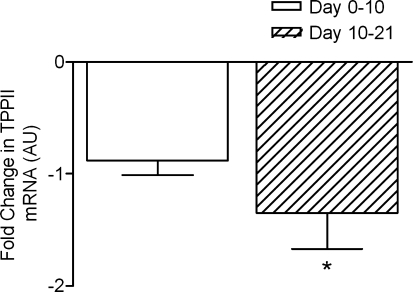
Figure 5.
Changes in tripeptidyl peptidase II mRNA between days 0 and 10 and 10 and 21 in the two groups of four subjects studied twice Values are means and standard errors. *P < 0.05 for change between times indicated.
The expression of mRNA myosin heavy chain isoforms showed marked increases in MHC IIx at 10 days which persisted at 21 days; there were no changes observed in MHC I or MHC IIa (see Supplemental material). Of the genes associated with proteolytic activity, MuRF-1 mRNA was elevated at day 10 but was expressed at a depressed amount by day 21, whereas MAFbx mRNA was unchanged at day 10 and depressed at day 21, a pattern also seen for TPPII (see Figs 3, 4 and 5). There were no changes in collagen I mRNA between days 0 and 10 but a marked increase in expression between 10 and 21 days (see Supplemental material). Myostatin mRNA was not changed at any point (see Supplemental material).
Muscle cell signalling (Fig. 6 and Supplemental material)
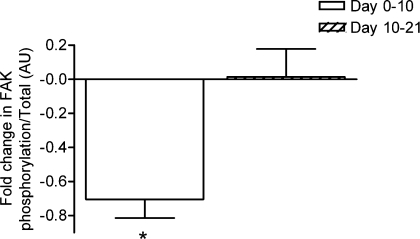
Figure 6.
Changes in FAK phosphorylation between days 0 and 10 and 10 and 21 in the two groups of four subjects studied twice Values are means and standard errors of the mean. *P < 0.05. (See Supplemental material for details of total protein amounts.)
None of the components controlling translation initiation or elongation, including PKB, TSC-2, p70, 4EBP1, eIF4E and eEF2, showed any changes at days 10 or 21 in either total protein or degree of phosphorylation (see Supplemental material). However, there were marked falls in the phosphorylation of FAK relative to total FAK (Fig. 6).
Discussion
The major novel results produced by this work concerning muscle are that, accompanying quadriceps muscle atrophy of 0.5% per day over 23 days, there is a halving of the fractional synthetic rate of myofibrillar proteins over 10 days, with no further decreases by 21 days, a substantial fall in the phosphorylation of FAK, a reversible initial increase in MuRF-1 mRNA, with decrements in this and mRNA for MAFbx and TPPII between days 10 and 21. The documentation of the lack of changes in phosphorylation of other muscle signalling proteins is also novel for human muscle. The increase in collagen I expression may reflect a greater expression generally of extracellular matrix proteins in atrophied muscle (Savolainen et al. 1987, 1988). Myostatin gene changes appeared not to be involved in the observed effects on muscle mass.
For tendon, the major finding is a progressive fall in collagen synthetic rate at days 10 and 21, despite no change in tendon dimensions, with the rate of fall being attenuated over the second period. Thus, as we hypothesized, the rates of decline of myofibrillar and tendon collagen protein synthesis do continue to fall but less rapidly with time. This is the first work to examine the time course of changes in factors affecting the mass of human muscle during disuse atrophy and the first to examine the effect of disuse on the rate of human tendon collagen synthesis. It is also the first work to examine the changes in muscle and tendon protein maintenance together, which is important given their closely related mechanical functions.
What do the results tell us about the process of alteration of physiological properties of muscle and tendon as a result of disuse? First, the marked fall in myofibrillar protein synthesis is consonant with the fall in actin concentration with disuse atrophy observed by previous workers (Widrick et al. 1999) although it is impossible for us to distinguish from our data whether or not actin or myosin synthesis were more greatly affected.
Secondly, it is plain that although the rates of myofibrillar protein synthesis were measured only in the postabsorptive state, rather than across the diurnal cycle which would normally include the results of a period of feeding, it is highly likely that daily rates of total muscle protein synthesis were markedly reduced. Normally, daily protein synthetic rates in human mixed muscle protein are about 2.0–2.5% per day (the resultant of about 12 h at 0.045–0.06% h−1 in the postabsorptive state) and 12 h at 0.12–0.15% h−1 in the fed state. The relationship between rates of synthesis of myofibrillar protein and total mixed muscle protein (i.e. including sarcoplasmic and mitochondrial) and the response to feeding with protein (Bohéet al. 2001; Cuthbertson et al. 2005; Mittendorfer et al. 2005) is such that if daily mixed muscle protein synthesis fell by 50% to 1.0–1.25% per day, and protein breakdown were preserved at its likely day 0 value of 2.0–2.5% per day, this would be more than enough to account for the observed fall of 0.5% per day in muscle CSA. In fact, we have preliminary data that in previously immobilized human quadriceps muscle, there is resistance to the anabolic effects of amino acids (E. Glover, S. Phillips, A. Selby, K. Smith and M. J. Rennie, unpublished work) strongly suggesting that the depressive effects would be seen across the diurnal span of feeding and fasting. Even if the effect on muscle protein FSR were confined to the fasting state (which is unlikely) the decrease could account for most of the observed wasting. This conclusion is in line with those of our previous work in human beings (Gibson et al. 1987) and those of others in rats (Tucker et al. 1981). Thus, it is likely that no substantial net increase in protein breakdown is required to account for the observed atrophy of human muscle over the extended periods of unilateral lower limb suspension we studied.
In contrast, it has been reported that, in young growing rats, immobilization is accompanied by an increase in muscle protein breakdown measured in vitro (Goldspink, 1977); however, it was only enhanced by about 20% during one week of limb immobilization and the validity of the method used has been questioned (Seider et al. 1980). It has also been reported that the activities of muscle lysosomal enzymes are elevated in immobilized rat muscle (Goldspink & Lewis, 1985), and in the past few years components of the ubiquitin–proteasome pathway, especially the ubiquitin ligases MAFbx and MuRF-1, have been suggested to be major regulators of muscle mass and promoters of atrophy in different circumstances, including immobilization (Gomes et al. 2001; Krawiec et al. 2005) in immature rodents. Also, it is reportedly possible to partially rescue muscle wasting by administration of proteasome inhibitors; indeed 53% of atrophy after 3 days immobilization in rats was prevented by provision of Velcade, an atrogin inhibitor (Gomes et al. 2001; Krawiec et al. 2005).
The relevance of these results to the human situation is not clear. First, the animals studied were immature and there may be substantial differences in the control of metabolism of immature and mature mammals. For example young animals show marked increases in muscle protein synthesis upon insulin administration whereas adult human beings do not (Gelfand & Barrett, 1987; Greenhaff et al. 2005). Also whereas rodents, lagomorphs and fowl show marked differentiation in rates of protein turnover between muscles of different fibre types, in human muscle there is no marked difference (Mittendorfer et al. 2005). Secondly, no study in human beings has ever identified increases in muscle protein breakdown using either direct or indirect measurements during immobilization atrophy (Ferrando et al. 1996; Stein & Schluter, 1997; Paddon-Jones et al. 2006). Thirdly, no study of human muscle protein turnover has investigated changes at a time point of less than 7 days, which opens up the possibility that previous workers, including ourselves and the Ferrando group have simply missed early increases in protein breakdown. This possibility is strengthened by the recent reports from studies of 2 days of immobilization in healthy individuals (Urso et al. 2006) and also of patients within 2–5 days of traumatic spinal cord transection showing increases in the expression of mRNA for enzymes involved both in the ubiquitin–proteasome pathway (UPP) and in the degradation of extracellular matrix proteins (Urso et al. 2007). However, neither of these reports measured protein breakdown directly and neither was able to discover increases in enzyme protein (apart from minor increases in concentrations of the proteasome subunit (PSMD11)) or activity although there was some suggestion of increased histochemical signs of increased PSMD11 and MAFbx protein on the periphery of muscle fibres at 5 days after spinal cord transection. Furthermore, in the studies of 2 days of immobilization of quadriceps in normal healthy subjects, no changes were observed in mRNA for MuRF-1 and MAFbx (Urso et al. 2006). This is in contrast to the findings of workers (Jones et al. 2004) who studied subjects who underwent 2 weeks of long leg casting (i.e. directly comparable to the first half of the suspension period of our subjects) with a similar rate of quadriceps wasting to that we report but also observed increased expression of the 20S proteasome 7-subunit by 26%, of MAFbx by 62% and a non-significant trend for up-regulation of MuRF1 by 34% without any change of the E3 ligase.
However, very recently (during the preparation of a revised version of this paper) a study of the expression of genes using a gene chip microarray for human muscle suffering disuse atrophy has been published (Chen et al. 2007). The authors found that in muscles immobilized after casting following ankle fracture, there was decreased expression in genes encoding proteolytic proteins, calpain 3 and calpastatin, as well as in several genes belonging to the ubiquitin–proteasome pathway. Nevertheless, there was also a modest up-regulation of MAFbx, similar to that observed by Jones and co workers (Jones et al. 2004).
It has been claimed that in most types of muscle atrophy, overall rates of protein synthesis are suppressed and rates of protein degradation are elevated, and these changes are linked to greater expression of mRNA for so called atrogenes, whose expression is coordinately induced or suppressed in muscle during systemic wasting states. (Sacheck et al. 2007). We are sceptical of this claim because (i) we have little faith that the animal models used are reflective of the human situation and (ii) the proponents of this view are unable to cite a single instance of the measurement of protein breakdown in the claimed circumstances in human muscle which supports their case, for example cancer cachexia, disuse atrophy, starvation. Indeed we have good data from a number of studies apart from those described here in which there is no rise of protein breakdown but a profound fall in protein synthesis with breakdown, as described in a review of the topic more than 20 years ago (Rennie, 1985). This is exactly the pattern seen in a study of weight losing cancer patients which we are preparing for publication (M. J. Rennie and others, unpublished information).
These results and our current findings cannot be easily reconciled without further experimentation but it is worth making a number of points which could guide future workers in experimental design. First, there are a number of physiological and pathological circumstances in human beings in which it is known that altered muscle proteolysis or muscle maintenance appears to be disassociated from the expression of mRNA for ubiquitin and components of the ubiquitin–proteasome pathway (UPP) with muscle wasting (Bossola et al. 2002; Jagoe et al. 2002; Roberts et al. 2002; Brodsky et al. 2004; Fareed et al. 2006; Constantin et al. 2007). Thus, the presumption that an increase in mRNA expression is matched by increases in enzyme activity, and in this case, bulk protein breakdown, may be unwarranted. Secondly, the histochemical evidence of Urso et al. (2006, 2007) suggests that if there is increased activity of the UPP and other proteases it may be confined to peripheral cell structures and not to compartments containing the bulk of muscle protein. The possibility therefore exists that the proteolytic activity is targeted at proteins whose degradation substantially decreases protein synthetic processes without marked increases in protein breakdown of myofibrillar protein. Thirdly, it is imperative that studies of both arms of protein turnover (i.e. protein synthesis and breakdown) are measured at an early time point (i.e. from 3 to 5 days of disuse) in the fed as well as the fasted states, since it may be that one of the problems associated with immobilization is an ‘anabolic resistance’ to feeding as seen in the elderly (Cuthbertson et al. 2005). In fact, as mentioned above, we have preliminary evidence for this in studies of immobilization of young healthy subjects for 10 days (E. Glover, S. Phillips, A. Selby, K. Smith and M. J. Rennie, unpublished work).
There were no large alterations in the concentration or degree of phosphorylation of any of the proteins associated with protein anabolic signalling pathways or elongation we measured (see Supplemental material). These results suggest that the signalling potential of skeletal muscle was not diminished, a finding previously reported by the Jefferson group (Gomes et al. 2001; Krawiec et al. 2005) in hindlimb-immobilized rats. However Urso and colleagues did report small decrements in the degree of phosphorylation of PKB (but no other components of the pathway) in healthy subjects undergoing 48 h of immobilization (Urso et al. 2006). It is therefore possible that any perturbations in activity of this pathway are only evident in the acute stages of immobilization or after feeding.
The marked and apparently persistent changes in FAK stand out among the general lack of changes in phosphorylation state of other proteins we measured. The results are similar to those reported by Booth's group in the soleus muscles of 7 days hindlimb-immobilized rats (Gordon et al. 2001). However, in their work no changes were observed for muscles with predominantly Type II fibres (plantaris and gastrocnemius) whereas the human quadriceps is equally composed of Types I and II. It may be that the changes we observed were only in Type I fibres, or due to a loss of Type I fibres. However the latter is unlikely as an explanation because the MHC Iβ expression did not change in our subjects (see Supplemental material). Indeed, the only indicator of an effect upon fibre type was an increase in mRNA for MHC IIX (see Supplemental material) which has been reported in unloaded rat soleus muscle (Giger et al. 2005).
These changes are likely to be of substantial importance in the mechanism of induction of the muscle atrophy we observed. According to a recent hypothesis, FAK signalling may lie upstream of serum response factor (SRF). SRF binds as a homodimer to a consensus sequence found in multiple muscle specific genes. The rationale for the potential relationship between FAK and SRF during altered muscle loading is that SRF exhibits increased mRNA abundance, increased protein concentration and altered SRF-SRE1 binding on the skeletal α-actin promoter in hypertrophying muscle of overloaded chicken anterior latissimus dorsi and presumably the opposite occurs in unloading (Gordon et al. 2001).
One of the most novel aspects of the results is the finding not reported before in human tendon of a fall in tendon collagen synthesis rate. Human musculoskeletal collagen metabolism is becoming recognized as being much more than physiologically responsive than hitherto thought (Kjaer, 2004; Miller et al. 2005) and the present results extend the knowledge in this area. Collagen synthesis per se has not been previously measured in human tendon during and after disuse, and the present result of a fall in the rate of collagen synthesis has never been reported previously, although it is consonant with predictions made from work in animals (Savolainen et al. 1987). However, it is particularly noteworthy that our data show that the rate of collagen synthesis falls progressively from days 0 through 21, with the rate slowing only slightly over the last 11 days. These results suggest that the driver for the fall in tendon persists more effectively in its effects over the entire period whereas for muscle the rate of myofibrillar synthesis apparently levels out after 10 days.
One puzzling aspect is that the mass of tendon, inferred from the dimensions, is apparently not affected by unilateral leg suspension. We would have been able to easily detect a fall of the same order as observed in quadriceps CSA (∼10%) had it occurred. If it indeed did not, then the fall in the rate of collagen protein synthesis must have been matched by an exactly equal fall in the rate of collagen proteolysis or mass would change; that it did not is a very unusual circumstance in our experience of alterations of musculoskeletal mass and protein turnover. It may be that the apparent lack of fall is an artefact of altered hydration of tendon, masking a fall in tendon cell and extracellular matrix mass. However, had tendon mass fallen, like muscle did, by 10%, this would only be a viable explanation if there had been an approximate doubling in tendon hydration to mask the loss of mass. The fact that we observed a decreased stiffness of the tendon argues against this, because only decreased hydration decreases stiffness in vitro (Haut & Haut, 1997). Unfortunately, we have no information on the water content of the patellar tendons of our subjects so this question cannot at present be resolved.
However the fall in stiffness in rat tendon is known to be accompanied by decreases in enzymes associated with collagen crosslinking (Savolainen et al. 1987). The method of measuring collagen synthesis depends upon the incorporation of proline into hydroxyproline so there is a possibility that the deficit we ascribed to decreased collagen synthesis is actually a measure of decreased hydroxy-proline synthesis. Against this hypothesis is the fact that the ratio of hydroxyproline : proline remained constant in our samples, as it did in the atrophied tendons examined by the Takala group (Savolainen et al. 1987). Unfortunately the small size of the tendon biopsies (7–10 mg) prohibited their further biochemical or molecular analysis and further interpretation must await new data.
Conclusion
Disuse atrophy consequent to unilateral lower limb suspension in young healthy men causes loss of quadriceps muscle mass and patellar tendon stiffness accompanied by large decreases in the rates of myofibrillar protein synthesis and tendon collagen synthesis, which are more rapid over the first 10 days than the latter 11. In muscle, the rates of decline are such as to make it unlikely that over the entire period there is a rise in muscle protein breakdown, and gene markers for proteolytic elements were not elevated over the whole period. The fall in FAK phosphorylation appears to persist and may be related to the catabolic loss of muscle via a decrease of mechanosensory input. It seems likely that countermeasures to reverse muscle wasting in immobilization should concentrate on the early period after onset of disuse, i.e. within 10 days.
Acknowledgments
The authors are particularly grateful to Jörn Rittweger for medical screening and monitoring of the subjects. Studies described in this paper were supported by the UK Biotechnology and Biological Sciences Research Council (BB/X510697/1 and BB/C516779/1), and the AJINOMOTO Co. Inc. to M.J.R. and the European Space Agency (ESA MESM grant 15097/01/NL/SH) to M.N.
Supplementary material
Online supplemental material for this paper can be accessed at:
http://jp.physoc.org/cgi/content/full/jphysiol.2007.142828/DC1 and http://www.blackwell-synergy.com/doi/suppl/10.1113/jphysiol.2007.142828
References
- Babraj J, Cuthbertson DJ, Rickhuss P, Meier-Augenstein W, Smith K, Bohe J, Wolfe RR, Gibson JN, Adams C, Rennie MJ. Sequential extracts of human bone show differing collagen synthetic rates. Biochem Soc Trans. 2002;30:61–65. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- Babraj JA, Cuthbertson DJ, Smith K, Langberg H, Miller B, Krogsgaard MR, Kjaer M, Rennie MJ. Collagen synthesis in human musculoskeletal tissues and skin. Am J Physiol Endocrinol Metab. 2005a;289:E864–E869. doi: 10.1152/ajpendo.00243.2005. [DOI] [PubMed] [Google Scholar]
- Babraj JA, Smith K, Cuthbertson DJ, Rickhuss P, Dorling JS, Rennie MJ. Human bone collagen synthesis is a rapid, nutritionally modulated process. J Bone Miner Res. 2005b;20:930–937. doi: 10.1359/JBMR.050201. [DOI] [PubMed] [Google Scholar]
- Balagopal P, Ljungqvist O, Nair KS. Skeletal muscle myosin heavy-chain synthesis rate in healthy humans. Am J Physiol Endocrinol Metab. 1997;35:E45–E50. doi: 10.1152/ajpendo.1997.272.1.E45. [DOI] [PubMed] [Google Scholar]
- Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3:1014–1019. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- Bohé J, Low JF, Wolfe RR, Rennie MJ. Latency and duration of stimulation of human muscle protein synthesis during continuous infusion of amino acids. J Physiol. 2001;532:575–579. doi: 10.1111/j.1469-7793.2001.0575f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossola M, Muscaritoli M, Costelli P, Nanni G, Tazza L, Panocchia N, Busquets S, Argiles J, Lopez-Soriano FJ, Grieco G, Baccino FM, Fanelli FR, Castagneto M, Luciani G. Muscle ubiquitin m-rNA levels in patients with end-stage renal disease on maintenance hemodialysis. J Nephrol. 2002;15:552–557. [PubMed] [Google Scholar]
- Brodsky I, Suzara D, Furman M, Goldspink P, Ford G, Nair K, Kukowski J, Bedno S. Proteasome production in human muscle during nutritional inhibition of myofibrillar protein degradation. Metabolism. 2004;53:340–347. doi: 10.1016/j.metabol.2003.10.015. [DOI] [PubMed] [Google Scholar]
- Chen YW, Gregory CM, Scarborough MT, Shi R, Walter GA, Vandenborne K. Transcriptional pathways associated with skeletal muscle disuse atrophy in humans. Physiol Genomics. 2007 doi: 10.1152/physiolgenomics.00115.2006. in press. [DOI] [PubMed] [Google Scholar]
- Constantin D, Constantin-Teodosiu D, Layfield R, Tsintzas K, Bennett A, Greenhaff P. PPARδ agonism induces a change in muscle fuel metabolism and activation of an atrophy programme, but does not impair mitochondrial function. J Physiol. 2007;583:381–390. doi: 10.1113/jphysiol.2007.135459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005;19:422–424. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- de Boer M, Maganaris C, Seynnes O, Rennie M, Narici M. Time course of neuromuscular and tendinous adaptations to 23-day unilateral lower-limb suspension in young men. J Physiol. 2007;583:1079–1091. doi: 10.1113/jphysiol.2007.135392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrichson P, Coakley J, Smith PEM, Griffiths RD, Helliwell TR, Edwards RHT. Conchotome and needle percutaneous biopsy of skeletal muscle. J Neurol Neurosurg Psychiatr. 1987;50:1461–1467. doi: 10.1136/jnnp.50.11.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareed MU, Evenson AR, Wei W, Menconi M, Poylin V, Petkova V, Pignol B, Hasselgren PO. Treatment of rats with calpain inhibitors prevents sepsis-induced muscle proteolysis independent of atrogin-1/MAFbx and MuRF1 expression. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1589–R1597. doi: 10.1152/ajpregu.00668.2005. [DOI] [PubMed] [Google Scholar]
- Ferrando AA, Lane HW, Stuart CA, Davis-Street J, Wolfe RR. Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. Am J Physiol Endocrinol Metab. 1996;270:E627–E633. doi: 10.1152/ajpendo.1996.270.4.E627. [DOI] [PubMed] [Google Scholar]
- Gelfand RA, Barrett EJ. Effect of physiologic hyperinsulinemia on skeletal muscle protein synthesis and breakdown in man. J Clin Invest. 1987;80:1–6. doi: 10.1172/JCI113033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson JNA, Halliday D, Watt PW, Stoward PJ, Morrison WL, Rennie MJ. Decrease in human quadriceps muscle protein turnover consequent upon leg immobilization. Clin Sci. 1987;72:503–509. doi: 10.1042/cs0720503. [DOI] [PubMed] [Google Scholar]
- Gibson JNA, Smith K, Rennie MJ. Prevention of disuse muscular atrophy by means of electrical stimulation: maintenance of protein synthesis. Lancet. 1988;ii:767–770. doi: 10.1016/s0140-6736(88)92417-8. [DOI] [PubMed] [Google Scholar]
- Giger JM, Haddad F, Qin AX, Zeng M, Baldwin KM. Effect of unloading on type I myosin heavy chain gene regulation in rat soleus muscle. J Appl Physiol. 2005;98:1185–1194. doi: 10.1152/japplphysiol.01099.2004. [DOI] [PubMed] [Google Scholar]
- Goldspink DF. The influence of immobilization and stretch on protein turnover of rat skeletal muscle. J Physiol. 1977;264:267–282. doi: 10.1113/jphysiol.1977.sp011667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldspink DF, Lewis SE. Age- and activity-related changes in three proteinase enzymes of rat skeletal muscle. Biochem J. 1985;230:833–836. doi: 10.1042/bj2300833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes MD, Lecker SH, Jagoe RT, Navon A, Goldberg AL. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci U S A. 2001;98:14440–14445. doi: 10.1073/pnas.251541198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon SE, Fluck M, Booth FW. Selected Contribution: Skeletal muscle focal adhesion kinase, paxillin, and serum response factor are loading dependent. J Appl Physiol. 2001;90:1174–1183. doi: 10.1152/jappl.2001.90.3.1174. [DOI] [PubMed] [Google Scholar]
- Greenhaff PL, Peirce N, Simpson E, Hazell M, Babraj J, Waddell T, Smith K, Rennie M. Dose–response relationship during hyperaminoacidaemia between insulin and leg protein turnover in healthy young men studied by tracer amino acid exchange. J Physiol. 2005;558P:C10. [Google Scholar]
- Haut TL, Haut RC. The state of tissue hydration determines the strain-rate-sensitive stiffness of human patellar tendon. J Biomech. 1997;30:79–81. doi: 10.1016/s0021-9290(96)00108-x. [DOI] [PubMed] [Google Scholar]
- Jagoe RT, Redfern CP, Roberts RG, Gibson GJ, Goodship TH. Skeletal muscle mRNA levels for cathepsin B, but not components of the ubiquitin-proteasome pathway, are increased in patients with lung cancer referred for thoracotomy. Clin Sci (Lond) 2002;102:353–361. [PubMed] [Google Scholar]
- Jones SW, Hill RJ, Krasney PA, O'Conner B, Peirce N, Greenhaff PL. Disuse atrophy and exercise rehabilitation in humans profoundly affects the expression of genes associated with the regulation of skeletal muscle mass. FASEB J. 2004;18:1025–1027. doi: 10.1096/fj.03-1228fje. [DOI] [PubMed] [Google Scholar]
- Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev. 2004;84:649–698. doi: 10.1152/physrev.00031.2003. [DOI] [PubMed] [Google Scholar]
- Krawiec BJ, Frost RA, Vary TC, Jefferson LS, Lang CH. Hindlimb casting decreases muscle mass in part by proteasome-dependent proteolysis but independent of protein synthesis. Am J Physiol Endocrinol Metab. 2005;289:E969–E980. doi: 10.1152/ajpendo.00126.2005. [DOI] [PubMed] [Google Scholar]
- Meier-Augenstein W. Applied gas chromatography coupled to isotope ratio mass spectrometry. J Chromatogr A. 1999;842:351–371. doi: 10.1016/s0021-9673(98)01057-7. [DOI] [PubMed] [Google Scholar]
- Miller BF, Olesen JL, Hansen M, Dossing S, Crameri RM, Welling RJ, Langberg H, Flyvbjerg A, Kjaer M, Babraj JA, Smith K, Rennie MJ. Coordinated collagen and muscle protein synthesis in human patella tendon and quadriceps muscle after exercise. J Physiol. 2005;567:1021–1033. doi: 10.1113/jphysiol.2005.093690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittendorfer B, Andersen JL, Plomgaard P, Saltin B, Babraj JA, Smith K, Rennie MJ. Protein synthesis rates in human muscles: neither anatomical location nor fibre-type composition are major determinants. J Physiol. 2005;563:203–211. doi: 10.1113/jphysiol.2004.077180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narici MV, Maganaris CN. Adaptability of elderly human muscles and tendons to increased loading. J Anat. 2006;208:433–443. doi: 10.1111/j.1469-7580.2006.00548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddon-Jones D, Sheffield-Moore M, Cree MG, Hewlings SJ, Aarsland A, Wolfe RR, Ferrando AA. Atrophy and impaired muscle protein synthesis during prolonged inactivity and stress. J Clin Endocrinol Metab. 2006;91:4836–4841. doi: 10.1210/jc.2006-0651. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennie MJ. Muscle protein turnover and the wasting due to injury and disease. Br Med Bull. 1985;41:257–264. doi: 10.1093/oxfordjournals.bmb.a072060. [DOI] [PubMed] [Google Scholar]
- Roberts RG, Redfern CP, Graham KA, Bartlett K, Wilkinson R, Goodship TH. Sodium bicarbonate treatment and ubiquitin gene expression in acidotic human subjects with chronic renal failure. Eur J Clin Invest. 2002;32:488–492. doi: 10.1046/j.1365-2362.2002.01008.x. [DOI] [PubMed] [Google Scholar]
- Sacheck JM, Hyatt JP, Raffaello A, Jagoe RT, Roy RR, Edgerton VR, Lecker SH, Goldberg AL. Rapid disuse and denervation atrophy involve transcriptional changes similar to those of muscle wasting during systemic diseases. FASEB J. 2007;21:140–155. doi: 10.1096/fj.06-6604com. [DOI] [PubMed] [Google Scholar]
- Savolainen J, Myllyla V, Myllyla R, Vihko V, Vaananen K, Takala TE. Effects of denervation and immobilization on collagen synthesis in rat skeletal muscle and tendon. Am J Physiol Regul Integr Comp Physiol. 1988;254:R897–R902. doi: 10.1152/ajpregu.1988.254.6.R897. [DOI] [PubMed] [Google Scholar]
- Savolainen J, Vaananen K, Vihko V, Puranen J, Takala TE. Effect of immobilization on collagen synthesis in rat skeletal muscles. Am J Physiol Regul Integr Comp Physiol. 1987;252:R883–R888. doi: 10.1152/ajpregu.1987.252.5.R883. [DOI] [PubMed] [Google Scholar]
- Seider MJ, Kapp R, Chen C-P, Booth FW. The effects of cutting or of stretching skeletal muscle in vitro on the rates of protein synthesis and degradation. Biochem J. 1980;188:247–254. doi: 10.1042/bj1880247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein TP, Schluter MD. Human skeletal muscle protein breakdown during spaceflight. Am J Physiol Endocrinol Metab. 1997;272:E688–E695. doi: 10.1152/ajpendo.1997.272.4.E688. [DOI] [PubMed] [Google Scholar]
- Thomason DB, Booth FW. Atrophy of the soleus muscle by hindlimb unweighting. J Appl Physiol. 1990;68:1–12. doi: 10.1152/jappl.1990.68.1.1. [DOI] [PubMed] [Google Scholar]
- Tucker KR, Seider MJ, Booth FW. Protein synthesis rates in atrophied gastrocnemius muscles after limb immobilization. J Appl Physiol. 1981;51:73–77. doi: 10.1152/jappl.1981.51.1.73. [DOI] [PubMed] [Google Scholar]
- Urso ML, Chen YW, Scrimgeour AG, Lee PC, Lee KF, Clarkson PM. Alterations in mRNA expression and protein products following spinal cord injury in humans. J Physiol. 2007;579:877–892. doi: 10.1113/jphysiol.2006.118042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urso ML, Scrimgeour AG, Chen YW, Thompson PD, Clarkson PM. Analysis of human skeletal muscle after 48 h immobilization reveals alterations in mRNA and protein for extracellular matrix components. J Appl Physiol. 2006;101:1136–1148. doi: 10.1152/japplphysiol.00180.2006. [DOI] [PubMed] [Google Scholar]
- Widrick JJ, Knuth ST, Norenberg KM, Romatowski JG, Bain JL, Riley DA, Karhanek M, Trappe SW, Trappe TA, Costill DL, Fitts RH. Effect of a 17 day spaceflight on contractile properties of human soleus muscle fibres. J Physiol. 1999;516:915–930. doi: 10.1111/j.1469-7793.1999.0915u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.