Abstract
Olfactory marker protein (OMP), a phylogenetically conserved protein, is highly, and almost exclusively, expressed in vertebrate olfactory receptor neurons (ORNs). Although OMP is widely used as a marker for ORNs, its function has remained largely elusive. Here we used suction-pipette recordings from isolated ORNs of OMP−/− mice to investigate its role in olfactory transduction. Vertebrate olfactory transduction is initiated when odourants bind to receptor proteins to activate an adenylyl cyclase via a G protein-coupled signalling pathway. This leads to an increase in cAMP and the opening of a cyclic nucleotide-gated (CNG), non-selective cation channel which depolarizes the cells. Ca2+ influx through the CNG channel in turn activates a Ca2+-activated Cl− channel, causing a Cl− efflux and further depolarization. In the absence of OMP, the time-to-transient-peak of the response, the latency to first spike, and the response termination were slowed 2- to 8-fold, indicating its role in regulating olfactory response kinetics and termination. This phenotype persisted in OMP−/− ORNs even in low external Ca2+ solution chosen to prevent Cl− channel activation, suggesting OMP acts upstream of Cl− channel activation. Furthermore, the response kinetics in cilia are virtually indistinguishable between OMP−/− and wild-type ORNs when intracellular cAMP level was elevated by the phospho-diesterase inhibitor, IBMX, suggesting OMP acts upstream of cAMP production. Together, our results suggest a role for OMP in regulating the kinetics and termination of olfactory responses, implicating a novel mechanism for fast and robust response termination to ensure the temporal resolution of the odour stimulus. These observations also help explain the deficits in odour detection threshold and odour quality discrimination seen in the OMP−/− mice.
Olfactory marker protein (OMP) is an abundant, small, cytoplasmic protein (Margolis, 1972) the expression of which is highly restricted to mature chemosensory neurons in the main olfactory epithelium, the vomeronasal organ, the septal organ and the Grueneberg ganglion (Johnson et al. 1993; Matsuoka et al. 2002; Ma et al. 2003; Fuss et al. 2005; Breer et al. 2006; Fleischer et al. 2006). This protein is derived from a single copy, intronless gene that gives an amino acid sequence > 50% identical across all vertebrate species (Keller & Margolis, 1975; Buiakova et al. 1994; Rössler et al. 1998; Ferrando et al. 2006). There is no evidence of an OMP orthologue in invertebrates, indicating that OMP was a vertebrate innovation. Its selective expression in chemosensory neurons and its phylogenetic conservation suggest that OMP serves a common function in vertebrate chemosensory transduction.
Homozygous OMP knockout (OMP−/−) mice exhibit much slower onset and decay kinetics of odour-induced electrical responses, based on electro-olfactographic (EOG) recordings (Buiakova et al. 1996). The successful rescue of this EOG deficit by the expression of OMP via adenoviral delivery of cDNA to olfactory receptor neurons (ORNs) of adult OMP−/− mice in vivo has confirmed that the deficit is indeed due to an acute absence of OMP rather than a developmental role of this protein (Ivic et al. 2000). The OMP−/− mice also have deficits in their ability to detect and discriminate odours, likewise reversible by adenoviral delivery of OMP cDNA (Youngentob & Margolis, 1999; Youngentob et al. 2001, 2003, 2004). In addition, OMP−/− mice exhibit altered innervation of the olfactory bulb (St John & Key, 2005) and OMP is reported to enhance mitosis of ORN precursors (Farbman et al. 1998). Despite these diverse phenotypic indications of OMP function in the olfactory system, the target of OMP in olfactory transduction remains unknown.
Olfactory transduction begins with the binding of an odourant molecule to a receptor protein on the ciliary membrane of ORNs. This binding triggers the exchange of GDP by GTP on the α-subunit of the olfactory G protein, Golf, and activates an adenylyl cyclase (type III), leading to elevated intraciliary cAMP (for review see Schild & Restrepo, 1998; Matthews & Reisert, 2003). The consequent opening of the olfactory cyclic nucleotide-gated (CNG) channel lets Ca2+ and Na+ into the cilia (Zufall & Firestein, 1993; Dzeja et al. 1999), depolarizing the cell. This depolarization is amplified by a substantial excitatory Ca2+-activated Cl− current at the ciliary membrane (Kleene & Gesteland, 1991; Kurahashi & Yau, 1993; Lowe & Gold, 1993; Reisert et al. 2005), owing to a high intracellular Cl− concentration maintained mostly by constitutive Cl− uptake through a Na+–K+–Cl− cotransporter (Kaneko et al. 2004; Nickell et al. 2006; Reisert et al. 2005). The Ca2+ influx through the CNG channels also leads to olfactory adaptation. One mechanism of Ca2+-mediated adaptation involves, through Ca2+–calmodulin, a reduction in the CNG channel's sensitivity to cAMP (Chen & Yau, 1994; Kurahashi & Menini, 1997; Bradley et al. 2004). Another adaptation mechanism involves an inhibition, via Ca2+–calmodulin and calmodulin-dependent protein kinase II, of the adenylyl cyclase (Wei et al. 1998; Leinders-Zufall et al. 1999). The termination of the ORN response requires all components of the signal transduction cascade to be inactivated. This begins with the separation of the odourant from the receptor, which occurs quickly (Bhandawat et al. 2005). The GTP bound to Gα,olf is hydrolysed to GDP, deactivating Gα,olf and allowing the adenylyl cyclase to return to its basal activity. The generated cAMP is hydrolysed by a phosphodiesterase (PDE) (Borisy et al. 1992; Yan et al. 1995). Finally, the excess intracellular Ca2+ is removed from the ciliary space via Na+–Ca2+ exchange (Reisert & Matthews, 1998) or a Ca2+-ATPase (Weeraratne et al. 2006) in order for the Ca2+-activated Cl− channel to close.
The action of OMP can be at any of the above steps in olfactory transduction. The experiments described here were aimed at identifying the target of OMP.
Methods
Tissue preparation
Female OMP−/− (Buiakova et al. 1996) and control mice (129SvEvTac) aged 2–7 months were killed using CO2 followed by decapitation, as approved by Johns Hopkins University School of Medicine and conforming with NIH guidelines. Olfactory turbinates were removed from the nasal cavity and stored in oxygenated Ringer solution at 4°C until use. A small piece of olfactory epithelium was freed from the underlying cartilage, placed in an Eppendorf tube containing 200 μl of Ringer solution and briefly vortexed (Reisert & Matthews, 2001a). The resulting cell suspension containing isolated ORNs was transferred to a recording chamber on an inverted microscope equipped with phase-contrast optics, and allowed to settle for 30 min before bath perfusion commenced.
Electrophysiological recordings
The suction-pipette technique was used to record from isolated ORNs (Baylor et al. 1979; Lowe & Gold, 1991; Reisert & Matthews, 2001a). The cell body of an isolated ORN was drawn into the tip of the recording pipette, leaving the cilia exposed to the bath solution. In this recording configuration, the recorded current represented the transduction current which entered at the cilia and exited at the cell body. Also, since the intracellular voltage was free to vary, the ORNs generated action potentials, which also were recorded as biphasic, fast current transients. Once an ORN was successfully sucked into the tip of the recording pipette, it was tested for odourant and IBMX responsiveness. The odour-induced receptor current was recorded with an Axopatch-1D patch clamp amplifier, digitized using a Digidata 1322A interface and pCLAMP software (Axon Instruments). All currents were sampled at 10 kHz. The slow receptor current was recorded with a bandwidth of DC to 50 Hz (−3dB, 8-pole Bessel filter), and action potentials were recorded with the wider bandwidth of DC to 5000 Hz (−3dB, 8-pole Bessel filter). Data were analysed with Origin software.
Solutions and solution changes
Mammalian Ringer solution contained (mm): 140 NaCl, 5 KCl, 1 MgCl2, 2 CaCl2, 0.01 EDTA, 10 Hepes, and 10 glucose. Low Ca2+ solution contained 2 mm nitriloacetic acid (NTA) (and no EDTA) and 0.115 mm CaCl2 to give a free Ca+ concentration of 20 μm. To keep the total concentration of free Ca2+ and Mg2+ at the same level as Ringer solution, 4.48 mm MgCl2 was added to give a free Mg2+ concentration of 3 mm in the low-Ca2+ Ringer solution. The free Ca2+ and Mg2+ concentrations were calculated using MaxChelator Winmaxc 2 (C. Patton, Stanford University, Palo Alto, CA, USA). The pH was adjusted to 7.5 with NaOH. Cineole was used as the odourant at 100 μm unless noted otherwise. When added, IBMX was at 1 mm. Forskolin was also tried at a concentration of 10 μm but was found to be inadequate for our intended experiments. Responses to forskolin, be it due to slow activation of the adenylyl cyclase or slow membrane permeability, occurred with a long (∼1 s) delay and only decayed over several seconds after forskolin removal.
Solutions were changed by transferring the tip of the recording pipette holding the cell across the interface of neighbouring streams of solutions using the Perfusion Fast-Step solution changer (Warner Instrument Corporation). When the stimulus duration was 1 s or longer, the flow rate was such that the solution change was complete within 15 ms. For shorter stimulus durations, the flow rate was increased to achieve solution change in < 5 ms, assessed from the junction current arising when stepping into a solution of different ionic composition. A delay of 40 ms occurred between the digital command and the actual solution change, and has been subtracted from all processed timing data in Fig. 2. Experiments were performed at 37°C. The solutions were heated just prior to entering the solution changer by a heater according to Matthews (1999). In low-Ca2+ experiments, ORNs were exposed to the low-Ca2+ Ringer solution for 3 s prior to, during, and for 4 s after, the odour stimulation.
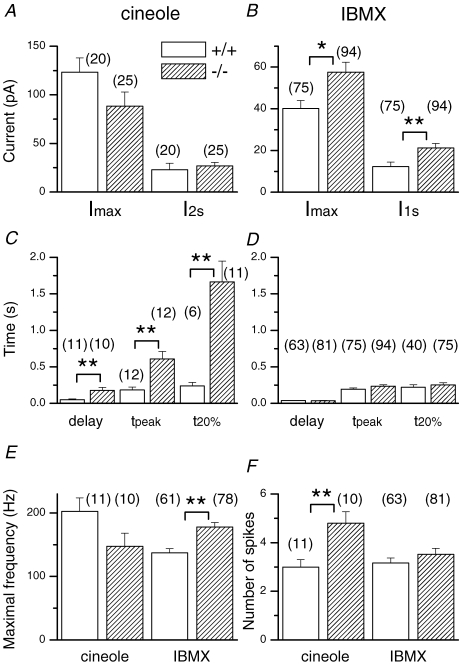
Figure 2. Averaged data for OMP+/+ and OMP−/− ORNs.
A, maximal current (Imax) and current at the end of the 2 s cineole stimulation (I2s). B, IBMX-induced peak current and current at the end of the 1 s IBMX (I1s) exposure. Kinetic parameters of the cineole-induced (C) and IBMX-triggered response (D). The response delay was taken as the time of the first spike to be generated and t20% represents the time for the response to fall to 20% of its value at the end of stimulation (2 s and 1 s for cineole and IBMX, respectively). The maximal firing frequency and number of spikes elicited by cineole or IBMX are shown in E and F, respectively. Symbols indicate values that are significantly different at the 0.05 (*) and 0.005 (**) level (Student's t test). Numbers in parentheses indicate the numbers of ORNs used in the analysis. Error bars represent s.e.m. Recordings included in this analysis were typically the first or second recording obtained from an ORN after it was sucked into the tip of the recording pipette to avoid possible stimulation-induced rundown. The cineole and IBMX concentrations were always 100 μm and 1 mm, respectively.
Results
Altered response kinetics in ORNs lacking OMP
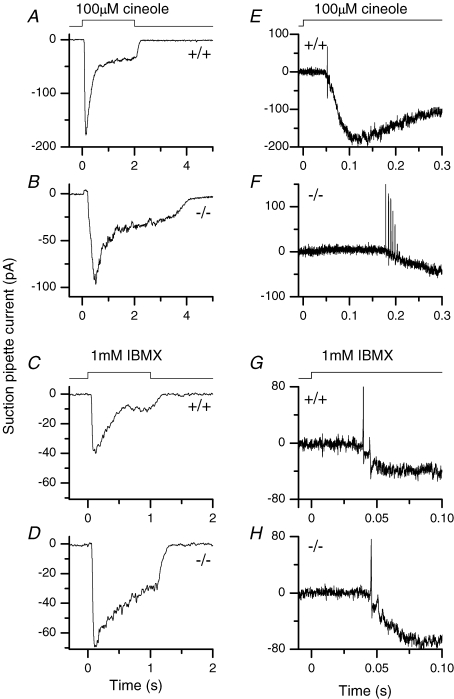
When stimulated by a 2 s pulse of the odourant cineole, a wild-type (WT) ORN gave a response that rose to a transient peak quickly before declining to a lower level, and rapidly terminated upon cineole removal (Fig. 1A). In some cells, the response also showed oscillations during the 2 s stimulus (Reisert & Matthews, 2001a; see Fig. 4). In contrast, the response of an OMP−/− ORN to the same stimulus rose more slowly and also declined from its transient peak more slowly. In some cells, the response did not even reach its peak during a 1 s stimulation, which explained why we chose a stimulus duration of 2 s. Most significantly, the response persisted for a substantial period upon stimulus removal. On average, the time for the response to fall to 20% (t20%) of its value at the end of stimulation increased 7-fold, from 0.23 to 1.7 s (Fig. 2C). This prolongation was apparently not due to a higher sensitivity of OMP−/− ORNs making the response activation over-ride the response termination because, from behavioural experiments, OMP−/− ORNs are much less sensitive than WT ORNs (Youngentob & Margolis, 1999; see also Fig. 2A). Oscillatory responses were never observed during a 2 s stimulation of OMP−/− ORNs. In Fig. 1E and F, the same traces as in Fig. 1A and B were filtered at a wider bandwidth and expanded on the time scale to reveal action potentials. WT ORNs typically gave a short burst of action potentials, consisting of 1–3 spikes during the early rising phase of the response (Figs 1E and 2F). OMP−/− ORNs, on the other hand, typically gave a longer spike train with approximately twice as many spikes (Figs 1F and 2F). The maximal spike frequency was lower in OMP−/− ORNs compared to wild-type ORNs, but this difference did not reach statistical significance (Fig. 2E). The latency (i.e. time delay) to the first spike was prolonged nearly 4-fold (Figs 1E and F, and 2C).
Figure 1. Suction pipette recordings of mouse olfactory receptor neurons.
Fast wild-type (A) and slow OMP−/− ORN (B) responses were recorded in response to a 2 s 100 μm cineole stimulation. C and D, suction pipette currents elicited by the phosphodiesterase inhibitor IBMX (1 mm for 1 s) in an OMP-positive and -negative ORN. All traces are filtered DC to 50 Hz to display the receptor current alone. E–F, the same recordings as in A–D at an expanded time scale, but filtered at DC to 5000 Hz to investigate action potential firing. The solution monitor at the top indicates the timing of the command pulse which initiated the solution change; the actual solution change occurred with a slight delay (see Methods).
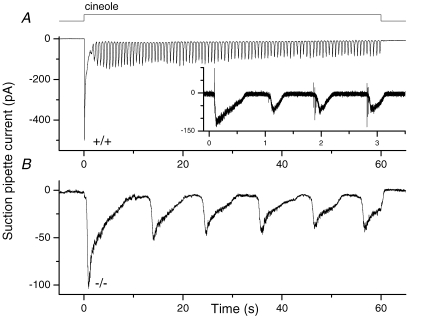
Figure 4. The oscillatory response during prolonged stimulation.
A, a wild-type ORN was exposed to 300 μm cineole for 60 s and exhibited a fast oscillatory response pattern with a mean oscillatory period of 0.64 s. The inset shows a recording from a different OMP-positive ORN, which was filtered DC to 5000 Hz. Action potentials are now visible at the rising phase of each repetitive oscillation. B, an OMP−/− ORN shows drastically slowed oscillations when exposed to cineole (100 μm) with the mean oscillation period being 10.6 s.
We have also used 3-isobutyl-1-methylxanthine (IBMX), a phosphodiesterase inhibitor, to elicit responses. In this case, the response came from a rise in cAMP concentration due to constitutive adenylyl cyclase activity not balanced by cAMP hydrolysis due to endogenous phosphodiesterase activity. Thus, the odourant receptor and the G protein were bypassed. Importantly, the responses of WT and OMP−/− ORNs to a 1 s exposure to IBMX were quite similar in time course, showing a comparable time to peak, latency to first spike and, in particular, t20% (Figs 1C and D, and 2D). The current at the transient peak and at the end of the 1 s IBMX exposure were significantly larger in the OMP−/− ORNs compared to control (Fig. 2B), explainable by slower adaptation and by the slower overall kinetics of the response (see next section). Unlike odourants (see previous paragraph), IBMX triggered a higher maximal spike frequency in OMP−/− than in WT ORNs (Fig. 1G and H; collected data in Fig. 2E and F).
From a total of 491 WT ORNs tested with 100 μm cineole, 4% of the cells (20 ORNs) responded, comparable as a percentage to the 6% (25 of 416) of cineole-responsive OMP−/− ORNs. The response to IBMX was 24% of WT (79 of 334) and 45% (97 of 217) of OMP−/− ORNs. Superficially, the higher percentage of OMP−/− ORNs responsive to IBMX together with the increased response to IBMX could imply either elevated adenylyl cyclase activity or an overall increase in adenylyl cyclase gene expression. RT-PCR analyses did not support the latter, since no up-regulation in transcription was observed (F. L. Margolis, unpublished observations).
OMP is required for fast recovery from adaptation
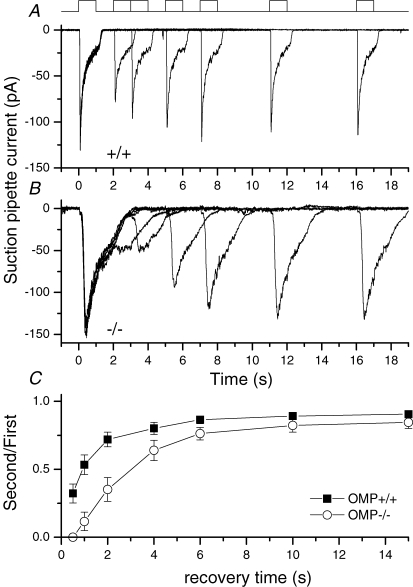
The much slower odour-induced responses in OMP−/− ORNs may also lead to a slower recovery from adaptation. We examined this property by exposing an ORN to two 1 s pulses of 100 μm cineole, separated by different time intervals, with the second serving as a test pulse for recovery from the first. In Fig. 3A, the WT neuron regained ∼75% of its sensitivity in 2 s after the first cineole pulse. By contrast, an OMP−/− neuron required ∼5 s for the sensitivity to recover by the same degree (Fig. 3B). Figure 3C shows collected data, giving the percentage recovery as a function of time. This finding confirmed previous observations in EOG recordings (Buiakova et al. 1996).
Figure 3. Double pulse experiment to investigate recovery from adaptation.
A wild-type (A) and an OMP−/− ORN (B) were exposed twice to 100 μm cineole for 1 s with increasing recovery time between the odour exposures as indicated by the solution monitor at the top. C, the recovery time course as a function of the recovery time, the ordinate displays the peak current of the second response normalized to the first. Individual data points represent the average of 2–8 measurements. Cineole concentration was 100 μm.
Oscillatory response during prolonged stimulation
A characteristic behaviour of ORNs is their ability to generate oscillatory responses during prolonged stimulation at intermediate odour concentrations (Reisert & Matthews, 2001a,b). In Fig. 4A, after an initial large response to a 60 s cineole exposure (300 μm), the response of the WT ORN displayed oscillations with a period of ∼0.6 s (0.83 ± 0.1 s, mean ± s.e.m., n = 11). The inset shows a record filtered at a wide bandwidth on an expanded time scale from a different cell. Action potentials were generated at the rising phase of each inward current wave. Hence the continuous presence of odour is translated into short, high-frequency bursts of action potentials to the olfactory bulb. In contrast, the response of an OMP−/− ORN to a similar prolonged odourant stimulus was typically non-oscillatory, with action potentials occurring only at the initial rising phase of the response. When oscillations did occur, their period lengthened by more than 10-fold (10.6 s in Fig. 4B and 100 μm cineole; 10.4 ± 1.5 s, mean ± s.e.m.n = 6); the same lengthening in periodicity would be expected for action potentials. The 3-fold difference in the cineole concentration used for the WT and the OMP−/− ORNs in Fig. 4 was not the reason for the difference in oscillation period, because the periodicity varies only slightly with odourant concentration (Reisert & Matthews, 2001a).
Decay of odour- and IBMX-induced responses
The connection between OMP and the recovery time course was examined more closely with 25–50 ms odourant stimulations. With such short stimulus durations, the odour exposure actually terminated before the response began to develop, so that the response can be considered as an impulse response (see Bhandawat et al. 2005). As with long odourant pulses, WT ORNs showed much faster olfactory response kinetics than OMP−/− ORNs (cf. Fig. 5A and C; note the compressed time scale in Fig. 5C). The final response decline (typically, fitting began at around 60% of the maximal peak current) followed a single exponential with a time constant of 26 ms for WT and 260 ms for OMP−/− neurons (grey traces in Fig. 5A and C; see also Fig. 6A for averaged data). The occurrence of action potentials distorted the rising phase of the response, so we did not attempt a curve fit, but the far more obvious asymmetry (about the transient peak) of the response in OMP−/− cells compared with WT cells suggested that not all of the steps of the transduction cascade were slowed by the absence of OMP. It should be mentioned here that the slow recovery of the OMP−/− response does not necessarily mean that the deactivation step of the response is differentially affected. All it means is that one step is significantly slowed down, and this could be one of the activation steps.
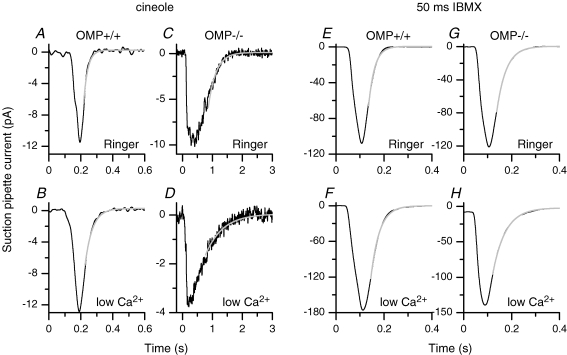
Figure 5. Analysis of the falling phase of the suction pipette current using short cineole or IBMX stimulations.
Wild-type (A) or OMP−/− (C) ORNs were exposed to cineole and the falling phases of the suction pipette current fitted with single exponentials (grey line) with time constants of 26 and 260 ms, respectively. To minimize the contribution of the Ca2+-activated Cl− conductance external Ca2+ was reduced to 20 μm (low Ca2+) for an OMP+/+ (B) and an OMP−/− (D) ORN. The fitted exponentials had time constants of 46 and 570 ms. Stimulation durations were chosen to generate peak currents of comparable magnitudes and were 40, 25, 50 and 25 ms in A–D, respectively, and chosen to elicit small currents, typically between 5 and 20 pA. Recordings in A and B are from the same OMP+/+ ORN, and in C and D are from the same OMP−/− ORN. Exposure to 1 mm IBMX for 50 ms in normal Ringer solution generated suction pipette currents with comparable time courses in wild-type (E) or OMP−/− (G) neurons (time constants of 25 and 39 ms). In low Ca2+ Ringer solution the kinetics of the falling phase also did not change much and could be fitted with time constants of 40 ms in the wild-type (F) and 53 ms in the OMP−/− ORN (G). Traces are in most cases averages of 5 recordings. Recordings in E–H are from different ORNs. Odourant or IBMX stimulations commenced at 40 ms.
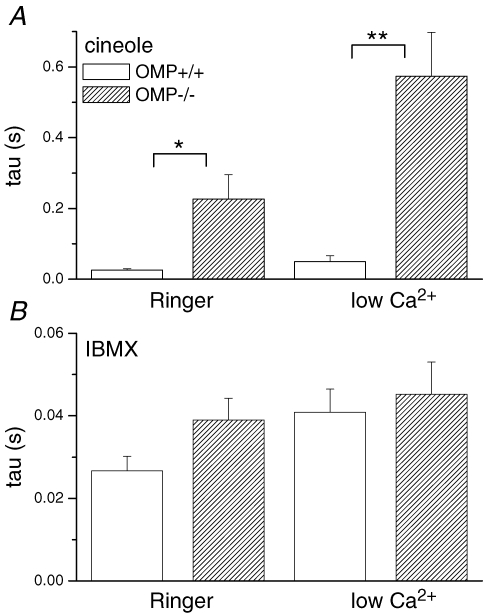
Figure 6. Averaged time constants of the falling phases of cineole or IBMX-triggered responses.
A, comparison of averaged time constants in OMP+/+ and OMP−/− ORNs when stimulated with cineole in either normal or low-Ca2+ Ringer solution. Values are averages of 8–10 cells. B, time constants of IBMX-triggered suction pipette currents in normal or low-Ca2+ Ringer solution. Symbols indicate values that are significantly different at the 0.05 (*) and 0.005 (**) level (Student's t test).
In low (20 μm)-Ca2+ solution, which simplifies the transduction process by attenuating the Ca2+-activated Cl− current in the response and the Ca2+-mediated adaptation (see Introduction), the decay of the WT response remained 10-fold faster than that of the OMP−/− response (time constant of 35 ms in Fig. 5Bversus 570 ms in Fig. 5D; note again the compressed time scale in Fig. 5D; averaged data in Fig. 6A) than in Ringer solution. Under low-Ca2+ conditions, the observed response kinetics should reflect mainly the change in cAMP concentration in the olfactory cilia.
As with long IBMX pulses described earlier, a 50 ms pulse of 1 mm IBMX elicited responses with similar decay rates in WT or OMP−/− ORNs, regardless of whether in Ringer or low-Ca2+ solution (Fig. 5E–H; see Fig. 6B for collected data). IBMX pulses of 25 and 35 ms gave similar results (data not shown). Because IBMX elicits a response by acting on (inhibiting) the phosphodiesterase, the fact that it gave essentially identical responses in WT and OMP−/− cells suggests that, again, the main target of OMP in olfactory cilia is at the step of adenylyl cyclase or earlier. This conclusion is consistent with the low-Ca2+ experiments above. Incidentally, the overall faster time course of the IBMX response compared to the odourant-induced response simply stems from the fact that the action of IBMX bypasses the steps of odourant receptor and Golf. Moreover, the permeation of IBMX through the ciliary plasma membrane is very rapid.
Discussion
Although OMP was discovered many years ago, its exact function remains unknown. Behavioural experiments on OMP−/− animals demonstrated a > 50-fold decrease in sensitivity to odours (Youngentob & Margolis, 1999) and an alteration in odourant quality perception (Youngentob et al. 2001). The first indication of its effect on olfactory response kinetics came from EOG recordings from OMP−/− mice which showed a slowing of odour-induced response onset and its termination. Curiously, OMP has also been implicated in regulating the mitotic activity of ORN precursors (Farbman et al. 1998) and in axon guidance in the olfactory bulb (St John & Key, 2005). To gain insight into the OMP function we have studied the connection between OMP and olfactory response kinetics more closely in this work.
The olfactory neuroepithelium of WT mice includes both mature and immature ORNs. Thus, would we have expected to record from an ORN of a WT mouse that has not yet expressed OMP and therefore displays slow response kinetics? The proportion of immature cells varies with the age of the animal (Farbman & Margolis, 1980; Miragall & Monti Graziadei, 1982; Margolis et al. 1991; Farbman, 1994). Maturation of ORNs in WT mice is associated with the expression of OMP, formation of cilia and expression of the dipeptide carnosine (Biffo et al. 1990; Farbman, 1994). In mice > 2 months old as used in this study, the immature ORNs are limited to a small population of the total that lie adjacent to the basal lamina (Margolis et al. 1991; Schwob et al. 1992). These immature ORNs lack cilia and do not express OMP or carnosine. In OMP−/− mice, the number of ORNs with cilia and expressing carnosine was unaltered (Buiakova et al. 1996). However, because the recorded currents reported in this study derive from the ORN cilia, it is highly unlikely that any of our recordings were from immature ORNs.
Our recordings from single ORNs confirmed the substantial slowing of their response to odourants in the absence of OMP. Furthermore, the experiments with IBMX and low-Ca2+ solution both pointed to the step of cAMP production or earlier in the transduction cascade as a major target of OMP action in the cilia of ORNs. Our results indicating that OMP acts very early in the transduction cascade are in apparent disagreement with the report that Ca2+ extrusion via the Na+–Ca2+ exchanger is slowed in the dendritic knobs of OMP−/− ORNs (Kwon et al. 2005). In principle, such an effect should have affected the response kinetics of OMP−/− ORNs much more in Ringer than in low-Ca2+ solution, because the involvement of Ca2+ should be strongly attenuated by the latter condition. However, we found that the two conditions made little difference (Figs 5 and 6). The disparity in findings could potentially arise from a difference in experimental approach or in the preparation. In particular, Kwon et al. (2005) studied the Ca2+ dynamics in the dendritic knob, whereas the electrical responses we studied came predominantly from the cilia, a compartment with its own Ca2+ dynamics compared to the dendritic knob (Leinders-Zufall et al. 1998). The small ciliary volume compared to its surface area means that even small amounts of Ca2+ entering the cilia through the CNG channel can lead to large local Ca2+ changes (Lindemann, 2001). Equally, this small absolute amount of Ca2+ can be quickly removed by Na+–Ca2+ exchange, which is abundantly expressed in ORNs and their cilia (Pyrski et al. 2007). This offers a parsimonious explanation for the difference in finding between the two studies. Na+–Ca2+ exchange could also be affected in the cilia, but, owing to the small amount of Ca2+ present in cilia, might not become apparent even when Na+–Ca2+ exchange was slowed due to the absence of OMP, thus allowing other more dominant effects of OMP to be uncovered as reported here. Nevertheless, we have demonstrated, using suction electrode recordings of individual dissociated ORNs that OMP is indeed a critical participant in modulating olfactory transduction. It appears to participate at a very early stage in the cascade, possibly at the level of the adenylyl cyclase (ACIII) where its absence leads to a delayed response to odour stimulation.
How could such a role of OMP explain not only the prolongation but also the sluggish onset of the response? In wild-type ORNs, any spontaneous activation of the receptor, the G protein or ACIII would cause a response, which would terminate quite quickly due to the presence of OMP and therefore remain small. If indeed OMP's role is to terminate transduction prior to cAMP production, any spontaneous activation of the receptor, G protein or ACIII would give rise to a longer and larger, although still small current in the absence of OMP and therefore a higher overall basal activity, which is indeed observed (increased response to IBMX). Furthermore, an increased basal activity in the OMP knockout can be mimicked by a low, continuous activation of the odourant receptor in wild-type ORNs, like a low, adapting level of odour, causing increased levels of cAMP. Adaptation of this kind does in fact bring about a slowing of the time to peak of frog odour responses (Reisert & Matthews, 1999), reminiscent of what is observed in OMP−/− ORNs. Alternatively, OMP might play a more subtle role and modulate a common regulator of several steps in the transduction cascade. For example, it might modulate the availability of calmodulin which is a critical effector of many stages of the transduction cascade (Wei et al. 1998; Bradley et al. 2004; Kaneko et al. 2006).
OMP, action potential firing and behavioural implications
The recorded alterations in action potential firing also shed light on reasons for the severe reduction in behaviourally determined odour sensitivity (Youngentob & Margolis, 1999), odour quality perception (Youngentob et al. 2001) and alterations in odourant-induced mucosal activity patterns (Youngentob et al. 2003). Constant, albeit low, levels of activity as that observed in the OMP−/− ORNs can desensitize ORNs quite effectively, leading to a shift of the odour dose–response relation to higher odour concentration and even complete failure to generate action potentials in response to odour stimulation (Reisert & Matthews, 1999). Furthermore, OMP−/− ORNs, when stimulated for extended durations, generate only occasionally, if at all, an oscillatory response pattern with a very low frequency. Consequently, action potentials, which can be associated with each repetitive response, only occur around every 10 s (instead of every second as in the WT). This severely reduces the number of action potentials and changes the action potential pattern signalled to the mitral cells, the olfactory second-order neurons in the olfactory bulb. Also, since OMP−/− ORNs recover only very slowly, any response following a previous stimulation is reduced, compromising rapid sampling (e.g. during sniffing) of an odour, a situation compounded by the increased latency to generate an action potential in response to odour stimulation in the OMP knockout. Together, these results on the level of a single cell can explain the reported increase in behaviourally determined odour detection threshold in the OMP knockout (Youngentob & Margolis, 1999) and odour quality perception deficits (Youngentob et al. 2001). They might also be related to the altered innervation patterns reported in the olfactory bulb of OMP−/− ORNs (St John & Key, 2005).
We have demonstrated that OMP plays a key role in one of the earliest steps of olfactory transduction in ORNs. However, the expression of OMP is highly restricted to chemosensory neurons in the main olfactory epithelium, the vomeronasal organ, the septal organ and the Grüneberg ganglion. This restricted cellular distribution of OMP coupled with the phylogenetic conservation of its amino acid sequence suggests that it plays a common role in these various loci where it is present. However, these different sites do not all use a common signal transduction pathway. It will be interesting to determine the role OMP plays in these various cells as this should help to explain why OMP has such a selective expression pattern.
Acknowledgments
We thank Drs T. Leinders-Zufall and F. Zufall for discussions and Dr C.-Y. Su for helpful comments on the manuscript. The work was supported by NIH grants DC-03112 (F.L.M.) and DC-06904 (K.-W.Y) and the Monell Chemical Senses Center.
References
- Baylor DA, Lamb TD, Yau KW. Responses of retinal rods to single photons. J Physiol. 1979;288:613–634. [PMC free article] [PubMed] [Google Scholar]
- Bhandawat V, Reisert J, Yau KW. Elementary response of olfactory receptor neurons to odorants. Science. 2005;308:1931–1934. doi: 10.1126/science.1109886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biffo S, Grillo M, Margolis FL. Cellular localization of carnosine-like and anserine-like immunoreactivities in rodent and avian central nervous system. Neuroscience. 1990;35:637–651. doi: 10.1016/0306-4522(90)90335-2. [DOI] [PubMed] [Google Scholar]
- Borisy FF, Ronnett GV, Cunningham AM, Juilfs D, Beavo J, Snyder SH. Calcium/calmodulin-activated phosphodiesterase expressed in olfactory receptor neurons. J Neurosci. 1992;12:915–923. doi: 10.1523/JNEUROSCI.12-03-00915.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley J, Bönigk W, Yau KW, Frings S. Calmodulin permanently associates with rat olfactory CNG channels under native conditions. Nat Neurosci. 2004;7:705–710. doi: 10.1038/nn1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breer H, Fleischer J, Strotmann J. The sense of smell: multiple olfactory subsystems. Cell Mol Life Sci. 2006;63:1465–1475. doi: 10.1007/s00018-006-6108-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buiakova OI, Baker H, Scott JW, Farbman A, Kream R, Grillo M, Franzen L, Richman M, Davis LM, Abbondanzo S, Stewart CL, Margolis FL. Olfactory marker protein (OMP) gene deletion causes altered physiological activity of olfactory sensory neurons. Proc Natl Acad Sci U S A. 1996;93:9858–9863. doi: 10.1073/pnas.93.18.9858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buiakova OI, Krishna NS, Getchell TV, Margolis FL. Human and rodent OMP genes: conservation of structural and regulatory motifs and cellular localization. Genomics. 1994;20:452–462. doi: 10.1006/geno.1994.1200. [DOI] [PubMed] [Google Scholar]
- Chen T-Y, Yau K-W. Direct modulation by Ca2+-calmodulin of cyclic nucleotide-activated channel of rat olfactory receptor neurons. Nature. 1994;368:545–548. doi: 10.1038/368545a0. [DOI] [PubMed] [Google Scholar]
- Dzeja C, Hagen V, Kaupp UB, Frings S. Ca2+ permeation in cyclic nucleotide-gated channels. EMBO J. 1999;18:131–144. doi: 10.1093/emboj/18.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farbman AI. Developmental biology of olfactory sensory neurons. Semin Cell Biol. 1994;5:3–10. doi: 10.1006/scel.1994.1002. [DOI] [PubMed] [Google Scholar]
- Farbman AI, Buchholz JA, Walters E, Margolis FL. Does olfactory marker protein participate in olfactory neurogenesis? Ann N Y Acad Sci. 1998;855:248–251. doi: 10.1111/j.1749-6632.1998.tb10576.x. [DOI] [PubMed] [Google Scholar]
- Farbman AI, Margolis FL. Olfactory marker protein during ontogeny: immunohistochemical localization. Dev Biol. 1980;74:205–215. doi: 10.1016/0012-1606(80)90062-7. [DOI] [PubMed] [Google Scholar]
- Ferrando S, Bottaro M, Gallus L, Girosi L, Vacchi M, Tagliafierro G. First detection of olfactory marker protein (OMP) immunoreactivity in the olfactory epithelium of a cartilaginous fish. Neurosci Lett. 2006;413:173–176. doi: 10.1016/j.neulet.2006.11.051. [DOI] [PubMed] [Google Scholar]
- Fleischer J, Hass N, Schwarzenbacher K, Besser S, Breer H. A novel population of neuronal cells expressing the olfactory marker protein (OMP) in the anterior/dorsal region of the nasal cavity. Histochem Cell Biol. 2006;125:337–349. doi: 10.1007/s00418-005-0077-x. [DOI] [PubMed] [Google Scholar]
- Fuss SH, Omura M, Mombaerts P. The Grueneberg ganglion of the mouse projects axons to glomeruli in the olfactory bulb. Eur J Neurosci. 2005;22:2649–2654. doi: 10.1111/j.1460-9568.2005.04468.x. [DOI] [PubMed] [Google Scholar]
- Ivic L, Pyrski MM, Margolis JW, Richards LJ, Firestein S, Margolis FL. Adenoviral vector-mediated rescue of the OMP-null phenotype in vivo. Nat Neurosci. 2000;3:1113–1120. doi: 10.1038/80632. [DOI] [PubMed] [Google Scholar]
- Johnson EW, Eller PM, Jafek BW. An immunoelectron microscopic comparison of olfactory marker protein localization in the supranuclear regions of the rat olfactory epithelium and vomeronasal organ neuroepithelium. Acta Otolaryngol. 1993;113:766–771. doi: 10.3109/00016489309135898. [DOI] [PubMed] [Google Scholar]
- Kaneko H, Möhrlen F, Frings S. Calmodulin contributes to gating control in olfactory calcium-activated chloride channels. J Gen Physiol. 2006;127:737–748. doi: 10.1085/jgp.200609497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko H, Putzier I, Frings S, Kaupp UB, Gensch T. Chloride accumulation in mammalian olfactory sensory neurons. J Neurosci. 2004;24:7931–7938. doi: 10.1523/JNEUROSCI.2115-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller A, Margolis FL. Immunological studies of the rat olfactory marker protein. J Neurochem. 1975;24:1101–1106. doi: 10.1111/j.1471-4159.1975.tb03883.x. [DOI] [PubMed] [Google Scholar]
- Kleene SJ, Gesteland RC. Calcium-activated chloride conductance in frog olfactory cilia. J Neurosci. 1991;11:3624–3629. doi: 10.1523/JNEUROSCI.11-11-03624.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurahashi T, Menini A. Mechanism of odorant adaptation in the olfactory receptor cell. Nature. 1997;385:725–729. doi: 10.1038/385725a0. [DOI] [PubMed] [Google Scholar]
- Kurahashi T, Yau K-W. Co-existence of cationic and chloride components in odorant-induced current of vertebrate olfactory receptor cells. Nature. 1993;363:71–74. doi: 10.1038/363071a0. [DOI] [PubMed] [Google Scholar]
- Kwon HJ, Leinders-Zufall T, Zufall F, Margolis FL. OMP is a modulator of Ca2+ clearance processes in mouse olfactory receptor neurons. Chem Senses. 2005;30:A43. [Google Scholar]
- Leinders-Zufall T, Greer CA, Shepherd GM, Zufall F. Imaging odor-induced calcium transients in single olfactory cilia: Specificity of activation and role in transduction. J Neurosci. 1998;18:5630–5639. doi: 10.1523/JNEUROSCI.18-15-05630.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinders-Zufall T, Ma M, Zufall F. Impaired odor adaptation in olfactory receptor neurons after inhibition of Ca2+/calmodulin kinase II. J Neurosci. 1999;19:11–16. doi: 10.1523/JNEUROSCI.19-14-j0005.1999. RC19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindemann B. Predicted profiles of ion concentrations in olfactory cilia in the steady state. Biophys J. 2001;80:1712–1721. doi: 10.1016/S0006-3495(01)76142-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe G, Gold GH. The spatial distributions of odorant sensitivity and odorant-induced currents in salamander olfactory receptor cells. J Physiol. 1991;442:147–168. doi: 10.1113/jphysiol.1991.sp018787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe G, Gold GH. Nonlinear amplification by calcium-dependent chloride channels in olfactory receptor cells. Nature. 1993;366:283–286. doi: 10.1038/366283a0. [DOI] [PubMed] [Google Scholar]
- Ma M, Grosmaitre X, Iwema CL, Baker H, Greer CA, Shepherd GM. Olfactory signal transduction in the mouse septal organ. J Neurosci. 2003;23:317–324. doi: 10.1523/JNEUROSCI.23-01-00317.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis FL. A brain protein unique to the olfactory bulb. Proc Natl Acad Sci U S A. 1972;69:1221–1224. doi: 10.1073/pnas.69.5.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis FL, Verhaagen J, Biffo S, Huang FL, Grillo M. Regulation of gene expression in the olfactory neuroepithelium: a neurogenetic matrix. Prog Brain Res. 1991;89:97–122. doi: 10.1016/s0079-6123(08)61718-5. [DOI] [PubMed] [Google Scholar]
- Matsuoka M, Osada T, Yoshida-Matsuoka J, Ikai A, Ichikawa M, Norita M, Costanzo RM. A comparative immunocytochemical study of development and regeneration of chemosensory neurons in the rat vomeronasal system. Brain Res. 2002;946:52–63. doi: 10.1016/s0006-8993(02)02823-8. [DOI] [PubMed] [Google Scholar]
- Matthews HR. A compact modular flow heater for the superfusion of mammalian cells. J Physiol. 1999;518:13P. [Google Scholar]
- Matthews HR, Reisert J. Calcium, the two-faced messenger of olfactory transduction and adaptation. Curr Opin Neurobiol. 2003;13:469–475. doi: 10.1016/s0959-4388(03)00097-7. [DOI] [PubMed] [Google Scholar]
- Miragall F, Monti Graziadei GA. Experimental studies on the olfactory marker protein. II. Appearance of the olfactory marker protein during differentiation of the olfactory sensory neurons of mouse: an immunohistochemical and autoradiographic study. Brain Res. 1982;239:245–250. doi: 10.1016/0006-8993(82)90846-0. [DOI] [PubMed] [Google Scholar]
- Nickell WT, Kleene NK, Gesteland RC, Kleene SJ. Neuronal chloride accumulation in olfactory epithelium of mice lacking NKCC1. J Neurophysiol. 2006;95:2003–2006. doi: 10.1152/jn.00962.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyrski M, Koo JH, Polumuri S, Ruknudin AM, Margolis JWS, Schulze DH, Margolis FL. Sodium/calcium exchanger expression in the mouse and rat olfactory systems. J Comp Neurol. 2007;501:944–958. doi: 10.1002/cne.21290. [DOI] [PubMed] [Google Scholar]
- Reisert J, Lai J, Yau KW, Bradley J. Mechanism of the excitatory Cl− response in mouse olfactory receptor neurons. Neuron. 2005;45:553–561. doi: 10.1016/j.neuron.2005.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisert J, Matthews HR. Na+-dependent Ca2+ extrusion governs response recovery in frog olfactory receptor cells. J Gen Physiol. 1998;112:529–535. doi: 10.1085/jgp.112.5.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisert J, Matthews HR. Adaptation of the odour-induced response in frog olfactory receptor cells. J Physiol. 1999;519:801–813. doi: 10.1111/j.1469-7793.1999.0801n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisert J, Matthews HR. Response properties of isolated mouse olfactory receptor cells. J Physiol. 2001a;530:113–122. doi: 10.1111/j.1469-7793.2001.0113m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisert J, Matthews HR. Responses to prolonged odour stimulation in frog olfactory receptor cells. J Physiol. 2001b;534:179–191. doi: 10.1111/j.1469-7793.2001.t01-1-00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rössler P, Mezler P, Breer H. Two olfactory marker proteins in Xenopus laevis. J Comp Neurol. 1998;395:273–280. [PubMed] [Google Scholar]
- St John JA, Key B. Olfactory marker protein modulates primary olfactory axon overshooting in the olfactory bulb. J Comp Neurol. 2005;488:61–69. doi: 10.1002/cne.20573. [DOI] [PubMed] [Google Scholar]
- Schild D, Restrepo D. Transduction mechanisms in vertebrate olfactory receptor cells. Physiol Rev. 1998;78:429–466. doi: 10.1152/physrev.1998.78.2.429. [DOI] [PubMed] [Google Scholar]
- Schwob JE, Szumowski KE, Stasky AA. Olfactory sensory neurons are trophically dependent on the olfactory bulb for their prolonged survival. J Neurosci. 1992;12:3896–3919. doi: 10.1523/JNEUROSCI.12-10-03896.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeraratne SD, Valentine M, Cusick M, Delay R, Van Houten JL. Plasma membrane calcium pumps in mouse olfactory sensory neurons. Chem Senses. 2006;31:725–730. doi: 10.1093/chemse/bjl014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Zhao AZ, Chan GCK, Baker LP, Impey S, Beavo JA, Storm DR. Phosphorylation and inhibition of olfactory adenylyl cyclase by CaM kinase II in neurons: a mechanism for attenuation of olfactory signals. Neuron. 1998;21:495–504. doi: 10.1016/s0896-6273(00)80561-9. [DOI] [PubMed] [Google Scholar]
- Yan C, Zhao AZ, Bentley JK, Loughney K, Ferguson K, Beavo JA. Molecular cloning and characterization of a calmodulin-dependent phosphodiesterase enriched in olfactory sensory neurons. Proc Natl Acad Sci U S A. 1995;92:9677–9681. doi: 10.1073/pnas.92.21.9677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngentob SL, Kent PF, Margolis FL. OMP gene deletion results in an alteration in odorant-induced mucosal activity patterns. J Neurophysiol. 2003;13:13. doi: 10.1152/jn.00806.2002. [DOI] [PubMed] [Google Scholar]
- Youngentob SL, Margolis FL. OMP gene deletion causes an elevation in behavioral threshold sensitivity. Neuroreport. 1999;10:15–19. doi: 10.1097/00001756-199901180-00003. [DOI] [PubMed] [Google Scholar]
- Youngentob SL, Margolis FL, Youngentob LM. OMP gene deletion results in an alteration in odorant quality perception. Behav Neurosci. 2001;115:626–631. doi: 10.1037//0735-7044.115.3.626. [DOI] [PubMed] [Google Scholar]
- Youngentob SL, Pyrski MM, Margolis FL. Adenoviral vector-mediated rescue of the OMP-null behavioral phenotype: enhancement of odorant threshold sensitivity. Behav Neurosci. 2004;118:636–642. doi: 10.1037/0735-7044.118.3.636. [DOI] [PubMed] [Google Scholar]
- Zufall F, Firestein S. Divalent cations block the cyclic nucleotide-gated channel of olfactory receptor neurons. J Neurophysiol. 1993;69:1758–1768. doi: 10.1152/jn.1993.69.5.1758. [DOI] [PubMed] [Google Scholar]