Abstract
Liddle's syndrome is an autosomal dominant form of human hypertension, caused by gain-of-function mutations of the epithelial sodium channel (ENaC) which is expressed in aldosterone target tissues including the distal colon. We used a mouse model for Liddle's syndrome to investigate ENaC-mediated Na+ transport in late distal colon by measuring the amiloride-sensitive transepithelial short circuit current (ΔISC-Ami) ex vivo. In Liddle mice maintained on a standard salt diet, ΔISC-Ami was only slightly increased but plasma aldosterone (PAldo) was severely suppressed. Liddle mice responded to a low or a high salt diet by increasing or decreasing, respectively, their PAldo and ΔISC-Ami. However, less aldosterone was required in Liddle animals to achieve similar or even higher Na+ transport rates than wild-type animals. Indeed, the ability of aldosterone to stimulate ΔISC-Ami was about threefold higher in Liddle animals than in the wild-type controls. Application of aldosterone to colon tissue in vitro confirmed that ENaC stimulation by aldosterone was not only preserved but enhanced in Liddle mice. Aldosterone-induced transcriptional up-regulation of the channel's β- and γ-subunit (βENaC and γENaC) and of the serum- and glucocorticoid-inducible kinase 1 (SGK1) was similar in colon tissue from Liddle and wild-type animals, while aldosterone had no transcriptional effect on the α-subunit (αENaC). Moreover, Na+ feedback regulation was largely preserved in colon tissue of Liddle animals. In conclusion, we have demonstrated that in the colon of Liddle mice, ENaC-mediated Na+ transport is enhanced with an increased responsiveness to aldosterone. This may be pathophysiologically relevant in patients with Liddle's syndrome, in particular on a high salt diet, when suppression of PAldo is likely to be insufficient to reduce Na+ absorption to an appropriate level.
Liddle's syndrome is an autosomal dominant form of human hypertension associated with hypokalaemia, suppressed plasma renin activity and low plasma aldosterone levels (pseudohyperaldosteronism) (Liddle et al. 1963; Warnock, 1998). Genetic analysis of kindred with Liddle's syndrome revealed that Liddle's syndrome is caused by gain-of-function mutations of the epithelial sodium channel (ENaC) which is expressed in aldosterone target tissues, including the distal colon and the aldosterone-sensitive distal nephron. The latter is important for the fine tuning of renal sodium absorption. Loss-of-function mutations of ENaC cause severe urinary sodium loss, hyperkalaemia and low blood pressure in patients with pseudohypoaldosteronism type 1 (PHA-1). These hereditary diseases caused by ENaC mutations clearly demonstrate that the appropriate regulation of ENaC is critically important for the maintenance of body sodium balance and hence for the long-term regulation of arterial blood pressure (Garty & Palmer, 1997; Rossier et al. 2002).
The bulk absorption of Na+ in the colon occurs through electroneutral Na+ transport rather than electrogenic absorption which is limited to the distal colon. In the distal colon, electrogenic sodium absorption mediated by ENaC is particularly important when dietary sodium intake is low and aldosterone levels are high. Under these conditions, the distal colon can efficiently absorb dietary sodium against a large concentration gradient (Sandle, 1998; Kunzelmann & Mall, 2002). ENaC was originally cloned from rat colon using an expression cloning strategy with mRNA isolated from tissue of animals exposed to a low sodium diet to up-regulate ENaC expression (Canessa et al. 1993). Enhanced ENaC function in the colon may contribute to Liddle's syndrome (Pradervand et al. 1999, 2003) while ENaC down-regulation and the resulting reduction of colonic sodium absorption may contribute to diarrhoea associated with inflammatory bowel disease (Amasheh et al. 2004; Greig et al. 2004).
ENaC is composed of three homologous subunits (α, β and γ) which form a heteromeric channel with a subunit stoichiometry that has been a matter of debate (Kellenberger & Schild, 2002). The recently published crystal structure of the related acid-sensing ion channel ASIC1 suggests that ENaC is a heterotrimer (Jasti et al. 2007). Each subunit has two transmembrane domains, a large extracellular loop and cytosolic N- and C-termini. The C-termini contain a proline-rich PPXY (PY) motif, which is believed to be important for the interaction with the ubiquitin-protein ligases Nedd4 and Nedd4-2, promoting the ubiquitination, endocytosis and proteasomal degradation of the channel. Studies in Xenopus laevis oocytes have demonstrated that Liddle's syndrome mutations, and/or deletions of the PY motif in β or γ ENaC, reduce the endocytic retrieval of ENaC from the membrane (Abriel & Horisberger, 1999). This results in an increase in the number of ENaC channels in the membrane. In addition, Liddle's syndrome mutation has been shown to increase channel open probability (Firsov et al. 1996; Anantharam et al. 2006) and to reduce sodium feedback inhibition of ENaC expressed in Xenopus laevis oocytes (Kellenberger et al. 1998). Recent evidence suggests that proteases may play a key role in ENaC regulation (Planes & Caughey, 2007) and that proteolytic processing and activation of the channel may be enhanced in Liddle's syndrome (Knight et al. 2006). Thus, the combined effect of increased surface expression and increased open probability of ENaC is thought to cause renal and colonic hyperabsorption of Na+, and consequently, arterial hypertension in patients with Liddle's syndrome.
Much of our present knowledge regarding the molecular mechanisms of ENaC regulation has been generated in insightful studies using Xenopus laevis oocytes or cell culture systems. However, the conclusions that can be drawn from heterologous expression systems are limited and their validation requires additional studies in native tissue. Therefore, a mouse model for Liddle's syndrome has been generated expressing C-terminally truncated β-subunits of ENaC, reproducing a typical mutation found in human patients with Liddle's syndrome. This animal model reproduces to a large extent a human form of salt-sensitive hypertension (Dahlmann et al. 2003; Pradervand et al. 2003).
In humans with Liddle's syndrome, aldosterone levels in plasma are below normal. This suggests that in Liddle's syndrome ENaC activity is at least partly aldosterone independent. Indeed, in Liddle mice maintained on a standard salt diet with suppressed plasma aldosterone levels, measurements of the rectal potential difference in vivo suggested that colonic sodium absorption is enhanced in these animals (Pradervand et al. 1999). In contrast, essentially no ENaC whole-cell currents were observed in microdissected renal tubules of Liddle mice maintained on a standard salt diet (Dahlmann et al. 2003). This latter finding was surprising and raises the question whether enhanced renal ENaC activity can be responsible for the arterial hypertension observed in Liddle mice on a high salt diet when plasma aldosterone levels are even lower than in animals on a standard salt diet. Thus, to understand the pathophysiology of Liddle's syndrome, it remains an important issue to determine whether Liddle's syndrome mutation does indeed increase ENaC activity in native epithelial tissue in the presence of suppressed plasma aldosterone levels.
In the present study we used short-circuit current measurements to investigate ENaC-mediated transepithelial sodium transport in native colon tissue of Liddle mice ex vivo. We determined the effect of Liddle's syndrome mutation on basal colonic sodium transport rates. Moreover, we studied the effect of Liddle's syndrome mutation on the responsiveness to aldosterone by using colon tissue from animals maintained on different sodium diets (low, standard or high), to vary the plasma aldosterone levels. To eliminate confounding effects of the diet, we also investigated the effect of aldosterone applied to colon tissue in vitro. We found that basal colonic sodium transport rates were increased in Liddle mice while plasma aldosterone levels were suppressed. Importantly, the responsiveness to aldosterone was greatly enhanced in colon tissue of mice with Liddle's syndrome mutation. This is likely to contribute to the pathophysiology of the disease.
Part of this work has been published in abstract form (Cuffe et al. 2002a; Bertog et al. 2004, 2006).
Methods
Ethical approval for animal studies
For this study, colon tissue preparations were obtained from mice (Mus musculus), killed by CO2 inhalation, followed by terminal interruption of circulation. Animals were cared for and experiments were performed in accordance with the principles of UK and German legislation. The study was initiated at the University Laboratory of Physiology (Oxford, UK) and completed at the Institut für Zelluläre und Molekulare Physiologie (Erlangen, Germany). The study was approved by the Home Office (Licence no. PPL/30/1443) and by the animal welfare officer for the University of Erlangen-Nürnberg (TS-03/01 ZellPhys). At Oxford and Erlangen care and housing of the animals was under the governance of the Home Office and the state veterinary health inspectorate, respectively.
Mouse model
An established mouse model for Liddle's syndrome was used with the β-subunit of ENaC truncated at position R564 (Pradervand et al. 1999). These animals develop a phenotype that reproduces most of the features of the human syndrome, when maintained on a high salt diet. To reduce the heterogeneity of the genetic background animals were backcrossed (N10) with C57BL/6J (Dahlmann et al. 2003). For the experiments, we used animals of both sexes, aged between 12 and 62 weeks, with an average age of 26 ± 1 weeks (n = 391). Animals were homozygous for the Liddle's syndrome mutation (L/L), heterozygous (+/L) or expressed wild-type ENaC (+/+). Mice of different genotypes were sex and age matched in the different experimental groups as closely as possible. Under control conditions (standard salt diet) mice received a chow containing 3.2 mg Na+ per gram and plain tap water. Alternatively, mice were kept on a low salt or high salt diet for 13–16 days prior to the experiments. The low salt animals received a chow containing 0.11 mg Na+ per gram and plain tap water. The high salt animals received a chow containing 16 mg Na+ per gram and 0.9% saline as drinking water ad libitum (Pradervand et al. 1999). Food was obtained from either Special Diet Services, Essex, UK or from Altromin, Lage, Germany.
Colon preparation for Ussing experiments
After opening the abdomen, the most distal 2 cm of colon were dissected. The lumen was flushed with ice-cold mouse Ringer solution before opening the colon longitudinally. With its exposed mucosal side facing downwards the colon tissue was placed onto a Perspex plate. The outer smooth muscle layer was removed using a scalpel and fine forceps. A Perspex ring was attached to the serosal side of the most distal part of the colon preparation using histoacryl tissue glue (B. Braun Aesculap, Tuttlingen, Germany). The tissue was subsequently mounted in a purpose-built chamber insert and transferred into a Ussing chamber system for continuous equivalent short-circuit current (ISC) measurements (Fromm et al. 1993; Cuffe et al. 2000; Zdebik et al. 2004). In our initial experiments, we used chamber inserts with an effective surface area of 0.28 cm2. With this relatively large surface area, we could obtain only one measurement per colon preparation (most distal 12 mm of colon). For paired measurements from the same animal, we developed smaller container inserts with an effective surface area of 0.08 cm2, using a design that minimizes edge damage (Stockmann et al. 1999). This made it possible to obtain two or three tissue samples from adjacent regions of the same circumferential late distal colon preparation (most distal 5 mm of the colon).
Solutions and chemicals
Colon tissue samples were routinely maintained in standard mouse Ringer solution, containing (mm): 119 NaCl, 21 NaHCO3, 1.2 CaCl2, 1.2 MgCl2, 0.6 KH2PO4, 2.4 K2HPO4, 10 d-glucose, 10 d-mannose, 2.5 l-glutamine, 0.5 β-hydroxybutyric acid and azlocillin (50 mg l−1), maintained at pH 7.4 by gassing with 95% O2 and 5% CO2 at 37°C (Fromm et al. 1993). To obtain a low Na+ (4 mm) and a high Na+ (123 mm) solution, 17 mm NaHCO3 of the mouse Ringer solution were replaced by 17 mm choline bicarbonate. In the low Na+ solution, 119 mm NaCl were osmotically replaced by mannitol. Amiloride hydrochloride and aldosterone were purchased from Sigma-Aldrich (Poole, Dorset, UK or Taufkirchen, Germany). Amiloride was prepared as a 100 mm stock solution in methanol. Aldosterone was made up as a 1 mm stock in ethanol and stored at −20°C.
Transepithelial measurements
Ussing experiments were performed using a CVC 6 clamp device (Fiebig, Berlin, Germany), as previously described (Bertog et al. 1999, 2000; Cuffe et al. 2002b). The transepithelial voltage (Vte) was continuously measured and transepithelial resistance (Rte) was evaluated by recording the voltage deflections induced by 200 ms symmetrical square current pulses of ± 10–20 μA. Data were acquired every 10–60 s. The equivalent short circuit current (ISC) was calculated according to Ohm's law. Conventionally, a lumen negative Vte corresponds to a positive ISC, which results from electrogenic cation absorption, anion secretion or a combination of both. Before each experiment, the electrical resistance of the fluid-filled Ussing chamber system and off-set potentials of the voltage electrodes were determined and were subtracted from the subsequent measurements.
Isolation of mRNA and real-time quantitative PCR
Total RNA was extracted from the same colon specimens as studied in the Ussing chamber experiments, by using RNeasy extraction kit (Qiagen, Hilden, Germany). First-strand cDNA was synthesized with TaqMan RT reagents (Applied Biosystems, Darmstadt, Germany) using random hexamers as primers, with a final RNA concentration in the reaction mixture adjusted to 0.1 ng μl−1. Real-time quantitative PCR was performed using the ABI PRISM. 7000 Sequence Detector System and the SYBR Green method (Applied Biosystems), according to the manufacturer's instructions. PCR reactions without Multiscribe reverse transcriptase were used as negative controls for genomic DNA contamination. In each set of experiments, one non-treated colon tissue from a wild-type mouse served as a ‘calibrator’. RT products were diluted 1 : 1 with dH2O before the PCR procedure. The sequences of the primer pairs are given in the Supplementary table available online. All primer pairs and probes for the detection of the genes investigated (except for mouse SGK1, for which we used a published sequence (Gumz et al. 2003)) were designed using Primer Express software (Perkin Elmer, Foster City, CA, USA) with uniform selection parameters for standard cycle conditions. All samples were analysed in triplicate. Threshold cycle (CT) values of target genes were normalized to mouse 18S rRNA, which served as the endogenous control. Differential expression was calculated according to the Standard curve method as specified in the manufacturer's manual.
Aldosterone measurements
Blood samples (approximately 500 μl) were removed by cardiac puncture and were allowed to clot for 30–60 min at 4°C. Samples were centrifuged at 10 000 g for 10 min at 4°C. The plasma was removed (100–300 μl samples) and frozen at −20°C. Plasma aldosterone (PAldo) concentration was quantified using the radioimmunoassay ALDOSTERONE MAIA Kit obtained from either Aldatis, Italy or from Diagnostic Products, Corp.-UK, Gwynedd, UK.
Data analysis
Data were analysed using PRISM 4.02 for Windows (Graph Pad Software Inc., San Diego, CA, USA). Unless stated otherwise, data are given as mean ± standard error of the mean (s.e.m.). Multiple comparisons were subjected to one-way ANOVA, followed by ad hoc post tests. Alternatively, appropriate versions of Student's t test were used. P values less than 0.05 were required to reject the null hypothesis; *, ** and *** represent P values smaller than 0.05, 0.01 and 0.001, respectively; n signifies the number of samples studied. The number of animals used per group was based on previous studies which indicated that in unpaired experiments a relatively large group size was necessary to obtain robust data, since the variability between individual animals was high compared with the expected differences between the groups. The required number in paired experiments was less because differences could be shown within rather than between individuals. Power analysis confirmed that the group sizes used were appropriate.
Results
On a standard salt diet baseline colonic ENaC activity is enhanced in mice with Liddle's syndrome mutation while plasma aldosterone levels are reduced
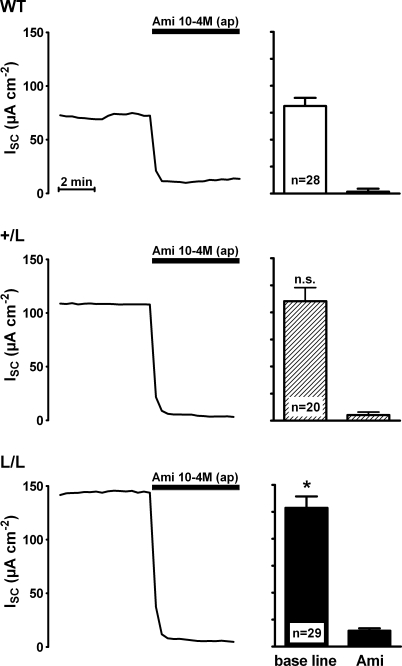
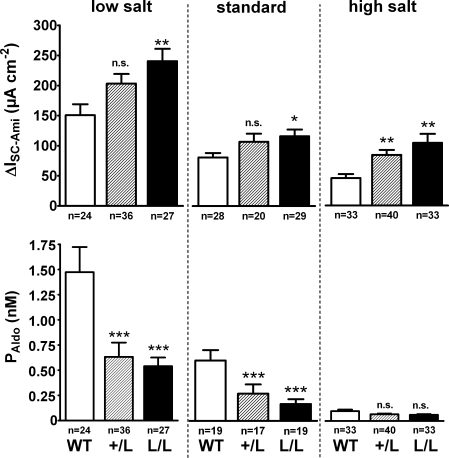
Transepithelial short circuit current (ISC) measurements were performed in late distal colon tissues obtained from animals that were homozygous for the Liddle's syndrome mutation (L/L), heterozygous (+/L) or expressed wild-type ENaC (+/+) (Fig. 1). In this initial set of experiments, animals were kept on a standard salt diet. After mounting the tissues into Ussing chambers, they were allowed to equilibrate for about 60 min to reach a stable ISC. At this point, amiloride (100 μm) was added to the apical bath solution to inhibit the ENaC-mediated ISC component. As shown in Fig. 1, application of amiloride largely abolished ISC with a similar effect in colon tissues from +/+, +/L and L/L animals. This indicates that in animals of all three genotypes ENaC-mediated sodium absorption is responsible for most of the observed ISC in late distal colon tissues. The average baseline ISC was significantly larger in late distal colon from L/L animals (129 ± 11 μA cm−2; n = 29) than the baseline ISC in tissues from wild-type control animals (81 ± 8 μA cm−2; n = 28; P < 0.01) with an intermediate value in tissues from +/L animals (Fig. 1). Similarly, the amiloride-sensitive ISC component (ΔISC-Ami) was significantly larger in L/L animals (114 ± 11 μA cm−2; n = 29) than in wild-type controls (79 ± 7 μA cm−2; n = 28; P < 0.05) with an intermediate value in +/L animals (105 ± 13 μA cm−2; n = 20; Fig. 2). Thus, the increased baseline ISC in colon tissues of L/L animals can be attributed to an increase in ENaC-mediated electrogenic sodium absorption. The amiloride-sensitive transepithelial potential difference (ΔVte-Ami) averaged −16.3 ± 1.8 mV in L/L animals, −15.5 ± 2.1 mV in +/L animals and −10.8 ± 1.2 mV in wild-type animals. These ex vivoΔVte-Ami values are similar to those reported previously in vivo (Pradervand et al. 1999). In the presence of amiloride, the transepithelial resistance (Rte) was similar in colon tissues from +/+, +/L and L/L animals, averaging 145 ± 10 Ωcm2 (n = 28), 166 ± 12 Ωcm2 (n = 20) and 174 ± 14 Ωcm2 (n = 29), respectively. The trend for slightly higher Rte values in L/L animals did not reach statistical significance and cannot be an explanation for the increased sodium transport rate observed in L/L colon tissues. Interestingly, measurements of the plasma aldosterone concentrations revealed larger differences between the three genotypes than the ΔISC-Ami measurements (Fig. 2). In L/L animals on a standard salt diet, the average plasma aldosterone concentration was reduced to 0.15 ± 0.04 nm (n = 19) compared with 0.55 ± 0.09 nm (n = 19) in wild-type control animals, with an intermediate value of 0.25 ± 0.09 nm (n = 17) in +/L animals. These findings indicate that animals with Liddle's syndrome mutation have a suppressed renin–angiotensin–aldosterone system. This can be interpreted as a compensatory regulation to counteract sodium retention and an expansion of the extracellular fluid volume caused by the hyperactive mutant ENaC. Thus, on a standard salt diet, the animals are able to maintain near-normal sodium transport rates but at the expense of suppressed plasma aldosterone levels.
Figure 1. Baseline short-circuit current (ISC) is increased in late distal colon tissue of mice with Liddle's syndrome mutation and is sensitive to amiloride.
Representative short-circuit current (ISC) traces are shown (left panels) which were recorded using colon tissue preparations from wild-type animals (WT) or from animals that were heterozygous (+/L) or homozygous (L/L) for Liddle's syndrome mutation. Animals used for these experiments were maintained on a standard salt diet. Amiloride (Ami) in a concentration of 10−4m was present in the apical (ap) bath solution during the period indicated by the black bar. The columns (right panels) summarize data from similar experiments, as shown in the corresponding traces. Average ISC values before (base line) and after the application of amiloride (Ami) are shown. The baseline ISC in L/L animals compared with that in wild-type animals was significantly increased (P < 0.05).
Figure 2. Effects of high and low dietary Na+ intake on ENaC-mediated colonic Na+ transport and on the plasma aldosterone concentration (PAldo).
Similar experiments to those described in Fig. 1 were performed to determine the amiloride-sensitive ISC component (ΔISC-Ami) in colon tissues from WT (open columns), +/L (hatched columns) and L/L animals (filled columns) exposed to a low salt or high salt diet. The ΔISC-Ami values for animals on a standard salt diet were calculated from the data shown in Fig. 1. Plasma aldosterone concentrations (PAldo) were determined in all groups of animals. In each dietary group, the mean ΔISC-Ami and PAldo values of the +/L and the L/L animals were compared to the corresponding values of the wild-type animals (*P < 0.05; **P < 0.01; ***P < 0.001; n.s., not significant).
Wild-type and Liddle animals respond to a low salt diet with an increase in plasma aldosterone and an increase in ENaC-mediated colonic sodium transport
To investigate the effect of an increase in plasma aldosterone on colonic ENaC activity, we exposed animals to a low salt diet for 13–16 days. As shown in Fig. 2, exposure to a low salt diet increased the plasma aldosterone levels in animals of all three genotypes by a factor of about two or three. Similarly, a low salt diet increased ΔISC-Ami about two- or threefold in animals of all three genotypes. Thus, the relative differences between the genotypes were preserved, i.e. ΔISC-Ami remained significantly higher in L/L animals compared with wild-type animals with an intermediate value in +/L animals. Our findings indicate that L/L animals can up-regulate their renin–angiotensin–aldosterone system in response to a low salt diet. However, compared with wild-type animals, the L/L animals can achieve higher sodium transport rates at lower aldosterone levels when challenged with a low salt diet. Thus, the L/L animals do not need to up-regulate their plasma aldosterone to the same extent as wild-type animals to maintain sodium balance on a low salt diet.
Exposure to a high salt diet reduces the plasma aldosterone concentration in wild-type animals relatively more than in Liddle mice
We also performed experiments in which animals of all three genotypes were exposed to a high salt diet. This has been reported to cause arterial hypertension in Liddle mice (Pradervand et al. 1999) (Fig. 2). Exposure to a high salt diet resulted in a marked suppression of plasma aldosterone levels in animals of all three genotypes. The largest fall in plasma aldosterone was observed in wild-type animals in which exposure to a high salt diet reduced the plasma aldosterone concentration to 0.10 ± 0.02 nm (n = 33). This concentration is about one fifth of that in wild-type animals maintained on standard salt diet (Fig. 2). The plasma aldosterone concentration reached in L/L animals on a high salt diet was not significantly lower than that in wild-type animals on a high salt diet and averaged 0.06 ± 0.01 nm (n = 33). Since the plasma aldosterone concentration was already quite low in L/L animals maintained on a standard salt diet, the overall fall of the plasma aldosterone concentration induced by exposure to a high salt diet was less pronounced in L/L animals than in wild-type animals (Fig. 2). Similarly, the reduction in ΔISC-Ami upon exposure to a high salt diet was more prominent in wild-type animals than in L/L animals, with a 43% and 10% reduction, respectively, compared with ΔISC-Ami in animals on a standard salt diet. The heterozygous +/L animals had an intermediate phenotype. Taken together, these findings indicate that in animals with Liddle's syndrome mutation, exposure to a high salt diet exceeds the regulatory range of the renin–angiotensin–aldosterone system since the plasma aldosterone levels are already suppressed under a standard salt diet. As a consequence, the sodium transport rates on a high salt diet remained about twice as high in L/L animals (103 ± 15 μA cm−2; n = 33) compared with wild-type animals (45 ± 7 μA cm−2; n = 33; P < 0.01).
In vitro stimulation of ENaC-mediated sodium absorption by aldosterone is preserved in colon tissue from mice with Liddle's syndrome mutation
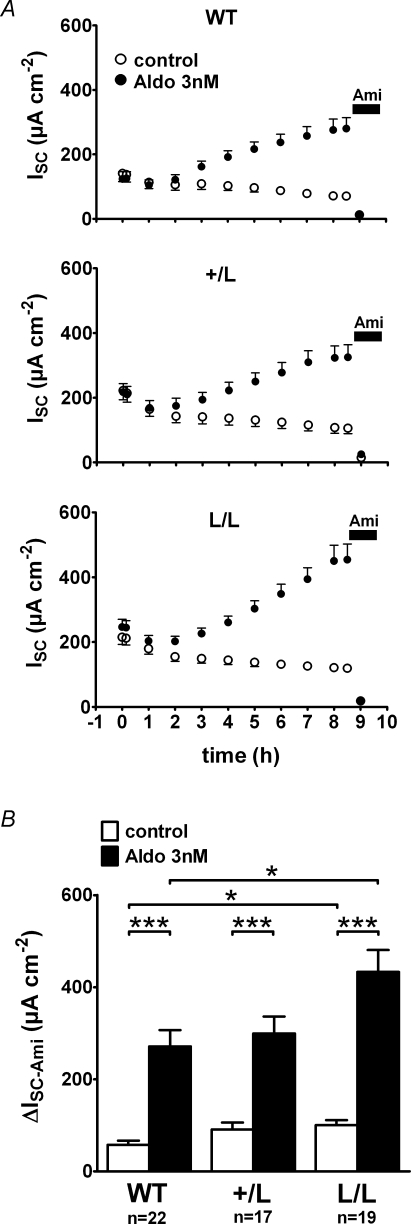
The findings so far suggest that the stimulatory effect of aldosterone on ENaC-mediated sodium transport is preserved or even enhanced in colon from mice with Liddle's syndrome mutation. However, changes in dietary sodium intake may not only change the plasma aldosterone level but may also affect colonic transport mechanisms by other signalling pathways. Therefore, we performed long-term Ussing chamber experiments (> 8 h) to investigate the stimulatory effect of physiological concentrations of aldosterone in vitro without confounding systemic effects (Fig. 3). For these experiments, matched pieces of tissue were obtained using the same circumferential colon segment and were mounted in parallel into Ussing chambers for continuous ISC measurements. After stabilization of the ISC, one tissue was treated with aldosterone (3 nm), while the corresponding control tissue from the same animal was treated with vehicle (0.0003% ethanol). As shown in Fig. 3A, ISC started to increase in aldosterone-treated tissues about 2 h after addition of the hormone and reached a maximum after about 8 h. The time course of the aldosterone-mediated stimulation of ISC was similar in tissues of wild-type, +/L and L/L animals. However, the initial ISC values were larger in colon preparations from L/L animals than in those from wild-type animals which is consistent with the findings reported in Fig. 1. On average, the maximum ISC reached in aldosterone-treated tissues was also higher in L/L animals (433 ± 48 μA cm−2; n = 22) than in wild-type control animals (271 ± 36 μA cm−2; n = 19; P < 0.05) with an intermediate value in the +/L animals (Fig. 3B). No stimulation of ISC was observed in matched control tissues. Instead, ISC continuously declined in the vehicle controls. This decline may be the result of the washout of endogenous aldosterone (see below) or may be caused by a gradual deterioration of the viability of the colon preparation in vitro. Application of amiloride at the end of the experiments confirmed that the stimulated ISC was indeed mediated by ENaC and that 3 nm aldosterone significantly stimulate ENaC-mediated sodium transport in the colon of wild-type, +/L and L/L mice (Fig. 3B). However, the ΔISC-Ami values reached in aldosterone-treated colon tissues from L/L mice were significantly higher than those in aldosterone-treated colon tissues from wild-type mice with intermediate values in the +/L animals. Similarly, the ΔISC-Ami values in the untreated control tissues were larger in the L/L animals than in wild-type controls, consistent with the data shown in Fig. 2. In conclusion, these in vitro data indicate that the stimulatory effect of aldosterone on ENaC-mediated sodium transport is qualitatively preserved in colon tissue of animals with Liddle's syndrome mutation. Compared with wild-type animals, the ΔISC-Ami values in L/L animals are higher in aldosterone-treated as well as in untreated colon tissues.
Figure 3. In vitro stimulation of ENaC by 3 nm aldosterone is enhanced in colon tissue from animals with Liddle's syndrome mutation.
Experiments were essentially performed as described in Fig. 1. In A, average ISC values are shown for every hour of the experiment. After an initial equilibration period of about 1 h, matched colon tissue preparations were exposed at time zero (0 h) to 3 nm aldosterone (•) or to vehicle control (○) added both to the apical and basolateral bath solution. About 8.5 h after exposure to aldosterone, amiloride (10−4m) was added to the apical bath solution to determine ΔISC-Ami. B, summary of the ΔISC-Ami values obtained from the experiments shown in A.
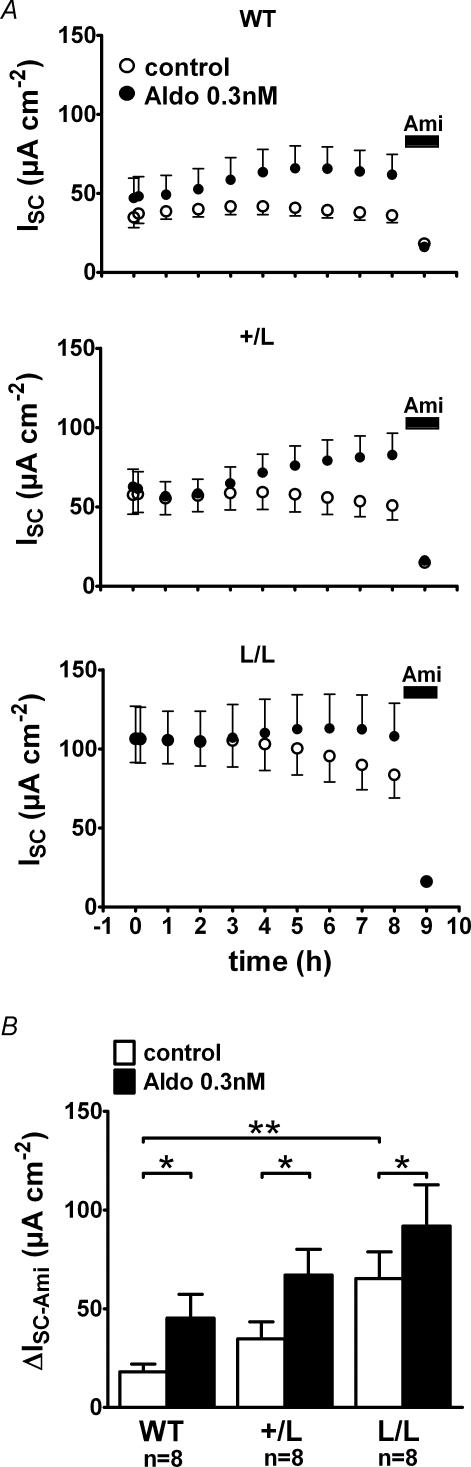
Our experiments with high and low salt diets suggested that the colon of L/L animals required lower aldosterone plasma levels to achieve similar transepithelial transport rates to wild-type animals. Therefore, we also tested the in vitro effect of 0.3 nm aldosterone (Fig. 4). This concentration is similar to that observed in the plasma of wild-type and +/L animals on a standard salt diet (Fig. 2). To reduce pre-stimulation of the colon tissues, we performed these experiments on tissues from animals that had been exposed for 2 weeks to a high salt diet to suppress endogenous plasma aldosterone. Results are summarized in Fig. 4B and demonstrate that we could detect a significant in vitro effect of 0.3 nm aldosterone in colon tissues from animals of all three genotypes. While the ISC in untreated tissues tended to decline, the ISC in tissues treated with 0.3 nm aldosterone slightly increased or was stable over time. At the end of the experiments ΔISC-Ami was significantly higher in all groups of aldosterone-treated tissues (+/+: 45 ± 12 μA cm−2, n = 8; +/L: 67 ± 13 μA cm−2, n = 8; L/L: 92 ± 21 μA cm−2, n = 8) compared with the matched control tissues (+/+: 18 ± 4 μA cm−2, n = 8, P < 0.05; +/L: 35 ± 9 μA cm−2, n = 8, P < 0.05; L/L: 65 ± 14 μA cm−2, n = 8, P < 0.05). There was also a trend that ΔISC-Ami was larger in aldosterone-treated tissues of L/L animals than ΔISC-Ami in aldosterone-treated tissues of wild-type animals with an intermediate value in +/L animals (Fig. 4B). Taken together, these in vitro findings demonstrate that the stimulatory effect of aldosterone is preserved in colon tissues from L/L animals with an aldosterone concentration of 0.3 nm, similar to that observed in animals on a standard salt diet.
Figure 4. Even at a concentration of 0.3 nmin vitro application of aldosterone has a significant stimulatory effect on colonic ISC in animals of all three genotypes (WT, +/L and L/L).
Animals were exposed to high salt diet for 2 weeks prior to the experiment in order to suppress endogenous aldosterone levels. Experiments were performed as in Fig. 3. Average ISC values are shown in A, while ΔISC-Ami values are summarized in B.
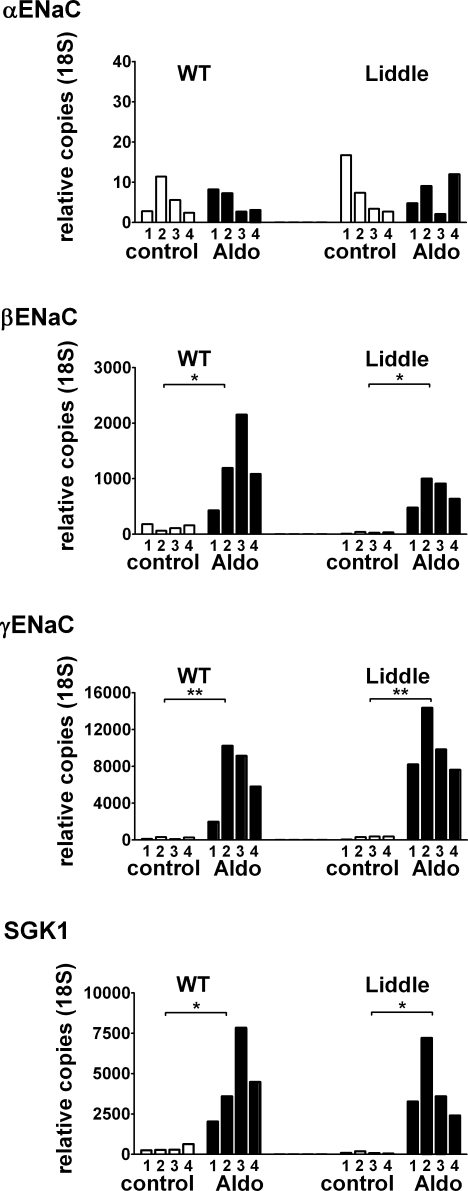
Aldosterone increases transcriptional expression of βENaC, γENaC and SGK1
To investigate whether transcriptional regulation contributes to the stimulatory effect of aldosterone on ENaC in our ex vivo experiments, we performed real-time quantitative PCR experiments (Fig. 5) using mRNA isolated from colon tissues that had been used in Ussing chamber experiments as shown in Fig. 3. Real-time quantitative PCR data, from a representative set of experiments using colon tissues from four wild-type and four L/L animals, are shown in Fig. 5. We detected a significant transcriptional up-regulation of the β- and γ-subunit of ENaC in the aldosterone (3 nm)-treated tissues in four out of four animals. In contrast, there was no apparent up-regulation of αENaC transcripts. Our in vitro data are consistent with previous findings that in colon a low salt diet or aldosterone treatment causes a transcriptional up-regulation of βENaC and γENaC but not of αENaC (Asher et al. 1996; Stokes & Sigmund, 1998). In non-treated tissues, the expression level of βENaC mRNA was markedly lower in L/L animals than in wild-type animals consistent with previously reported findings (Pradervand et al. 1999; Randrianarison et al. 2007). Interestingly, this difference appeared to be reduced in tissues treated with 3 nm aldosterone (Fig. 5). There is a growing body of evidence that SGK1 (Lang et al. 2006) is a mediator of aldosterone action in classical mineralocorticoid target tissues, including the colon, where it is induced by aldosterone at the transcriptional and translational level (Kunzelmann & Mall, 2002). Phosphorylation of the ENaC regulatory protein Nedd4-2 by SGK1 has been shown to reduce the interaction of Nedd4-2 and the PY motif of ENaC, thereby inhibiting ENaC retrieval (Debonneville et al. 2001). Therefore, we also investigated the effect of aldosterone on SGK1 mRNA levels. In non-treated tissues, the expression of SGK1 mRNA in colon of L/L mice appeared to be lower than in wild-type mice. This finding may be explained by the lower baseline PAldo of L/L animals. Treatment of colon tissues in vitro with 3 nm aldosterone caused a large increase in SGK1 transcripts which reached similar levels in +/+ and L/L animals (Fig. 5).
Figure 5. In vitro exposure to aldosterone (3 nm) up-regulates mRNA expression of βENaC, γENaC and SGK1 in L/L and WT animals.
Depicted, are results from one set of real-time quantitative PCR experiments on colon tissue preparations from four wild-type (WT) and four L/L animals processed in parallel. Prior to RNA extraction, the same tissue preparations had been studied in Ussing chamber experiments, as shown in Fig. 3, to confirm functional up-regulation of ENaC-mediated Na+ transport by aldosterone. Tissues were either treated with 3 nm aldosterone (Aldo) or with vehicle (control). Numbers (1–4) correspond to the four WT and four L/L animals used and indicate corresponding values in the Aldo and control groups. Exposure to 3 nm aldosterone for 8.5 h resulted in an up-regulation of the mRNAs for βENaC, γENaC, SGK1, but not for αENaC.
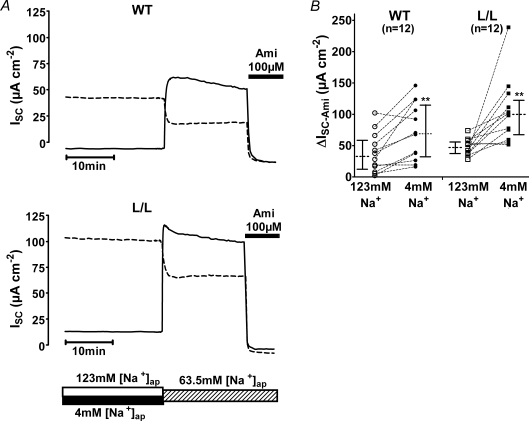
Sodium feedback regulation is largely preserved in colon tissue from mice with Liddle's syndrome mutation
Studies in heterologous expression systems have indicated that impaired sodium feedback regulation may contribute to the hyperactivity of ENaC with Liddle's syndrome mutation (Kellenberger et al. 1998). Therefore, we investigated sodium feedback regulation in native colon of wild-type and L/L mice (Figs 6 and 7). We speculated that a prolonged exposure of the apical side of the colon tissue to a bath solution containing a low sodium concentration should reduce sodium feedback inhibition and should consequently stimulate ENaC-mediated sodium transport. To test this hypothesis, paired pieces of late distal colon tissue from the same animal were mounted in Ussing chambers and were exposed either to an apical bath solution containing 4 mm Na+ or to a bath solution containing 123 mm Na+ for a period of 8 h. The basolateral bath solution was standard mouse Ringer solution. The representative ISC traces shown in Fig. 6A were recorded after an 8 h pre-incubation period and show the ISC response to a change of the apical sodium concentration to 63.5 mm. In tissues pre-incubated in 123 mm Na+, the reduction of the apical sodium concentration to 63.5 mm resulted in a concomitant decrease of the ISC. This is not surprising since ENaC-mediated sodium transport across the apical membrane is a saturable function of the extracellular sodium concentration (Letz et al. 1995; Garty & Palmer, 1997). This also explains why in tissues maintained in the presence of 4 mm apical Na+ the baseline ISC was very low. In contrast, exposure of the tissues pre-incubated with 4 mm apical Na+ to an apical Na+ concentration of 63.5 mm caused a rapid and sustained ISC increase. Importantly, the ISC values reached in the presence of 63.5 mm apical Na+ were higher in the tissues pre-incubated with low sodium than in matched tissues pre-incubated in high sodium. This was the case in colon tissues from wild-type animals (Fig. 6A, upper panel) as well as in colon tissues from L/L animals (Fig. 6A, lower panel). Application of amiloride confirmed that the observed difference in ISC can be attributed toΔISC-Ami and therefore reflects a different activity of ENaC in the low and high Na+-pre-treated tissues. Data from similar experiments, as shown in Fig. 6A, are summarized in Fig. 6B. On average, ΔISC-Ami was significantly larger after pre-incubating colon preparations with a low apical Na+ concentration than after pre-incubating them with a high apical Na+ concentration. Overall, the ΔISC-Ami values were higher in L/L animals than in wild-type animals which is consistent with our other data. Interestingly, the relative increase in ΔISC-Ami, achieved by low Na+ pre-incubation compared with high Na+ pre-incubation, was similar in wild-type and L/L animals.
Figure 6. Prolonged exposure of colon tissue to low apical Na+ results in an up-regulation of ENaC which is preserved in Liddle mice.
Representative traces are shown in A for one wild-type animal (WT; top traces) and one L/L animal (bottom traces). Paired colon tissue preparations obtained from the same animal were mounted into Ussing chambers and were exposed either to an apical solution containing 4 mm Na+ (low sodium) or to an apical solution containing 123 mm Na+ (high sodium). The basolateral compartment contained standard mouse Ringer solution. Tissue preparations were maintained under these conditions for 8 h. At the time indicated by the bar below the traces, the apical bath solution was switched to a bath solution containing 63.5 mm NaCl. This was achieved either by replacing 50% of the low sodium bath solution by high sodium bath solution (ISC traces with continuous lines) or by replacing 50% of the high sodium bath solution by low sodium bath solution (ISC traces with dashed lines). Application of 10−4m amiloride (Ami), as indicated by the black bar, revealed the ENaC-mediated ISC component (ΔISC-Ami). B, summary of ΔISC-Ami values obtained in similar experiments as shown in A. Each circle or square represents an individual ΔISC-Ami value measured either in colon tissue pre-incubated in high sodium (123 mm; open symbols) or in low sodium (4 mm; filled symbols), using colon tissue from WT (circles) or L/L (squares) animals. ΔISC-Ami values measured in matched tissue preparations from the same animal are connected by dotted lines. In addition to the individual values, medians with interquartiles are shown.
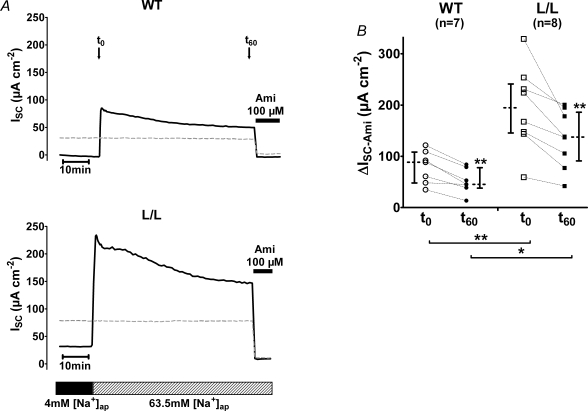
Figure 7. Sodium feedback inhibition is at least partially preserved in colon tissue of L/L mice.
The experimental protocol was similar to that described in Fig. 6. Tissue preparations were pre-incubated for 8 h using an apical low sodium (4 mm) bath solution. A, representative ISC traces from colon tissue of WT or L/L animals are shown. To investigate sodium feedback inhibition, ISC was monitored for 60 min after increasing the NaCl concentration in the apical bath solution to 63.5 mm (as indicated by the bar below the traces). ΔISC-Ami was estimated at time zero (t0) immediately after switching the apical bath solution from 4 mm to 63.5 mm NaCl and 60 min later (t60) when 10−4m amiloride (Ami) were applied as indicated by the black bar. The ISC traces, shown as dashed lines, represent control experiments in which colon tissues were pre-incubated with an apical bath solution containing 63.5 mm NaCl. Mock solution exchanges were performed at t0 to demonstrate that a solution exchange per se has no significant effect on ISC. B, summary of experiments as shown in A. Each circle or square represents an individual ΔISC-Ami value estimated at time zero (t0; open symbols) or after 60 min (t60; filled symbols) in colon tissue from WT (circles) or L/L (squares) animals. ΔISC-Ami values measured in matched tissues from the same animal are connected by dotted lines. In addition to the individual values, medians with interquartiles are shown.
The acute ISC increase observed after exposing low Na+-pre-incubated tissues to 63.5 mm Na+ was followed by a gradual decline of the ISC, probably reflecting sodium-dependent down-regulation of ENaC. To further investigate this phenomenon, we performed experiments as shown in Fig. 7A in which low Na+-pre-treated tissues were exposed to 63.5 mm Na+ for 1 h before amiloride was applied. Data from similar experiments are summarized in Fig. 7B. A significant sodium-induced decline in ΔISC-Ami was apparent in wild-type as well as in L/L animals. The relative decrease of ΔISC-Ami observed within 1 h of exposure to 63.5 mm Na+ was similar in wild-type and L/L animals. In conclusion, our findings indicate that sodium feedback inhibition of ENaC is largely preserved in colon of animals with Liddle's syndrome mutation in the β-subunit of ENaC.
Discussion
The key findings of the present study are (1) that ENaC-mediated colonic sodium transport rates are enhanced in Liddle mice under all dietary conditions (low, standard and high salt diet), and (2) that the colon of Liddle mice is about three times more responsive to aldosterone than that of wild-type animals. Moreover, we demonstrate that in colon of Liddle mice transcriptional up-regulation of βENaC, γENaC and SGK1 by aldosterone is normal and that sodium feedback regulation is largely preserved.
Liddle's syndrome mutation is partially compensated by suppressed plasma aldosterone
Our finding that ΔISC-Ami is only about 44% larger in L/L animals than in +/+ control animals is in contrast to the large stimulatory effect of Liddle's syndrome mutations on ENaC currents in heterologous expression systems and in cultured renal collecting duct cells. Amiloride-sensitive whole-cell currents are about threefold larger in Xenopus laevis oocytes expressing ENaC with the βR564stop mutation than in control oocytes expressing wild-type ENaC (Schild et al. 1995; Kellenberger et al. 1998). Similarly, compared with control cells, baseline transepithelial ENaC currents were found to be two- to fourfold higher in cultured cortical collecting duct (CCD) cells expressing a β-subunit of ENaC harbouring a Liddle's syndrome mutation (Auberson et al. 2003; Pradervand et al. 2003). These large stimulatory effects are in contrast to the moderate effects of the Liddle's syndrome mutation we observed in native colon tissue ex vivo under baseline conditions.
It is likely that in vivo a number of regulatory mechanisms, triggered by increased Na+ reabsorption and hypervolaemia, lead to the repression of the constitutively hyperactive mutant ENaC. In contrast, in vitro these repressor mechanisms are lost and the gain-of-function effect of the Liddle's syndrome mutation becomes fully apparent. Liddle mice in vivo are able to respond to an increase in Na+ absorption by down-regulating their renin–angiotensin–aldosterone system to limit ENaC expression and/or activation. Indeed, we found that the moderately elevated ΔISC-Ami values in Liddle animals on a standard salt diet are associated with markedly suppressed plasma aldosterone levels. Our findings are in good agreement with previous plasma aldosterone measurements in Liddle mice (Pradervand et al. 1999, 2003) and the finding that patients with Liddle's syndrome have low plasma aldosterone levels (Liddle et al. 1963; Warnock, 1998).
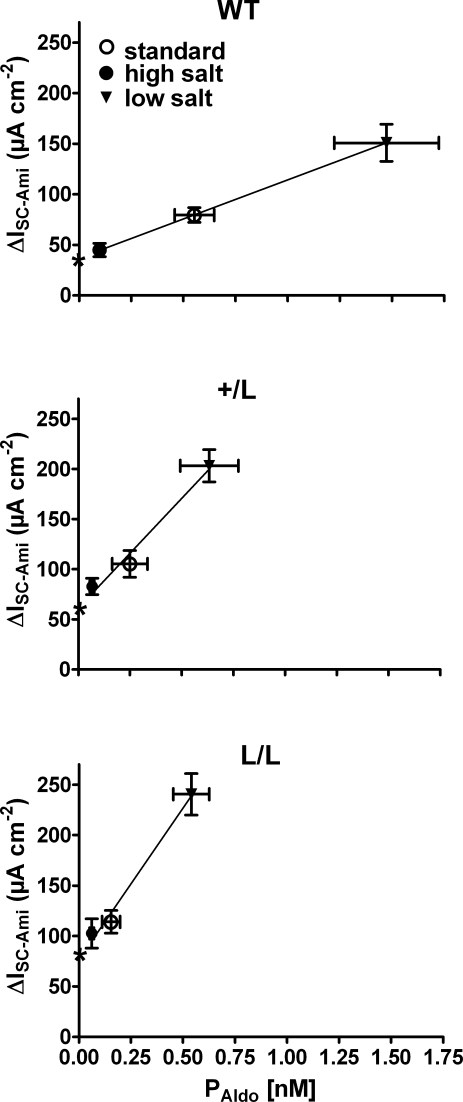
The responsiveness to aldosterone is increased in Liddle's syndrome
Our findings demonstrate that the regulatory response of the renin–angiotensin–aldosterone system to changes in dietary salt intake is intact in Liddle mice. However, the system operates at plasma aldosterone levels that are shifted to lower values. In other words, L/L animals require lower plasma aldosterone levels than wild-type animals to achieve a certain Na+ transport rate in the colon. As can be seen in Fig. 8, the relationship between the plasma aldosterone concentration (PAldo) and the colonic sodium transport rate (ΔISC-Ami) is significantly steeper in L/L animals than in wild-type animals with an intermediate slope in +/L animals (data taken from Fig. 2). Linear regression of the data suggests that increasing PAldo by 0.1 nm increases ΔISC-Ami on average by 7.5 μA cm−2, 22 μA cm−2 and 30 μA cm−2 in wild-type, +/L and L/L animals, respectively. Thus, in Liddle animals, the responsiveness to aldosterone is about threefold higher than in wild-type animals.
Figure 8. Responsiveness to aldosterone is increased in colon of mice with Liddle's syndrome mutation.
ΔISC-Ami and PAldo values were taken from the experiments summarized in Fig. 2. For each genotype (WT, top panel; +/L, middle panel; L/L, lower panel), the average ΔISC-Ami values are plotted against the corresponding average PAldo values from animals maintained on a standard (○), high (•) or low (▾) salt diet (vertical and horizontal bars indicate s.e.m. values). Linear regression of the mean values revealed a significantly (P < 0.01) steeper slope in L/L and +/L animals compared with the wild-type animals. The predicted intercepts of the regression lines with the y-axis are indicated by stars and suggest that baseline ENaC activity at a hypothetical PAldo of zero is higher in L/L and +/L animals than in wild-type animals.
Moreover, we can estimate from Fig. 8 the hypothetical ENaC-mediated transport rates at a plasma aldosterone level of zero. The estimated values of 77 μA cm−2 (L/L), 61 μA cm−2 (+/L) and 37 μA cm−2 (+/+) are not too different from the ΔISC-Ami values of 65 ± 14 μA cm−2 (L/L), 35 ± 9 μA cm−2 (+/L) and 18 ± 4 μA cm−2 (+/+) observed in colon tissues from animals on a high Na+ diet, after maintaining the tissues for a period of 8 h in Ussing chambers in the absence of aldosterone (Fig. 4). These findings indicate that in late distal colon of mice, a baseline ENaC activity is preserved, even at very low plasma aldosterone concentrations. Importantly, even when aldosterone is essentially absent or largely suppressed, ENaC with Liddle's syndrome mutation is more active in colon tissue than wild-type ENaC. Thus, in colon, the gain-of-function mutation of Liddle's syndrome appears to be effective at all plasma aldosterone concentrations with an exaggerated stimulation of ENaC at high levels of plasma aldosterone.
Different effects of Liddle's syndrome mutation on ENaC activity in colon, kidney and lung
Our findings indicate that in colon, the consequences of Liddle's syndrome mutation of ENaC are somewhat different from those in microdissected renal CCD (Dahlmann et al. 2003) and in lung (Randrianarison et al. 2007). Since human patients with Liddle's syndrome have high blood pressure, presumably reflecting increased Na+ absorption, together with low levels of aldosterone, it was hypothesized that the CCDs from L/L animals would escape from tight mineralocorticoid control and would have high ENaC activity, even on a standard salt diet with suppressed plasma aldosterone levels. However, in animals fed a standard salt diet, the principal cells of microdissected CCDs were found to have very small amiloride-sensitive whole-cell currents that were not different in WT and L/L animals. Only when the animals were fed a low salt diet, or were infused with aldosterone, ENaC currents were five to seven times higher in the L/L animals than in wild-type controls (Dahlmann et al. 2003). This increased responsiveness of ENaC to aldosterone in the CCDs from Liddle mice is in good agreement with our findings in colon. However, the finding that ENaC currents are not increased in the CCD of Liddle mice on a standard salt diet is in contrast to our observation that ENaC currents are significantly enhanced in late distal colon of Liddle mice compared with wild-type mice under all dietary conditions (low, standard and high salt diet). Our data, in mouse colon, demonstrate for the first time that Liddle's syndrome mutation of ENaC results in a gain-of-function effect in a native aldosterone-sensitive epithelial tissue, even when plasma aldosterone levels are low, as in patients with Liddle's syndrome. Since we have to assume that an increased ENaC activity in the kidney is essential for the development of Liddle's syndrome, it is tempting to speculate that the situation in proximal parts of the aldosterone-sensitive distal nephron (i.e. in the late distal tubule and connecting tubule) may be similar to that in colon. Unlike the CCD (Dahlmann et al. 2003), these nephron segments have not yet been studied in Liddle mice but are thought to contribute substantially to ENaC-mediated sodium absorption in the kidney (Rubera et al. 2003; Ronzaud et al. 2007).
In the lung of Liddle mice, ENaC-dependent alveolar fluid clearance was found to be significantly increased by about threefold in L/L animals compared with wild-type animals under control conditions on a standard salt diet (Randrianarison et al. 2007). Thus, in the lung, the stimulatory effect of the Liddle's syndrome mutation was even more prominent under control conditions, i.e. in the presence of low plasma aldosterone levels, than in late distal colon. This is not surprising since in the lung ENaC is regulated by glucocorticoids rather than by aldosterone (Renard et al. 1995; Stokes & Sigmund, 1998). Thus, suppression of plasma aldosterone levels in Liddle's syndrome may help to limit the hyperactivity of the mutant channel in mineralocorticoid target tissues, such as the aldosterone-sensitive distal nephron and colon, but not in the lung.
Transcriptional regulation of βENaC, γENaC and SGK1 by aldosterone is preserved in the colon of Liddle mice
Our in vitro studies demonstrated that the time course of ENaC activation by aldosterone in colon tissue from Liddle mice is similar to that in wild-type mice and also similar to that previously described for rat colon (Fromm et al. 1993). This finding indicates that the molecular mechanisms that contribute to ENaC stimulation by aldosterone are not compromised by the mutant β-subunit of ENaC in Liddle mice. Our finding that aldosterone-mediated ENaC stimulation is preserved in colon of Liddle mice is consistent with early clinical observations made by Dr Liddle, who found that patients with pseudohyperaldosteronism (Liddle's syndrome) responded to a challenge with aldosterone by a decrease in renal sodium excretion (Liddle et al. 1963). In aldosterone-sensitive epithelia, the stimulation of Na+ transport by aldosterone involves the transcriptional regulation of ENaC itself but also the transcriptional regulation of other aldosterone-induced proteins possibly involved in ENaC regulation (Verrey et al. 2000; Robert-Nicoud et al. 2001). Transcriptional regulation of the different ENaC subunits by aldosterone has a characteristic tissue-specific pattern (Escoubet et al. 1997; Stokes & Sigmund, 1998). Our study confirms that in colon αENaC is constitutively expressed, while expression of β- and γENaC subunits is up-regulated by aldosterone (Asher et al. 1996; Epple et al. 2000; Fuller et al. 2000; Greig et al. 2002). Moreover, we detected lower baseline expression levels of β-ENaC transcripts in colon tissue from L/L mice compared to +/+ which is consistent with previously reported Northern blot results (Pradervand et al. 1999). However, treatment of colon tissue with 3 nm aldosterone in vitro markedly increased βENaC mRNA expression to similar levels in L/L and +/+ animals. Thus, the different βENaC mRNA expression levels observed in Liddle and wild-type mice under baseline conditions may be caused by reduced plasma aldosterone levels in Liddle animals. In any case, our results demonstrate that transcriptional up-regulation of βENaC and γENaC by aldosterone is largely preserved in colon tissue of Liddle mice.
As yet, only a few aldosterone-induced proteins have been shown to have an impact on ENaC function. These include SGK1 (Lang et al. 2006), K-Ras2 (Mastroberardino et al. 1998), GILZ (Soundararajan et al. 2005), and most recently, Usp2-45 which has the ability to deubiquitylate ENaC (Fakitsas et al. 2007). In the present study, we demonstrated that up-regulation of SGK1 by aldosterone is fully preserved in colon tissue of mice with Liddle's syndrome mutation. The stimulatory effect of aldosterone on SGK1 expression in colon was similar to that observed in microdissected tubule segments (Fakitsas et al. 2007). Phosphorylation of Nedd4-2 by SGK1 has been reported to reduce the ability of the WW domains of Nedd4-2 to interact with the C-terminal PY motifs of ENaC, thereby preventing ubiquitination, retrieval and proteasomal degradation of the channel (Debonneville et al. 2001; Snyder et al. 2002). Our findings indicate that in colon an inhibition of the Nedd4-2 interaction with the C-terminus of βENaC by SGK1 is not essential for the stimulatory effect of aldosterone. However, since the PY motifs in αENaC and γENaC are intact in the mouse model of Liddle's syndrome, we cannot rule out the possibility that an inhibition of their interaction with Nedd4-2 contributes to the stimulatory effect of SGK1 in colon. Thus, the intact PY motifs in the α- and γ-subunit may well explain the preserved aldosterone responsiveness in mice with Liddle's syndrome mutation. Moreover, additional Nedd4-2-independent pathways are likely to contribute to the stimulatory effect of SGK1 on ENaC and may involve specific phosphorylation sites in the C-terminus of αENaC (Diakov & Korbmacher, 2004). Interestingly, recent findings indicate that stimulation of ENaC-mediated electrogenic sodium transport is preserved in SGK1 knock-out mice (Rexhepaj et al. 2006). This indicates that the transcriptional up-regulation of SGK1 is not essential for mediating the stimulatory effect of aldosterone on electrogenic sodium transport in the colon.
Sodium feedback inhibition is largely preserved in colon of Liddle mice
Liddle's syndrome mutation has been shown to reduce sodium feedback inhibition of ENaC expressed in Xenopus laevis oocytes (Kellenberger et al. 1998; Volk et al. 2004). Thus, reduced Na+ feedback inhibition of the mutant channel may contribute to the hyperactivity of ENaC in Liddle's syndrome. In contrast to our expectation, we did not find any evidence for reduced Na+ feedback regulation in native colon tissue of Liddle mice. An increase in intracellular Na+ is thought to contribute to Na+ feedback inhibition, but the mechanism of inhibition is still unclear and may be rather complex. Studies in ‘cut open’ oocytes have demonstrated that this probably is not a direct effect of Na+ on the channel (Abriel & Horisberger, 1999). Instead, Na+ feedback inhibition is likely to involve modulation of the channel by other intracellular factors or proteins which can impinge on channel gating, trafficking or both. Interestingly, a recent study performed in Xenopus laevis oocytes comes to the conclusion that in ENaC with Liddle's syndrome mutation, feedback inhibition is at least partially preserved and achieved primarily by reducing single-channel open probability. In contrast, in wild-type channels, the process of feedback inhibition relies on reducing both channel open probability and channel density, to limit Na+ entry when intracellular Na+ is high (Anantharam et al. 2006). In our experiments, we may have missed the latter component of Na+ feedback inhibition, since channel retrieval may occur with a slower time course. Thus, our finding that Na+ feedback regulation was at least partially preserved in colon tissue of Liddle mice may be explained by the ability of an increase in intracellular Na+ to reduce the channel open probability of both wild-type ENaC and of ENaC with Liddle's syndrome mutation. Interestingly, recent evidence suggests that proteolytic cleavage of the channel by trypsin reduces Na+ self-inhibition (Sheng et al. 2006; Bize & Horisberger, 2007). Since proteolytic cleavage is thought to occur within the extracellular loops of ENaC, this component of Na+ feedback inhibition should not be affected by Liddle's syndrome mutation. Finally, a Nedd4-2-mediated component of Na+ feedback inhibition (Dinudom et al. 1998) may be preserved in animals with Liddle's syndrome mutation since the PY motifs in the α- and γ-subunits are intact and may be sufficient for Nedd4-2-mediated channel retrieval.
Pathophysiological relevance of increased sodium transport in Liddle's syndrome
Our data suggest that in Liddle mice, colonic ENaC activity cannot be sufficiently reduced when animals are maintained on a high salt diet, since aldosterone levels are already suppressed to near-minimal values on a standard salt diet. Thus, on a high salt diet, the regulatory capacity of the renin–angiotensin–aldosterone-system is exceeded in Liddle mice, and ENaC-mediated renal and colonic Na+ absorption remains inappropriately high. According to Guytons's hypothesis, the animals have to respond under these circumstances with an increase in arterial blood pressure to increase renal Na+ excretion in order to maintain Na+ balance. Indeed, an increase in arterial blood pressure has been observed in Liddle mice maintained on a high Na+ diet (Pradervand et al. 1999). The enhanced responsiveness to aldosterone, which we have demonstrated in colon tissue from Liddle mice, is likely to aggravate sodium retention in Liddle's syndrome in all circumstances in which aldosterone secretion is stimulated, e.g. by an activation of renal sympathetic nerve activity. The concept of an enhanced aldosterone sensitivity of ENaC as a contributing factor to salt-sensitive hypertension is also supported by clinical studies demonstrating the effectiveness of a therapy with amiloride or spironolactone to treat hypertension (Saha et al. 2005).
In conclusion, we have demonstrated that ENaC-mediated sodium absorption is enhanced in native colon tissue of Liddle mice which confirms the gain-of-function nature of Liddle's syndrome mutation. Importantly, in colon of Liddle mice, the responsiveness to aldosterone is increased by about threefold and a relatively high ENaC activity is maintained, even when plasma aldosterone is very low. This is likely to contribute to the pathophysiology of Liddle's syndrome, in particular, when dietary Na+ intake is high and aldosterone levels cannot be suppressed sufficiently to down-regulate the hyperactive mutant ENaC to an appropriate level. It will be an important task for future research to determine the molecular mechanisms that cause the increased aldosterone sensitivity in Liddle's syndrome.
Acknowledgments
The expert technical assistance of Jessica Ott is gratefully acknowledged. This work was supported by The Wellcome Trust (C.K.), the EPA Cephalosporin Fund (M.B.) and the Deutsche Forschungsgemeinschaft (SFB 423, TP-A12; C.K.).
Supplemental material
Online supplemental material for this paper can be accessed at: http://jp.physoc.org/cgi/content/full/jphysiol.2007.140459/DC1 and http://www.blackwell-synergy.com/doi/suppl/10.1113/jphysiol.2007.140459
References
- Abriel H, Horisberger JD. Feedback inhibition of rat amiloride-sensitive epithelial sodium channels expressed in Xenopus laevis oocytes. J Physiol. 1999;516:31–43. doi: 10.1111/j.1469-7793.1999.031aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amasheh S, Barmeyer C, Koch CS, Tavalali S, Mankertz J, Epple HJ, Gehring MM, Florian P, Kroesen AJ, Zeitz M, Fromm M, Schulzke JD. Cytokine-dependent transcriptional down-regulation of epithelial sodium channel in ulcerative colitis. Gastroenterology. 2004;126:1711–1720. doi: 10.1053/j.gastro.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Anantharam A, Tian Y, Palmer LG. Open probability of the epithelial sodium channel is regulated by intracellular sodium. J Physiol. 2006;574:333–347. doi: 10.1113/jphysiol.2006.109173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asher C, Wald H, Rossier BC, Garty H. Aldosterone-induced increase in the abundance of Na+ channel subunits. Am J Physiol Cell Physiol. 1996;271:C605–C611. doi: 10.1152/ajpcell.1996.271.2.C605. [DOI] [PubMed] [Google Scholar]
- Auberson M, Hoffmann-Pochon N, Vandewalle A, Kellenberger S, Schild L. Epithelial Na+ channel mutants causing Liddle's syndrome retain ability to respond to aldosterone and vasopressin. Am J Physiol Renal Physiol. 2003;285:F459–F471. doi: 10.1152/ajprenal.00071.2003. [DOI] [PubMed] [Google Scholar]
- Bertog M, Hummler E, Rossier BC, Korbmacher C. Aldosterone induced stimulation of colonic Na+ absorption is preserved in a mouse model for Liddle's syndrome. Pflugers Arch. 2004;447(Suppl.):S120. [Google Scholar]
- Bertog M, Letz B, Kong W, Steinhoff M, Higgins MA, Bielfeld Ackermann A, Fromter E, Bunnett NW, Korbmacher C. Basolateral proteinase-activated receptor (PAR-2) induces chloride secretion in M-1 mouse renal cortical collecting duct cells. J Physiol. 1999;521:3–17. doi: 10.1111/j.1469-7793.1999.00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertog M, Pradervand S, Hummler E, Rossier BC, Korbmacher C. Sodium feedback regulation is partially preserved in a mouse model for Liddle's syndrome. Acta Physiol. 2006;186(Suppl. 650):P243. [Google Scholar]
- Bertog M, Smith DJ, Bielfeld Ackermann A, Bassett J, Ferguson DJ, Korbmacher C, Harris A. Ovine male genital duct epithelial cells differentiate in vitro and express functional CFTR and ENaC. Am J Physiol Cell Physiol. 2000;278:C885–C894. doi: 10.1152/ajpcell.2000.278.5.C885. [DOI] [PubMed] [Google Scholar]
- Bize V, Horisberger JD. Sodium self-inhibition of human epithelial sodium channel: selectivity and affinity of the extracellular sodium sensing site. Am J Physiol Renal Physiol. 2007;293:F1137–F1146. doi: 10.1152/ajprenal.00100.2007. [DOI] [PubMed] [Google Scholar]
- Canessa CM, Horisberger JD, Rossier BC. Epithelial sodium channel related to proteins involved in neurodegeneration. Nature. 1993;361:467–470. doi: 10.1038/361467a0. [DOI] [PubMed] [Google Scholar]
- Cuffe JE, Bertog M, Bunnett NW, Korbmacher C. Basolateral proteinase-activated receptor-2 modifies transepithelial ion transport in mouse distal colon. J Physiol. 2000;527:34P–35P. [Google Scholar]
- Cuffe JE, Bertog M, Koch JP, Regardsoe E, Pradervand S, Hummler E, Rossier BC, Korbmacher C. Colonic Na+ absorption is enhanced in a mouse model for Liddel's syndrome. Pflugers Arch. 2002a;443(Suppl.):S282. [Google Scholar]
- Cuffe JE, Bertog M, Velazquez-Rocha S, Dery O, Bunnett N, Korbmacher C. Basolateral PAR-2 receptors mediate KCl secretion and inhibition of Na+ absorption in the mouse distal colon. J Physiol. 2002b;539:209–222. doi: 10.1113/jphysiol.2001.013159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlmann A, Pradervand S, Hummler E, Rossier BC, Frindt G, Palmer LG. Mineralocorticoid regulation of epithelial Na+ channels is maintained in a mouse model of Liddle's syndrome. Am J Physiol Renal Physiol. 2003;285:F310–F318. doi: 10.1152/ajprenal.00016.2003. [DOI] [PubMed] [Google Scholar]
- Debonneville C, Flores SY, Kamynina E, Plant PJ, Tauxe C, Thomas MA, Münster C, Chraibi A, Pratt JH, Horisberger J-D, Pearce D, Loffing J, Staub O. Phosphorylation of Nedd-4-2 by Sgk1 regulates epithelial Na+ channel cell surface expression. EMBO J. 2001;20:7052–7059. doi: 10.1093/emboj/20.24.7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diakov A, Korbmacher C. A novel pathway of ENaC activation involves an SGK1 consensus motif in the C-terminus of the channel's α-subunit. J Biol Chem. 2004;279:38134–38142. doi: 10.1074/jbc.M403260200. [DOI] [PubMed] [Google Scholar]
- Dinudom A, Harvey KF, Komwatana P, Young JA, Kumar S, Cook DI. Nedd4 mediates control of an epithelial Na+ channel in salivary duct cells by cytosolic Na+ Proc Natl Acad Sci U S A. 1998;95:7169–7173. doi: 10.1073/pnas.95.12.7169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epple HJ, Amasheh S, Mankertz J, Goltz M, Schulzke JD, Fromm M. Early aldosterone effect in distal colon by transcriptional regulation of ENaC subunits. Am J Physiol Gastrointest Liver Physiol. 2000;278:G718–G724. doi: 10.1152/ajpgi.2000.278.5.G718. [DOI] [PubMed] [Google Scholar]
- Escoubet B, Coureau C, Bonvalet JP, Farman N. Noncoordinate regulation of epithelial Na channel and Na pump subunit mRNAs in kidney and colon by aldosterone. Am J Physiol Cell Physiol. 1997;272:C1482–C1491. doi: 10.1152/ajpcell.1997.272.5.C1482. [DOI] [PubMed] [Google Scholar]
- Fakitsas P, Adam G, Daidie D, van Bemmelen MX, Fouladkou F, Patrignani A, Wagner U, Warth R, Camargo SM, Staub O, Verrey F. Early aldosterone-induced gene product regulates the epithelial sodium channel by deubiquitylation. J Am Soc Nephrol. 2007;18:1084–1092. doi: 10.1681/ASN.2006080902. [DOI] [PubMed] [Google Scholar]
- Firsov D, Schild L, Gautschi I, Merillat AM, Schneeberger E, Rossier BC. Cell surface expression of the epithelial Na+ channel and a mutant causing Liddle syndrome: a quantitative approach. Proc Natl Acad Sci U S A. 1996;93:15370–15375. doi: 10.1073/pnas.93.26.15370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromm M, Schulzke JD, Hegel U. Control of electrogenic Na+ absorption in rat late distal colon by nanomolar aldosterone added in vitro. Am J Physiol Endocrinol Metab. 1993;264:E68–E73. doi: 10.1152/ajpendo.1993.264.1.E68. [DOI] [PubMed] [Google Scholar]
- Fuller PJ, Brennan FE, Burgess JS. Acute differential regulation by corticosteroids of epithelial sodium channel subunit and Nedd4 mRNA levels in the distal colon. Pflugers Arch. 2000;441:94–101. doi: 10.1007/s004240000366. [DOI] [PubMed] [Google Scholar]
- Garty H, Palmer LG. Epithelial sodium channels: function, structure, and regulation. Physiol Rev. 1997;77:359–396. doi: 10.1152/physrev.1997.77.2.359. [DOI] [PubMed] [Google Scholar]
- Greig ER, Baker EH, Mathialahan T, Boot-Handford RP, Sandle GI. Segmental variability of ENaC subunit expression in rat colon during dietary sodium depletion. Pflugers Arch. 2002;444:476–483. doi: 10.1007/s00424-002-0828-7. [DOI] [PubMed] [Google Scholar]
- Greig ER, Boot-Handford RP, Mani V, Sandle GI. Decreased expression of apical Na+ channels and basolateral Na+, K+-ATPase in ulcerative colitis. J Pathol. 2004;204:84–92. doi: 10.1002/path.1613. [DOI] [PubMed] [Google Scholar]
- Gumz ML, Popp MP, Wingo CS, Cain BD. Early transcriptional effects of aldosterone in a mouse inner medullary collecting duct cell line. Am J Physiol Renal Physiol. 2003;285:F664–F673. doi: 10.1152/ajprenal.00353.2002. [DOI] [PubMed] [Google Scholar]
- Jasti J, Furukawa H, Gonzales EB, Gouaux E. Structure of acid-sensing ion channel 1 at 1.9 A resolution and low pH. Nature. 2007;449:316–323. doi: 10.1038/nature06163. [DOI] [PubMed] [Google Scholar]
- Kellenberger S, Gautschi I, Rossier BC, Schild L. Mutations causing Liddle syndrome reduce sodium-dependent downregulation of the epithelial sodium channel in the Xenopus oocyte expression system. J Clin Invest. 1998;101:2741–2750. doi: 10.1172/JCI2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev. 2002;82:735–767. doi: 10.1152/physrev.00007.2002. [DOI] [PubMed] [Google Scholar]
- Knight KK, Olson DR, Zhou R, Snyder PM. Liddle's syndrome mutations increase Na+ transport through dual effects on epithelial Na+ channel surface expression and proteolytic cleavage. Proc Natl Acad Sci U S A. 2006;103:2805–2808. doi: 10.1073/pnas.0511184103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunzelmann K, Mall M. Electrolyte transport in the mammalian colon: mechanisms and implications for disease. Physiol Rev. 2002;82:245–289. doi: 10.1152/physrev.00026.2001. [DOI] [PubMed] [Google Scholar]
- Lang F, Bohmer C, Palmada M, Seebohm G, Strutz-Seebohm N, Vallon V. (Patho) physiological significance of the serum- and slucocorticoid-inducible kinase isoforms. Physiol Rev. 2006;86:1151–1178. doi: 10.1152/physrev.00050.2005. [DOI] [PubMed] [Google Scholar]
- Letz B, Ackermann A, Canessa CM, Rossier BC, Korbmacher C. Amiloride-sensitive sodium channels in confluent M-1 mouse cortical collecting duct cells. J Membrane Biol. 1995;148:127–141. doi: 10.1007/BF00207269. [DOI] [PubMed] [Google Scholar]
- Liddle GW, Bledsoe T, Coppage WS., Jr A familial renal disorder simulating primary aldosteronism but with negligible aldosterone secretion. Trans Assoc Am Physicians. 1963;76:199–213. [Google Scholar]
- Mastroberardino L, Spindler B, Forster I, Loffing J, Assandri R, May A, Verrey F. Ras pathway activates epithelial Na+ channel and decreases its surface expression in Xenopus oocytes. Mol Biol Cell. 1998;9:3417–3427. doi: 10.1091/mbc.9.12.3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planes C, Caughey GH. Regulation of the epithelial Na+ channel by peptidases. Curr Top Dev Biol. 2007;78:23–46. doi: 10.1016/S0070-2153(06)78002-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradervand S, Vandewalle A, Bens M, Gautschi I, Loffing J, Hummler E, Schild L, Rossier BC. Dysfunction of the epithelial sodium channel expressed in the kidney of a mouse model for Liddle syndrome. J Am Soc Nephrol. 2003;14:2219–2228. doi: 10.1097/01.asn.0000080204.65527.e6. [DOI] [PubMed] [Google Scholar]
- Pradervand S, Wang Q, Burnier M, Beermann F, Horisberger JD, Hummler E, Rossier BC. A mouse model for Liddle's syndrome. J Am Soc Nephrol. 1999;10:2527–2533. doi: 10.1681/ASN.V10122527. [DOI] [PubMed] [Google Scholar]
- Randrianarison N, Escoubet B, Ferreira C, Fontayne A, Fowler-Jaeger N, Clerici C, Hummler E, Rossier BC, Planes C. β-Liddle mutation of the epithelial sodium channel increases alveolar fluid clearance and reduces the severity of hydrostatic pulmonary oedema in mice. J Physiol. 2007;582:777–788. doi: 10.1113/jphysiol.2007.131078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renard S, Voilley N, Bassilana F, Lazdunski M, Barbry P. Localization and regulation by steroids of the alpha, beta and gamma subunits of the amiloride-sensitive Na+ channel in colon, lung and kidney. Pflugers Arch. 1995;430:299–307. doi: 10.1007/BF00373903. [DOI] [PubMed] [Google Scholar]
- Rexhepaj R, Artunc F, Grahammer F, Nasir O, Sandu C, Friedrich B, Kuhl D, Lang F. SGK1 is not required for regulation of colonic ENaC activity. Pflugers Arch. 2006;453:97–105. doi: 10.1007/s00424-006-0111-4. [DOI] [PubMed] [Google Scholar]
- Robert-Nicoud M, Flahaut M, Elalouf JM, Nicod M, Salinas M, Bens M, Doucet A, Wincker P, Artiguenave F, Horisberger JD, Vandewalle A, Rossier BC, Firsov D. Transcriptome of a mouse kidney cortical collecting duct cell line: effects of aldosterone and vasopressin. Proc Natl Acad Sci U S A. 2001;98:2712–2716. doi: 10.1073/pnas.051603198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronzaud C, Loffing J, Bleich M, Gretz N, Grone HJ, Schutz G, Berger S. Impairment of sodium balance in mice deficient in renal principal cell mineralocorticoid receptor. J Am Soc Nephrol. 2007;18:1679–1687. doi: 10.1681/ASN.2006090975. [DOI] [PubMed] [Google Scholar]
- Rossier BC, Pradervand S, Schild L, Hummler E. Epithelial sodium channel and the control of sodium balance: Interaction between genetic and environmental factors. Annu Rev Physiol. 2002;64:877–897. doi: 10.1146/annurev.physiol.64.082101.143243. [DOI] [PubMed] [Google Scholar]
- Rubera I, Loffing J, Palmer LG, Frindt G, Fowler-Jaeger N, Sauter D, Carroll T, McMahon A, Hummler E, Rossier BC. Collecting duct-specific gene inactivation of αENaC in the mouse kidney does not impair sodium and potassium balance. J Clin Invest. 2003;112:554–565. doi: 10.1172/JCI16956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha C, Eckert GJ, Ambrosius WT, Chun TY, Wagner MA, Zhao Q, Pratt JH. Improvement in blood pressure with inhibition of the epithelial sodium channel in blacks with hypertension. Hypertension. 2005;46:481–487. doi: 10.1161/01.HYP.0000179582.42830.1d. [DOI] [PubMed] [Google Scholar]
- Sandle GI. Salt and water absorption in the human colon: a modern appraisal. Gut. 1998;43:294–299. doi: 10.1136/gut.43.2.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schild L, Canessa CM, Shimkets RA, Gautschi I, Lifton RP, Rossier BC. A mutation in the epithelial sodium channel causing Liddle disease increases channel activity in the Xenopus laevis oocyte expression system. Proc Natl Acad Sci U S A. 1995;92:5699–5703. doi: 10.1073/pnas.92.12.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng S, Carattino MD, Bruns JB, Hughey RP, Kleyman TR. Furin cleavage activates the epithelial Na+ channel by relieving Na+ self-inhibition. Am J Physiol Renal Physiol. 2006;290:F1488–F1496. doi: 10.1152/ajprenal.00439.2005. [DOI] [PubMed] [Google Scholar]
- Snyder PM, Olson DR, Thomas BC. Serum and glucocorticoid-regulated kinase modulates Nedd4-2-mediated inhibition of the epithelial Na+ channel. J Biol Chem. 2002;277:5–8. doi: 10.1074/jbc.C100623200. [DOI] [PubMed] [Google Scholar]
- Soundararajan R, Zhang TT, Wang J, Vandewalle A, Pearce D. A novel role for glucocorticoid-induced leucine zipper protein (GILZ) in ENaC-mediated sodium transport. J Biol Chem. 2005;280:39970–39981. doi: 10.1074/jbc.M508658200. [DOI] [PubMed] [Google Scholar]
- Stockmann M, Gitter AH, Sorgenfrei D, Fromm M, Schulzke JD. Low edge damage container insert that adjusts intestinal forceps biopsies into Ussing chamber systems. Pflugers Arch. 1999;438:107–112. doi: 10.1007/s004240050886. [DOI] [PubMed] [Google Scholar]
- Stokes JB, Sigmund RD. Regulation of rENaC mRNA by dietary NaCl and steroids: organ, tissue, and steroid heterogeneity. Am J Physiol Cell Physiol. 1998;274:C1699–C1707. doi: 10.1152/ajpcell.1998.274.6.C1699. [DOI] [PubMed] [Google Scholar]
- Verrey F, Hummler E, Schild L, Rossier BC. Control of Na+ transport by aldosterone. In: Seldin DW, Giebisch G, editors. The Kidney, Physiology and Pathophysiology. 3. Philadelphia, USA: Lippencott Williams and Wilkins; 2000. pp. 1441–1471. Chap. 53. [Google Scholar]
- Volk T, Konstas AA, Bassalay P, Ehmke H, Korbmacher C. Extracellular Na+ removal attenuates rundown of the epithelial Na+-channel (ENaC) by reducing the rate of channel retrieval. Pflugers Arch. 2004;447:884–894. doi: 10.1007/s00424-003-1193-x. [DOI] [PubMed] [Google Scholar]
- Warnock DG. Liddle syndrome: an autosomal dominant form of human hypertension. Kidney Int. 1998;53:18–24. doi: 10.1046/j.1523-1755.1998.00728.x. [DOI] [PubMed] [Google Scholar]
- Zdebik AA, Cuffe JE, Bertog M, Korbmacher C, Jentsch TJ. Additional disruption of the ClC-2 Cl− channel does not exacerbate the cystic fibrosis phenotype of cystic fibrosis transmembrane conductance regulator mouse models. J Biol Chem. 2004;279:22276–22283. doi: 10.1074/jbc.M309899200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.