Abstract
Luteinizing hormone (LH) regulates testosterone synthesis in Leydig cells by inducing an intracellular increase in cAMP concentration. LH also increases the intracellular calcium concentration ([Ca2+]i), dependent on the presence of Ca2+ in the extracellular medium ([Ca2+]e) for its effect. Despite these evidences, the identity of a pathway for calcium entry has remained elusive and the relationship between cAMP and [Ca2+]i has been questioned. Here we show that mice Leydig cells do have an inward Ca2+ current carried by T-type Ca2+ channels. In 10 mm [Ca2+]e, the currents start to be activated at −60 mV, reaching maximal amplitude of 1.8 ± 0.3 pA pF−1 at −20 mV. Currents were not modified by Ba2+ or Sr2+, were suppressed in Ca2+-free external solution, and were blocked by 100 μm nickel or 100 μm cadmium. The Ki for Ni2+ is 2.6 μm and concentrations of Cd2+ smaller than 50 μm have a very small effect on the currents. The calcium currents displayed a window centred at −40 mV. The half-voltage (V0.5) of activation is −30.3 mV, whereas the half-voltage steady-state inactivation is −51.1 mV. The deactivation time constant (τdeactivation) is around 3 ms at −35 mV. Confocal microscopy experiments with Fluo-3 loaded cells reveal that both LH and dibutyryl-cAMP (db-cAMP) increase [Ca2+]i. The db-cAMP induced calcium increase was dependent on Ca2+ influx since it was abolished by removal of extracellular Ca2+ and by 400 μm Ni2+. [Ca2+]i increases in regions close to the plasma membrane and in the cell nucleus. Similar effects are seen when Leydig cells are depolarized by withdrawing K+ from the extracellular solution. Altogether, our studies show that Ca2+ influx through T-type Ca2+ channels in the plasma membrane of Leydig cells plays a crucial role in the intracellular calcium concentration changes that follow binding of LH to its receptor.
The Leydig cells in the testis are the main source of testosterone in mammalian species. The synthesis and secretion of this steroid is controlled essentially by the luteinizing hormone (LH) produced by the hypophysis. Binding of LH to its receptor leads primarily to an increase in the intracellular cAMP level followed by an increase in [Ca2+]i (Dufau et al. 1980). However, there are other indications that LH also increases [Ca2+]i and its effect is dependent on the presence of calcium in the extracellular medium (Mendelson et al. 1975; Janszen et al. 1976) whose influx through the plasma membrane is a necessary step for the steroidogenic process (Moger, 1983). The latter point is, however, quite controversial. Kumar et al. (1994) and Sullivan & Cooke (1986) suggested that LH leads to an increase in [Ca2+]i by activation of Ca2+ entry through voltage-sensitive calcium channels. The presence of voltage-dependent Ca2+ currents in rat Leydig cells was firstly reported by Kawa (1987), but their characterization was incompletely described. Since then, no other electrophysiological study reported Ca2+ currents in Leydig cells. In fact, a few papers, using electrophysiology (Desaphy et al. 1996) or calcium indicator dyes (Tomić et al. 1995), reported either the absence of calcium entry through the plasma membrane or that they were unable to detect a calcium influx pathway. Nevertheless it is widely known that the stimulus–secretion coupling mechanism in other steroid producing cells requires an increase in [Ca2+]i in order to activate mitochondrial metabolism. In particular, an increase in [Ca2+]i activates the StAR protein facilitating the transport of cholesterol into the mitochondria (Spät et al. 2001). Calcium is also transported into the mitochondria, enhancing the synthesis of NADH and NADPH. The later is required by P450 for the cleavage of cholesterol to pregnenolone (Pitter et al. 2002; Spät & Pitter, 2004). In this context the increase in [Ca2+]i has a dual stimulatory effect on steroid production: (1) acting on the supply of the main substrate for testosterone synthesis; and (2) modifying the redox state of the mitochondria.
Despite the existence of such compelling evidences for the participation of intracellular calcium ions in the response to LH, the nature of the relationships between intracellular calcium stores, plasma membrane channels and cAMP still remains not completely understood.
In this paper we describe calcium currents through the plasma membrane of murine Leydig cells as well as the involvement of cAMP in the overall [Ca2+]i dynamics. Our results clearly demonstrate the presence of low voltage activated calcium currents in Leydig cells, whose electrophysiological properties can be associated with T-type calcium channels. We also show, using the calcium indicator dye Fluo-3 in confocal microscopy experiments, that the influx of calcium is a necessary step for the dramatic increase in intracellular calcium concentrations that follows the stimulation of the cells with LH or db-cAMP.
Methods
Cells
All experimental protocols used in this work were reviewed and approved by the Institutional Ethical Committee on Animal Experimentation of the School of Medicine of Ribeirão Preto/University of São Paulo (no. 092/2006).
Leydig cells were isolated from testes of 50- to 60-day-old mice (Swiss). The mice were killed by cervical dislocation and the testes rapidly removed, decapsulated and placed in Hanks' balanced salt solution (mm): 140 NaCl, 4.6 KCl, 2 CaCl2, 1 MgCl2, 10 Hepes, 5 NaHCO3, 10 glucose, pH adjusted to 7.4 with NaOH; osmolality 300 mosmol (kg H2O)−1. Cells were isolated by infusion of Hanks' solution into the interstitial spaces (Carnio & Varanda, 1995; Kawa, 1987) and collected in the superfusate. Thereafter, the cells where seeded onto glass coverslips, bathed with the same solution, and allowed to adhere. Leydig cells are easily identified under the microscope due to their size and presence of lipid droplets in their cytoplasm and they readily respond to stimulation by LH, as shown in Fig. 4A. Besides this, they were also identified in separate experiments by cytochemical detection of the enzyme 3β-hydroxysteroid dehydrogenase (Klinefelter et al. 1987).
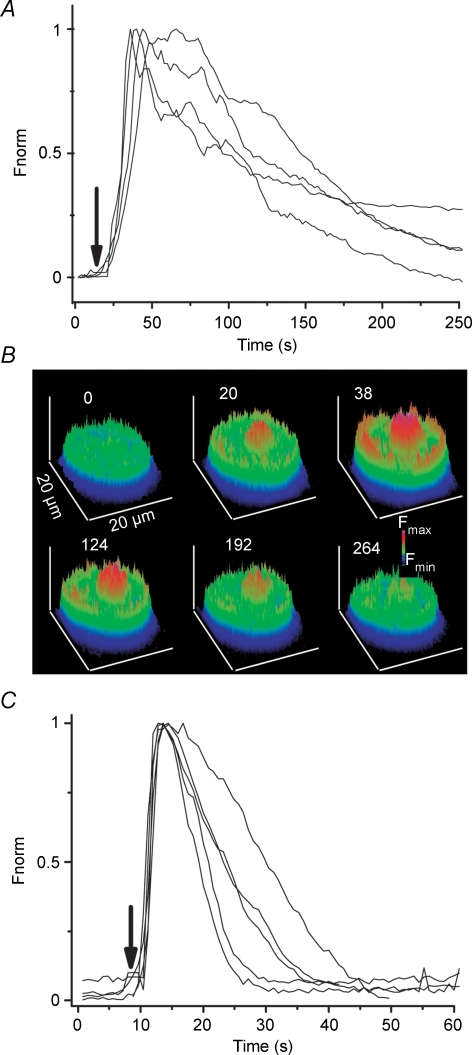
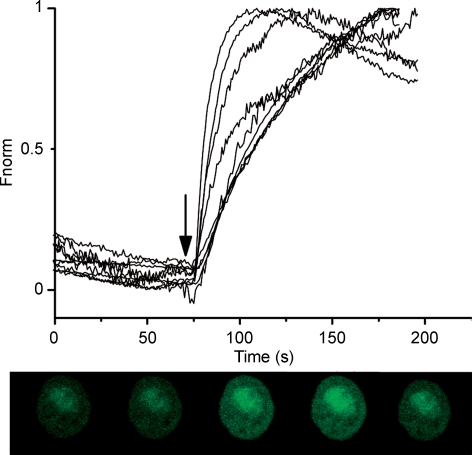
Figure 4. LH and cAMP increase intracellular calcium concentration.
A, time course of the fluorescence changes induced by addition (arrow) of 1 microg/ml LH to the solution superfusing 4 cells (lines represent different cells). Total number of cells analysed was 67 in 5 different preparations. Fluorescence increases sharply after treatment with LH and lasts for several minutes. Fluorescence was baseline subtracted and normalized to the maximum fluorescence measured in each cell (Fnorm). B, a series of surface plot pictures from a cell exposed to LH at the indicated times (number on top of each picture, in seconds). C, time course of fluorescence changes before and after application of 400 μm db-cAMP to 5 different cells, from a total of 20 analysed in at least 4 preparations.
Experiments were performed at room temperature (24 ± 1°C).
Electrophysiology
Coverslips with Leydig cells were transferred to a Lucite chamber mounted on the stage of an inverted microscope (CK2, Olympus, Center Valley, PA, USA) for patch clamp experiments. Whole-cell currents were measured with an Axopatch 200B amplifier (Molecular Devices Corp., Union City, CA, USA), as described by Hamill et al. (1981). Ca2+ currents were low pass filtered at 2 kHz (4-pole Bessel Filter), sampled at 5 kHz and fed to a microcomputer through a Digidata 1440A board (Molecular Devices Corp.) controlled by the software pCLAMP 10 (Molecular Devices Corp.). The software was also used to generate and apply the voltage protocols used throughout the paper. Patch pipettes with resistance ranging from 2 to 5 MΩ were pulled from borosilicate glass capillaries (BF150–86-10, Sutter Instrument Co., Novato, CA, USA) using a P-97 puller (Sutter Instrument Co.). Average series resistance and membrane capacitance for 47 cells were 16.3 ± 0.8 MΩ and 22.3 ± 0.7 pF. Series resistance was usually compensated by 30–60%.
The standard pipette solution contained (mm): 130 CsCl, 0.8 MgCl2, 5 EGTA, 10 Hepes, 2 CaCl2, 2 ATPMg. The free calcium concentration was 1.02 × 10−7 m (estimated with the program MaxChelator (Patton et al. 2004). pH was adjusted to 7.2 with CsOH and osmolality was 282–290 mosmol (kg H2O)−1 The external bath solution had the following composition (mm): 126 NaCl, 5 KCl, 10 CaCl2, 1 MgCl2, 6 sodium lactate, 10 Hepes. The pH was adjusted to 7.3 with NaOH and osmolality was 290–300 mosmol (kg H2O)−1. Solutions and drugs were delivered by gravity into the experimental chamber at a rate of 1 ml min−1.
Calcium currents were elicited by applying voltage steps between −80 mV and +50 mV from a holding potential of −80 mV, for 300 ms, every 2 s. I–V relationships were generated and the voltage dependency of activation evaluated by fitting a Boltzmann function to the experimental points:
Where I denotes the peak current obtained in response to each voltage pulse, Gmax the maximal slope conductance, Gmin the minimal conductance, V the clamped voltage, Vr the estimated reversal potential, V0.5 the voltage giving the half-point of the I–V curve and k a slope factor related to the voltage dependency of the system.
The steady-state inactivation was analysed using 2 s conditioning prepulses to various voltages (from −100 to −30 mV) followed by a test pulse to −10 mV with 100 ms duration. The experimental data were normalized to the peak current obtained after the −100 mV prepulse and plotted versus the respective conditioning prepulse potential. Data were analysed using a Boltzmann function similar to that described above.
Deactivation of the calcium currents was evaluated by analysing the tail currents upon repolarization to different membrane potentials ranging from −100 mV to −30 mV, for 50 ms, after a 15 ms conditioning pulse to −20 mV. The time course of the tail currents were fitted with a single exponential function and the time constants plotted against the repolarization potential.
Measurement of changes in intracellular Ca2+ concentration
Freshly isolated Leydig cells were seeded on poly l-lysine coated coverslips (22 mm in diameter, 0.17 mm thickness) and loaded with 5 μm Fluo-3 AM by incubation for 30–45 min at room temperature in Hanks' solution. Changes in [Ca2+]i were observed by fluorescence detection using an SPC 5 confocal microscope (Leica, Bensheim, Germany). Fluo-3 was excited with 488 nm light supplied by an argon laser and the emitted fluorescence was collected at wavelengths >505 nm. Cellular fluorescence was recorded in x–y confocal plane and image frames were obtained at a resolution of 512 × 512 or 1024 × 512 pixels every 300 ms. A 63× water immersion objective was used. The control and test solutions were carefully applied by using multiple barrels to a single output glass capillary (200 μm in diameter) manifold, placed as close as possible to the cells to be tested. Note that the flow regime is maintained throughout the experiment. The images were recorded on disk and analysed offline using the software ImageJ (W. S. Rasband/National Institutes of Health, Bethesda, MD, USA, http://rsb.info.nih.gov/ij/).
Temporal changes of the mean [Ca2+]i, such as those presented in Figs 4–6, were obtained directly from the fluorescence counts measured by the confocal microscope. For cell to cell comparison, the resulting counts were baseline subtracted and normalized with respect to the maximum fluorescence value. The actual fluorescence data were not filtered, nor smoothed or averaged. Surface plots shown in Fig. 6 were calculated from the raw image sequence by using the surface plot function from ImageJ. The pseudocolour scale was also chosen from within ImageJ.
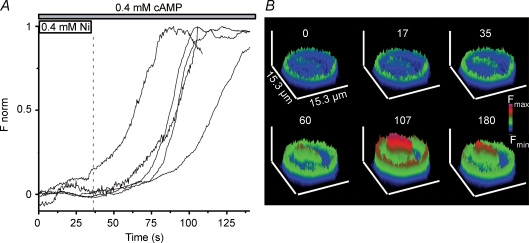
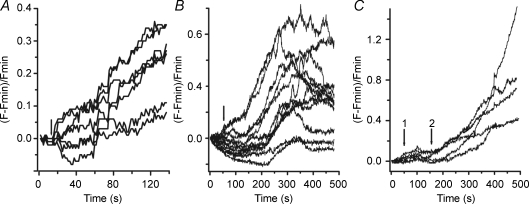
Figure 6. Influx of calcium is needed for the effect of cAMP.
A, blocking calcium channels with 400 μm Ni2+ impairs the effect of cAMP shown in Fig. 5. Ni2+ and cAMP were present in the superfusing solution from the beginning of the measurement up to 37 s. Fluorescence only increased after Ni2+ was washed out from the bathing solution (horizontal bars at the top). Different traces illustrate the behaviour of different cells (24 analysed cells). B, representative surface plots for a cell treated with Ni2+ and cAMP (upper row: 0, 17 and 35 s) and after removal of Ni2+ from the bathing solution (lower row: 60, 107 and 180 s).
Measurement of changes in membrane potential
Cells were prepared essentially as described for the intracellular calcium measurements, but loaded with a potentiometric dye by incubation with 5 μm DiBAC4(3). The dye was excited at 488 nm and the fluorescence read at 516 nm. According to the manufacturer's instructions (The Handbook – A Guide to Fluorescent Probes and Labeling Technologies, http://probes.invitrogen.com/handbook/sections/2203.html) a 1% increase in fluorescence should correspond approximately to a 1 mV depolarization in membrane potential. Images were treated as described in the previous paragraph.
Drugs and reagents
Fluo-3 AM and DiBAC4(3) were obtained from Invitrogen Corporation (Carlsbad, CA, USA). LH, db-cAMP and nifedipine and all other reagent grade salts were obtained from Sigma (St Louis, MO, USA).
Statistics
Data are expressed as means ± s.e.m. and n is the number of observations, as indicated under each result. Where pertinent the statistical test used to compare data is specified under the particular result.
Results
Calcium Currents
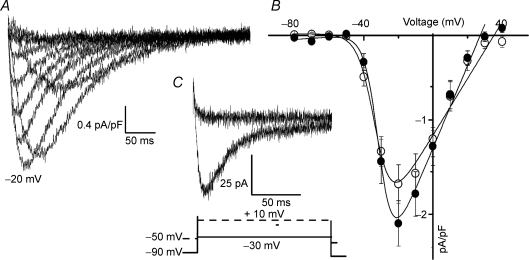
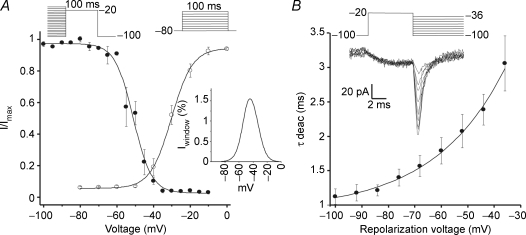
Whole-cell Ca2+ currents were evoked by 300 ms depolarizing voltage steps from −80 mV to +40 mV. From a total of 103 cells recorded, we could detect inward current in 59% of them. Typical recordings are shown in Fig. 1A. Currents began to activate at voltages positive to −60 mV and were fully activated at −20 mV. The current inactivated with time and displayed the typical criss-crossing signature observed for calcium currents carried by T-type calcium channels. Average peak currents at each voltage, normalized to the cell capacitance, are plotted in Fig. 1B against voltage. Although small in amplitude the currents are increased by approximately ∼27% after treating the cells with 400 μm db-cAMP (n = 13). Fitting of a Boltzmann function to the data points resulted in a k of 5.3 ± 0.5 mV in the control and 4.1 ± 0.5 in the presence of db-cAMP, and a V0.5 of −31.7 ± 1.5 mV that did not change with the db-cAMP.
Figure 1. Calcium currents in Leydig cells.
A, representative current traces elicited by depolarizing potentials from a holding potential of −80 mV to +40 mV in 10 mV steps. Note the typical criss-crossing of the traces. The trace at −20 mV is indicated. B, average I–V relationship for the peak currents obtained at each potential, normalized by the capacitance of the cell, in standard external solution (○) and in the presence of 400 μm db-cAMP (•). The continuous line is the fit of a Boltzmann function to the experimental points, giving V0.5 = −31.8 ± 1.4 mV, k = 5.5 ± 0.3 mV and Gmax = 2.0 ± 0.2 nS in control condition and V0.5 = −34.7 ± 1.8 mV, k = 4.1 ± 0.5 mV and Gmax = 2.6 ± 0.2 nS in the presence of cAMP (means ± s.e.m., n = 43). C, holding the cell at a more depolarized potential completely inactivates the current. We chose to depolarize from −90 mV to −30 mV and then from −50 mV to +10 mV because at +10 mV potential L-type currents can be activated (see voltage protocol under the current traces). All traces were obtained with 10 mm Ca2+ as charge carrier. The capacitive transients were suppressed from the traces for presentation purposes only.
The mean amplitude of the currents at the peak were −1.8 ± 0.2 pA pF−1 (n = 43) under control conditions and −2.2 ± 0.3 pA pF−1 (n = 14) after db-cAMP. Due to the large scattering in the amplitude of the currents, observed from cell to cell, these values were not statistically different (P = 0.3, Mann–Whitney U test). Figure 1C shows that inactivation is strongly voltage dependent. The current observed in response to a voltage pulse from −90 mV to −30 mV inactivated almost completely during the time of the pulse. Maintaining the cells at a holding potential of −50 mV resulted in a complete inactivation of the calcium channels, as shown by the absence of current response to a voltage pulse from −50 mV to +10 mV (n = 9). This last protocol indicates that high voltage activated (HVA) calcium currents are not present in Leydig cells. Besides this, 1 μm nifedipine (see online supplemental material, Supplemental Fig. 1A) has only a very weak effect, reinforcing the idea that HVA calcium channels make no important contribution to the observed currents.
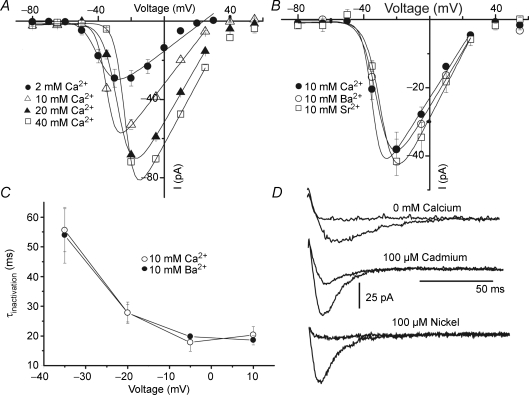
In order to show that our measurements refer to calcium currents, the extracellular calcium concentration was varied and I–V curves constructed for each condition. The results are shown in Fig. 2A. As can be seen, increasing the calcium concentration resulted in increases in the peak inward currents. The current–voltage relationship shifted to the right along the voltage axis when varying [Ca2+]e from 2 mm to 40 mm. This shift possibly reflects changes in surface potential due to surface charges in the plasma membrane (Ohmori & Yoshi, 1977). Substitution of Ca2+ for Ba2+ or Sr2+ as charge carriers did not appreciably change the I–V relationships, indicating that the channel did not discriminate between these divalent ions (Fig. 2B). The inactivation time constants are not dependent on the presence of calcium ions and have the same values when Ba2+ is the charge carrier, as shown in Fig. 2C, suggesting that the inactivation is a process dependent solely on the membrane voltage. Figure 2D shows that absence of [Ca2+]e, or addition of either of the Ca2+ channel blockers Cd2+ (100 μm) or Ni2+ (100 μm), led to suppression of the inward currents. Cd2+ at concentrations below 50 μm has no appreciable effects on the calcium currents and the KD for Ni2+, derived from the fitting of the Hill equation to a concentration–response relationship is around 2.6 μm (see Supplemental Fig. 1B and C). Substitution of external NaCl by equimolar LiCl (data not shown) did not produce any changes in the inward currents. Altogether these data strongly suggest that the observed inward currents are carried by T-type calcium channels.
Figure 2. Characteristics of the inward currents.
A, average I–V relationships for currents measured at the indicated calcium concentrations. Points are means ± s.e.m. (n = 7). The continuous line shows a fit of the Boltzmann function to the experimental points. Increasing the calcium concentration shifts V0.5 from −38.5 ± 0.8 for 2 mm Ca2+ to −37.0 ± 0.2 for 10 mm Ca2+, to −29.6 ± 1.3 for 20 mm Ca2+ and to −23.5 ± 0.7 mV for 40 mm Ca2+, without major changes in the voltage dependency of the currents. B, an average I–V plot for currents measured in the presence of 10 mm Ca2+ (V0.5 = −31.7 ± 1.6 mV, k = 3.7 ± 0.3 mV) or 10 mm Ba2+ (V0.5 = −29.9 ± 0.9 mV; k = 3.8 ± 0.3 mV) or 10 mm Sr2+ (V0.5 = −32.8 ± 1.1 mV; k = 3.4 ± 0.4 mV) (n = 6). C, the inactivation time constants, obtained by fitting a single exponential function to the decay phase of the currents obtained at different potentials, in the presence of 10 mm calcium or 10 mm Ba2+. Note that the constants are equal in both conditions, have the same voltage dependency and are independent of the presence of calcium. D, representative traces showing the absence of current when Ca2+ is withdrawn from the external solution, or is blocked by 100 μm Cd2+ and 100 μm Ni2+.
Voltage-dependent activation and inactivation
In order to assess the activation properties of the channels, the peaks of the inward currents were normalized in relation to the maximum current observed in each cell and plotted against voltage (Fig. 3A). The fitting of the Boltzmann function to the points resulted in V0.5 = −30.2 ± 0.5 mV and slope factor k = 4.9 ± 0.6 mV (n = 6). Currents start to be activated at voltages above −60 mV and are fully activated at −20 mV. Steady state inactivation was analysed by applying conditioning prepulses (−100 mV to −30 mV) followed by test pulses to −20 mV, as described in the legend to Fig. 3A. The data points are well described by a Boltzmann function with V0.5 = −51.1 ± 0.7 mV and k = 6.2 ± 0.4 mV (n = 7). The activation and inactivation relationships show a significant area overlap disclosing the presence of a putative window current. The inset in Fig. 3A shows the window current, computed by multiplying the fittings for the activation and inactivation curves expressed as a percentage of the activation current, against voltage. In this case, the calculated window current, was maximum at −37 mV, having amplitude of 1.8% of the peak current at this same voltage.
Figure 3. Electrophysiological properties of the T-type calcium currents in Leydig cells.
A, activation of the channels (○) was analysed by applying voltage pulses from −80 mV to 0 mV in 10 mV steps. Resulting peak currents measured at each voltage were normalized with respect to the maximum observed value (I/Imax). Continuous line represents the fit of a Boltzmann function to the experimental points giving V0.5 = −30.2 ± 0.5 mV and k = 4.9 ± 0.6 mV. Inactivation properties were analysed by applying prepulses from −100 mV to −10 mV in steps of 5 mV, for 2 s, and then to a test pulse of −20 mV. The normalized currents (I/Imax) are plotted against the pre-pulse voltage. A Boltzmann relation was fitted to these points (means ± s.e.m., n = 6) resulting in V0.5 = −51.1 ± 0.7 mV and k = 6.2 ± 0.4 mV. The inset shows the calculated relative window current (Iwin percentage) computed as the product of the activation and the inactivation fits. Calculations did not take into consideration leakage currents. B, deactivation properties of the calcium currents. Upper traces show the tail currents elicited by a short pre-pulse of 10 ms duration from −100 mV to −20 mV, and then observing their deactivation with pulses ranging from −100 mV to −10 mV in steps of 10 mV. Tail currents were fitted with a single exponential and the decay time constants were plotted against the pulse voltage (lower plot). Experimental points (means ± s.e.m.; n = 7) are well fitted by a single exponential showing a shallow voltage dependency as expected from T-type calcium channels. Calcium at 10 mm was used as charge carrier in all experiments.
Deactivation kinetics of the ICa
The voltage-dependent kinetics of channel closing was measured by applying a short depolarizing pulse, sufficient to drive most of the channels into the open configuration, and then by repolarizing to different voltages in order to observe the relaxation of the tail currents, as shown in Fig. 3B (upper panel). The deactivation time constants were estimated by fitting a single exponential to the decay phase of the currents. Figure 3B (lower panel) shows that deactivation is not a strongly voltage-dependent phenomenon, changing the time constants from 1.1 ms at −100 mV to 2.1 ms at −50 mV. The inward calcium current in Leydig cells deactivates relatively slowly when compared to other types of Ca2+ currents, again suggesting the presence of T-type calcium channels in these cells.
Fluorescence measurements of intracellular Ca2
The experiments below were designed in order to show that calcium entry through plasma membrane channels is responsible for the changes in [Ca2+]i induced by cAMP, a well known second messenger involved in the response of Leydig cells to LH. Treatment of the cells with LH (1 μg ml−1) readily increases [Ca2+]i, as shown in Fig. 4A. The effect is seen a few seconds after addition of LH to the superfusing solution and reaches a peak in 41.0 ± 2.5 s which is 2.5 ± 0.1 times (n = 50 cells) the baseline fluorescence. The fluorescence slowly decays with time. Electrophysiological experiments also reveal that application of LH to the cells leads to an increase in the calcium currents with a similar time course. Although variability from cell to cell is somewhat large, the calcium currents increase 25% in about 55–60 s (see Supplemental Fig. 2). Figure 4B shows that fluorescence increases in regions close to the plasma membrane and also in the nucleus of the cell. The effect of LH is clearly mimicked by the application of 400 μm db-cAMP (Fig. 4C). Fluorescence rises faster, time to peak equal 17 s (Fig. 4D), than with LH. The amplitude of the fluorescence at the peak is 2.9 ± 0.4 times (n = 20) the baseline fluorescence before application of db-cAMP. During the transient, [Ca2+]i increases at the inner side of the plasma membrane and inside the nucleus (see Figs 4 and 5). Interestingly, the increase in fluorescence lasts longer at the nucleus than at places close to the plasma membrane. During the course of our experiments, we noticed that this type of response was more frequent (62%) in Leydig cells isolated from 45- to 65-day-old mice than in Leydig cells isolated from younger (40- to 45-day-old) mice.
Figure 5.
External calcium is needed for the db-cAMP effect Fluorescence changes against time for several cells (different traces representative of 30 cells) before and after addition of 10 mm Ca2+ (arrow) to the solution superfusing the cells. db-cAMP at 400 μm was present in the superfusing solution all the time. Note that db-cAMP alone did not induce any rise in fluorescence. The fast increase occurs only after addition of calcium. Fnorm has the same meaning as described in Fig. 4.
In order to assess the involvement of the [Ca2+]e on the db-cAMP-induced [Ca2+]i we treated the cells with db-cAMP in the absence of [Ca2+]e and no increase in fluorescence was observed. Then, 10 mm calcium was added to the superfusion solution. This led to a sharp increase in the baseline fluorescence, reaching a maximum fluorescence value at 82 ± 4 s with an amplitude of 1.6 ± 0.2 times the baseline fluorescence (n = 30 cells) as shown in Fig. 5. The influx of Ca2+ through the plasma membrane was blocked by the addition of 400 μm Ni2+ to the bathing solution in the presence of 400 μm db-cAMP and 2 mm Ca2+ (Fig. 6A). The fluorescence increased 1.8 ± 0.1 times (n = 24 cells) from the baseline value only after washing out Ni2+ from the bathing solution. Figure 6B illustrates the fluorescence changes in a single cell exposed to the protocol described above.
Since T-type calcium channels respond to depolarization of the membrane, we also carried out experiments to measure [Ca2+]i in response to manoeuvres that change the resting potential of Leydig cells. This was done by measuring the membrane potential changes, induced by withdrawing K+ from the extracellular solution, with the fluorescent dye DiBAC4(3), and recording the concomitant changes in [Ca2+]i. Figure 7A shows that indeed the membrane potential strongly depolarized (by 20–30 mV) upon withdrawing K+ from the external solution, as expected from block of the electrogenic Na+/K+-ATPase. The same treatment also led to increases in [Ca2+]i, measured with Fluo3 (Fig. 7B), which were blocked by 200 μm Ni2+ (Fig. 7C).
Figure 7.
Depolarization of the cell membrane increases [Ca2+]i A, removal of K+ from the extracellular [K+]e solution (arrow) depolarizes the membrane potential in Leydig cells. The magnitude of the depolarization, estimated from the increase in fluorescence of diBAC4(3) is around 20–30 mV. The slow time course reflects the gradual block of the electrogenic Na+/K+ pump. Fluorescence points were collected at every 1.4 s. B, depolarization of the cell membrane, by removal of extracellular K+ (arrow) induces increase in [Ca2+]i. C, nickel blocks the effect of the depolarization on [Ca2+]i. Nickel (200 μm) was added to the superfusing solutions at the beginning of the experiment. Arrow 1 indicates the time when [K+]e was made equal to zero. Arrow 2 indicates the time when Ni2+ was washed out of the external solution maintaining [K+]e = 0. Fluorescence measurements were done every 0.8 s. Each trace in the figures represents measurements made on different cells.
Discussion
LH plays a major role in the hormonal regulation of testosterone synthesis and secretion. It has been shown by several authors that its action in Leydig cells is mainly dependent on the activation of a cAMP signalling pathway. In fact, cAMP has been taken as the sole or the most important intracellular mediator of the LH effect (Dufau et al. 1980). Nevertheless it has also long been known that extracellular Ca2+ is necessary for the LH induced synthesis of testosterone under certain conditions (Janszen et al. 1976; Moger, 1983; Sullivan & Cooke, 1986). The occurrence of Ca2+ influx through the plasma membrane of Leydig cells has been the subject of some controversy and it has been suggested that purinergic receptors may serve this function (Foresta et al. 1996; Poletto Chaves et al. 2006). To our knowledge the presence of specific calcium channels was reported only once for rat Leydig cells by Kawa (1987), but their characterization was far from complete. Other authors have even questioned their existence (Tomić et al. 1995; Desaphy et al. 1996; Rossato et al. 1997).
In this study we demonstrate the presence of calcium channels in mice Leydig cells by using measurements of both whole cell currents and fluorescence from calcium sensitive dyes in single isolated cells. Our results clearly demonstrate that Leydig cells do indeed present calcium currents as first suggested by Kawa (1987). Results presented in Figs 1–3 show that these currents resemble those carried by T-type channels. They had a low activation threshold, activated and inactivated rapidly, deactivated slowly and showed a high sensitivity to low concentrations of nickel ions, a known blocker of T-type calcium channels. Besides this, and in contrast to L-type Ca2+ channels, the inactivation process is not Ca2+ dependent, since changing the current carrying species for Ba2+ or Sr2+ does not significantly alter the rate of macroscopic inactivation (Fig. 2). Inward currents were very weakly sensitive to nifedipine (Supplemental Fig. 1A), ruling out their relationship with a typical HVA calcium channel. T-type channels are usually activated between −70 mV and −50 mV, with half-maximal activation from −75 mV to −55 mV and a slow deactivation time constant in the range 1–10 ms. The reversal potentials observed in our experiments (around +40 mV) are also evidence for a T-type calcium channel, since HVA channels reverse at potentials more positive than +60 mV (for a review see Talavera & Nilius, 2006).
Other types of voltage-dependent calcium channels have faster deactivation time constants and high activation voltages from −30 to 10 mV. Moreover, the activation and inactivation curves shown in Fig. 3 superimpose in a range of voltages, indicating the presence of a window current, which peaks at about −37 mV. Interestingly, the reported resting potential of Leydig cells is around −32 mV (Del Corsso & Varanda, 2003) indicating that a small fraction of channels is ready to open at this voltage. Therefore, our electrophysiological data are consistent with the classical properties shown by a low voltage activated calcium channel (T-type). These results are in agreement with the reported T-type currents observed in Sertoli cells of the testes (Lalevée et al. 1997), and other cell types like the glomerulosa from the adrenal gland and granulosa from the ovary, in which the activation of these channels is required for the synthesis of steroids (Rossier et al. 1996; Lesouhaitier et al. 2001; Lotshaw, 2001).
In certain instances, R-type HVA currents may resemble T-currents, especially if the subunit α1E is present. This is because they exhibit a few similar features, such as a negative threshold for activation and sensitivity to block by Ni2+ (Randall & Tsien, 1997). Nevertheless, in our case the currents do not deactivated as fast as R-type (8-fold faster), they require more negative potentials to be activated and they show a typical criss-cross pattern, absent in R-type channels (Carbone & Lux, 1984). Moreover, they are highly sensitive to block by Ni2+, with a Ki around 2.6 μm (Supplemental Fig. 1C). On the other side, significant block by Cd2+ requires concentrations above 50 μm (Supplemental Fig. 1B) Finally the overlap between the inactivation and activation curves is larger than the reported for R-type channels (Randall & Tsien, 1997).
Another feature of the inward currents is their up-regulation by cAMP, which causes an increase in both maximal current observed at −20 mV and the maximal conductance (see Fig. 1). LH also increases the calcium current (Supplemental Fig. 2). Although highly variable from cell to cell the maximum increase in current is around 25% and a steady level is reached after about 60 s. This time course is consistent with the effect of both LH and cAMP shown in Fig. 4. This effect was indirectly predicted in the work of Carnio & Varanda (1995) by showing that the effect of cAMP on the activity of BKCa channels was dependent on the presence of calcium in the extracellular solution.
The increase in amplitude of T-type calcium currents by cAMP was also observed in freshly isolated glomerulosa cells (Lenglet et al. 2002). In cultured cells from the zona fasciculata of the rat adrenal gland, Barbara & Takeda (1995) also found an increased expression of T-type currents upon incubation of the cells for 3 days with ACTH or VIP. Nevertheless, Durroux et al. (1991) reports inhibition of T-type currents by ACTH, which partially recovers with time, and Drolet et al. (1997) report inhibition of T-type calcium currents by dopamine in rat cultured glomerulosa cells. Possibly the discrepancies between these results are due to the different experimental conditions used (fresh vs. cultured cells). In any case recent literature shows that cAMP can either inhibit or stimulate T-type channels depending on cells type and specific stimulus (for a review see Chemin et al. 2006). The physiological relevance of the observed currents and their possible involvement in steroidogenesis is unknown. As a first step in this direction we decided to use both LH and db-cAMP, and monitor the intracellular calcium concentration by using confocal imaging techniques. The addition of LH and db-cAMP in the presence of 2 mm Ca2+ triggered a highly heterogeneous spatial and temporal Ca2+ response in a given cell. This may be due to the presence of scattered lipid droplets in the cytoplasm, which exclude the fluorescent dye. The action of cAMP is, however, only evident when calcium ions are present in the extracellular solution. Removal of calcium from the extracellular medium impairs the action of cAMP, suggesting that influx of calcium through the plasma membrane is a necessary requirement for the subsequent increase in cytoplasmic calcium levels. This is also supported by the fact that the response to cAMP has no appreciable delay, and that addition of Ni2+ to the bath, a known calcium channel blocker, or removal of Ca2+ from the external solution abolished the response to cAMP. Subsequent addition of extracellular Ca2+ to the incubation medium, or removal of Ni2+, restored db-cAMP stimulated [Ca2+]i increase (Figs 5 and 6). Since T-type calcium channels are voltage dependent we also tested whether depolarization of the plasma membrane could induce similar results, as shown for other cell types. In our case the membrane potential was changed by withdrawing K+ from the extracellular solution. We chose this manoeuvre, in contrast to increasing K+, because the resting potential of Leydig cells is largely determined by an electrogenic Na+/K+ pump (Del Corsso & Varanda, 2003). The results shown in Fig. 7 clearly demonstrate that depolarization also leads to [Ca2+]i changes, similar to those observed in the other experiments reported here. As a matter of fact, Ni2+ also blocks the changes in [Ca2+]i. These results suggest that calcium influx is occurring via calcium channels present in the membrane of Leydig cells and that they play a fundamental role in the cAMP induced intracellular changes in calcium concentrations that follows. Similar results were seen in Sertoli cells where testosterone and FSH increased cytosolic Ca2+ by inducing influx through the plasma membrane (Grasso & Reichert, 1989; Gorczynska et al. 1994). More recently, the Ca2+ influx via T-type channels has been shown to be necessary for the hCG-dependent progesterone synthesis via a G protein coupled receptor in granulosa cells of the ovary (Agoston et al. 2004). In glomerulosa cells of the adrenal gland, the maximal aldosterone secretion in response to stimulus by 8-bromo-cAMP is only achieved in the presence of external calcium, and T-type calcium channels are clearly involved (Uebele et al. 2004). Whatever the mechanism, the increase in intracellular Ca2+, either released from intracellular stores or induced by entry from the extracellular compartment, is known to play an important role in steroidogenesis (Cooke, 1999).
To our knowledge, the present work is the first to show direct evidence that both LH and cAMP induce increases in [Ca2+]i in individual Leydig cells with clear spatial and fast temporal resolutions. Our measurements show calcium transients in a time scale of seconds, much faster than those described in previous studies (Kumar et al. 1994; Tomić et al. 1995). Interestingly, the addition of LH or db-cAMP in the presence of extracelular Ca2+ produces a substantial and fast Ca2+ increase in the nucleus suggesting some sort of interconnection between the plasma membrane and the organelle events. Although, the magnitude of the Ca2+ signal appears to be larger in the nucleus, care has to be taken because Fluo-3 may accumulate in this compartment and its properties, such as affinity for Ca2+, may differ from in the cytoplasm (Perez-Terzic et al. 1997). The increase in the nuclear Ca2+ concentration may control specific nuclear processes related to steroidogenesis such as gene transcription and protein transport.
In conclusion, using the whole-cell configuration of the patch-clamp technique, a low voltage-gated calcium channel with properties of a T-type current has been identified in the plasma membrane of Leydig cells. Confocal microscopy experiments reveal that LH/cAMP induce an increase in [Ca2+]i, which is dependent on the influx of Ca2+ from the extracellular solution. The latter seems to occur through the voltage-gated channels described here.
Acknowledgments
This research was supported by a grant from Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP – 06/50954-7) to WAV. The confocal microscope facility is maintained by a grant from FAPESP (04/08868-0). R.R.C. was the recipient of a fellowship from CNPq (no. 151886/2006-7). We would like to thank Dr Raul Martinez-Zaguilan and Dr Alan Marty for critical reading of the manuscript and Mr José Fernando Aguiar for technical assistance.
Supplementary material
Online supplemental material for this paper can be accessed at:
http://jp.physoc.org/cgi/content/full/jphysiol.2007.137950/DC1 and http://www.blackwell-synergy.com/doi/suppl/10.1113/jphysiol.2007.137950
References
- Agoston A, Kunz L, Krieger A, Mayerhofer A. Two types of calcium channels in human ovarian endocrine cells: Involvement in steroidogenesis. J Clin Endocrinol Metab. 2004;89:4503–4512. doi: 10.1210/jc.2003-032219. [DOI] [PubMed] [Google Scholar]
- Barbara JG, Takeda K. Voltage-dependent currents and modulation of calcium channel expression in zona fasciculata cells from rat adrenal gland. J Physiol. 1995;488:609–622. doi: 10.1113/jphysiol.1995.sp020994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone E, Lux HD. A low voltage-activated calcium conductance in embryonic chick sensory neurons. Biophys J. 1984;46:413–418. doi: 10.1016/S0006-3495(84)84037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnio EC, Varanda WA. Calcium-activated potassium channels are involved in the response of mouse Leydig cells to human chorionic gonadotropin. Braz J Med Biol Res. 1995;28:813–824. [PubMed] [Google Scholar]
- Chemin J, Traboulsie A, Lory P. Molecular pathways underlying the modulation of T-type calcium channels by neurotransmitters and hormones. Cell Calcium. 2006;40:121–134. doi: 10.1016/j.ceca.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Cooke BA. Signal transduction involving cyclic AMP-dependent and cyclic AMP-independent mechanisms in the control of steroidogenesis. Mol Cell Endocrinol. 1999;151:25–35. doi: 10.1016/s0303-7207(98)00255-x. [DOI] [PubMed] [Google Scholar]
- Del Corsso C, Varanda WA. The resting potential of mouse Leydig cells: Role of an electrogenic Na+/K+ pump. J Membr Biol. 2003;191:123–131. doi: 10.1007/s00232-002-1048-y. [DOI] [PubMed] [Google Scholar]
- Desaphy JF, Christian R, Joffre M. Modulation of K+ conductances by Ca2+ and human chorionic gonadotrophin in Leydig cells from mature rat testis. J Physiol. 1996;495:23–35. doi: 10.1113/jphysiol.1996.sp021571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drolet P, Bilodeau L, Chorvatova A, Laflamme L, Gallo-Payet N, Payet MD. Inhibition of the T-type Ca2+ current by the dopamine D1 receptor in rat adrenal glomerulosa cells: Requirement of the combined action of the Gβγ protein subunit and cyclic adenosine 3′,5′-monophosphate. Mol Endocrinol. 1997;11:503–514. doi: 10.1210/mend.11.4.9910. [DOI] [PubMed] [Google Scholar]
- Dufau ML, Baukal AJ, Catt KJ. Hormone-induced guanyl nucleotide binding and activation of adenylate cyclase in the Leydig cell. Proc Natl Acad Sci U S A. 1980;77:5837–5841. doi: 10.1073/pnas.77.10.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durroux T, Gallo-Payet N, Payet MD. Effects of adrenocorticotropin on action potential and calcium currents in cultured rat and bovine glomerulosa cells. Endocrinol. 1991;129:2139–2147. doi: 10.1210/endo-129-4-2139. [DOI] [PubMed] [Google Scholar]
- Foresta C, Rossato M, Nogara A, Gottaderlo F, Bordon P, Di Virgilio F. Role of P2 purinergic receptors in rat Leydig cell steroidogenesis. Biochem J. 1996;320:499–504. doi: 10.1042/bj3200499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorczynska E, Spaliviero J, Handelsman DJ. The relationship between 3′,5′ cyclic adenosine monophosphate and calcium in mediating follicle-stimulating hormone signal transduction in Sertoli cells. Endocrinology. 1994;134:293–300. doi: 10.1210/endo.134.1.8275946. [DOI] [PubMed] [Google Scholar]
- Grasso P, Reichert LE. Follicle-stimulating hormone receptor mediated uptake of Ca2+ by proteolisomes and cultured rat Sertoli cells; evidence for involvement of voltage-activated and voltage-independent calcium channels. Endocrinology. 1989;125:3029–3036. doi: 10.1210/endo-125-6-3029. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Janszen FHA, Cooke BA, van Driel MJA, van der Molen HJ. The effect of calcium ions on testosterone production in Leydig cells from rat testis. Biochem J. 1976;160:433–437. doi: 10.1042/bj1600433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawa K. Existence of calcium channels and intercellular couplings in the testosterone-secreting cells of the mouse. J Physiol. 1987;393:647–666. doi: 10.1113/jphysiol.1987.sp016846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinefelter GR, Hall PF, Ewing LL. Effect of luteinizing hormone deprivation in situ on steroidogenesis of rat Leydig cells purified by a multistep procedure. Biol Reprod. 1987;36:769–783. doi: 10.1095/biolreprod36.3.769. [DOI] [PubMed] [Google Scholar]
- Kumar S, Blumberg DL, Canas JA, Maddaiah VT. Human chorionic gonadotropin (hCG) increases cytololic free calcium in adult rat Leydig cells. Cell Calcium. 1994;15:349–355. doi: 10.1016/0143-4160(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Lalevée N, Pluciennik F, Jofre M. Voltage-dependent calcium current with properties of T-type current in Sertoli cells from immature rat testis in primary cultures. Biol Reprod. 1997;56:680–687. doi: 10.1095/biolreprod56.3.680. [DOI] [PubMed] [Google Scholar]
- Lenglet S, Louiset E, Delarue C, Vaudry H, Contesse V. Activation of 5-HT7 receptor in rat glomerulosa cells is associated with an increase in adenylyl cyclase activity and calcium influx through T-type calcium channels. Endocrinology. 2002;143:1748–1760. doi: 10.1210/endo.143.5.8817. [DOI] [PubMed] [Google Scholar]
- Lesouhaitier O, Chiappe A, Rossier MF. Aldosterone increases T-type calcium currents in human adenocarcinoma (H295R) cells by inducing channel expression. Endocrinology. 2001;142:4320–4330. doi: 10.1210/endo.142.10.8435. [DOI] [PubMed] [Google Scholar]
- Lotshaw DP. Role of membrane depolarization and T-type channels in angiotensin II and K+ stimulated aldosterone secretion. Mol Cell Endocrinol. 2001;175:157–171. doi: 10.1016/s0303-7207(01)00384-7. [DOI] [PubMed] [Google Scholar]
- Mendelson C, Dufau M, Catt K. Gonadotropin binding and stimulation of cyclic adenosine 3′:5′-monophosphate and testosterone production in isolated Leydig cells. J Biol Chem. 1975;250:8818–8823. [PubMed] [Google Scholar]
- Moger WH. Effects of the calcium-channel blockers cobalt, verapamil, and D600 on Leydig cell steroidogenesis. Biol Reprod. 1983;28:528–535. doi: 10.1095/biolreprod28.3.528. [DOI] [PubMed] [Google Scholar]
- Ohmori H, Yoshii M. Surface potential reflected in both gating and permeation mechanisms of sodium and calcium channels of the tunicate egg cell membrane. J Physiol. 1977;267:429–463. doi: 10.1113/jphysiol.1977.sp011821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton C, Thompson S, Epel D. Some precautions in using chelators to buffer metals in biological solutions. Cell Calcium. 2004;35:427–431. doi: 10.1016/j.ceca.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Perez-Terzic C, Stehno-Bittel L, Clapham DE. Nucleoplasmic and cytoplasmic differences in the fluorescence properties of the calcium indicator Fluo-3. Cell Calcium. 1997;21:275–282. doi: 10.1016/s0143-4160(97)90115-9. [DOI] [PubMed] [Google Scholar]
- Pitter JG, Maechler P, Wollheim CB, Spät A. Mitochondria respond to Ca2+ already in the submicromolar range: correlation with redox state. Cell Calcium. 2002;31:97–104. doi: 10.1054/ceca.2001.0264. [DOI] [PubMed] [Google Scholar]
- Poletto Chaves LA, Pontelli EP, Varanda WA. P2X receptors in mouse Leydig cells. Am J Physiol Cell Physiol. 2006;290:C1009–C1017. doi: 10.1152/ajpcell.00506.2005. [DOI] [PubMed] [Google Scholar]
- Randall A, Tsien RW. Contrasting biophysical and pharmacological properties of T-type and R-type calcium channels. Neuropharmacology. 1997;36:879–893. doi: 10.1016/s0028-3908(97)00086-5. [DOI] [PubMed] [Google Scholar]
- Rossato M, Nogara A, Gottardello F, Bordon P, Foresta C. Pituitary adenylate cyclase activating polypeptide stimulates rat Leydig cell steroidogenesis through a novel transduction pathway. Endocrinology. 1997;38:3228–3235. doi: 10.1210/endo.138.8.5314. [DOI] [PubMed] [Google Scholar]
- Rossier MF, Burnay MM, Valloton MB, Capponi AM. Distinct functions of T-type and L-type calcium channels during activation of bovine adrenal glomerulosa cells. Endocrinology. 1996;137:4817–4826. doi: 10.1210/endo.137.11.8895352. [DOI] [PubMed] [Google Scholar]
- Spät A, Pitter JG. The effect of cytoplasmic Ca2+ signal redox state of mitochondrial pyridine nucleotides. Mol Cell Endocrinol. 2004;215:115–118. doi: 10.1016/j.mce.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Spät A, Pitter JG, Rohás T, Szabadkai G. Stimulus-secretion coupling and mitochondrial metabolism in steroid-secreting cells. News Physiol Sci. 2001;16:197–200. doi: 10.1152/physiologyonline.2001.16.5.197. [DOI] [PubMed] [Google Scholar]
- Sullivan MH, Cooke BA. The role of Ca2+ in steroidogenesis in Leydig cells. Stimulation of intracellular free Ca2+ by lutoprin (LH), luliberin (LHRH) agonist and cyclic AMP. Biochem J. 1986;236:45–51. doi: 10.1042/bj2360045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talavera K, Nilius B. Biophysics and structure-function relationship of T-type Ca2+ channels. Cell Calcium. 2006;40:97–114. doi: 10.1016/j.ceca.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Tomić M, Dufau ML, Catt KJ, Stanko SS. Calcium signaling in single rat Leydig cells. Endocrinology. 1995;136:3422–3429. doi: 10.1210/endo.136.8.7628378. [DOI] [PubMed] [Google Scholar]
- Uebele VN, Nuss CE, Renger JJ, Connolly TM. Role of voltage-gated calcium channels in potassium-stimulated aldosterone secretion from rat adrenal zona glomerulosa cells. J Steroid Biochem Mol Biol. 2004;92:209–218. doi: 10.1016/j.jsbmb.2004.04.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.