Abstract
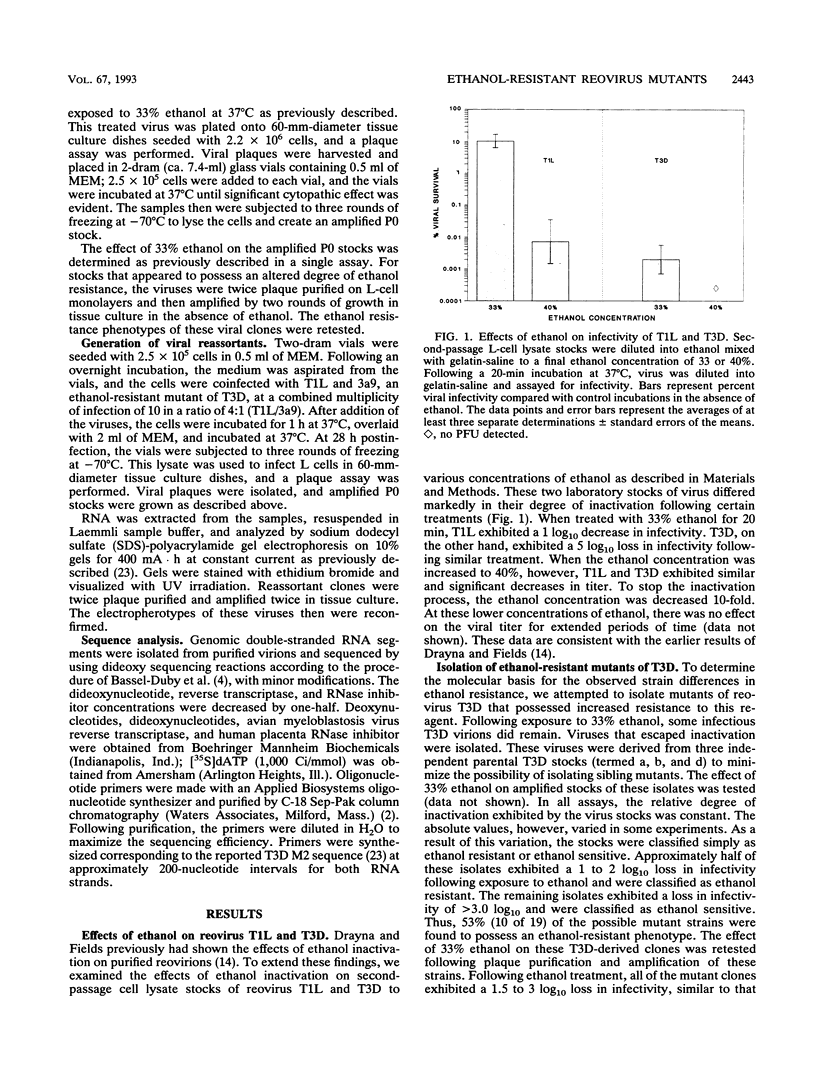
To better understand the mechanism(s) by which viruses respond to chemical or physical treatments, we isolated a series of mutant strains of reovirus type 3 Dearing that exhibit increased ethanol resistance. Following exposure to 33% ethanol for 20 min, the parental strain exhibited a 5 log10 decrease in infectivity. The mutant strains, however, exhibited a 2 to 3 log10 decrease in titer following identical treatment. Through the use of reassortant viruses, we mapped this increased ethanol resistance mutation to the M2 gene segment, which encodes a major outer capsid protein, mu1C. Sequence analysis of mutant M2 genes revealed that six of seven unique mutants possessed single-point mutations in this gene. In addition, the change in six of seven mutants caused a predicted amino acid change in a 35-amino-acid region of the gene product between amino acids 425 and 459. The identification of ethanol resistance mutations within a discrete region of this outer capsid protein identifies that portion of the protein as important in reovirus stability. The presence of viral particles possessing altered stability also suggests that subpopulations of viruses may possess altered environmental stability, which, in turn, could affect viral transmission.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson-Sillman K., Bartal S., Tershak D. R. Guanidine-resistant poliovirus mutants produce modified 37-kilodalton proteins. J Virol. 1984 Jun;50(3):922–928. doi: 10.1128/jvi.50.3.922-928.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass D. M., Bodkin D., Dambrauskas R., Trier J. S., Fields B. N., Wolf J. L. Intraluminal proteolytic activation plays an important role in replication of type 1 reovirus in the intestines of neonatal mice. J Virol. 1990 Apr;64(4):1830–1833. doi: 10.1128/jvi.64.4.1830-1833.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassel-Duby R., Spriggs D. R., Tyler K. L., Fields B. N. Identification of attenuating mutations on the reovirus type 3 S1 double-stranded RNA segment with a rapid sequencing technique. J Virol. 1986 Oct;60(1):64–67. doi: 10.1128/jvi.60.1.64-67.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodkin D. K., Nibert M. L., Fields B. N. Proteolytic digestion of reovirus in the intestinal lumens of neonatal mice. J Virol. 1989 Nov;63(11):4676–4681. doi: 10.1128/jvi.63.11.4676-4681.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsa J., Copps T. P., Sargent M. D., Long D. G., Chapman J. D. New intermediate subviral particles in the in vitro uncoating of reovirus virions by chymotrypsin. J Virol. 1973 Apr;11(4):552–564. doi: 10.1128/jvi.11.4.552-564.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARKE N. A., KABLER P. W., STEVENSON R. E. The inactivation of purified type 3 adenovirus in water by chlorine. Am J Hyg. 1956 Nov;64(3):314–319. doi: 10.1093/oxfordjournals.aje.a119844. [DOI] [PubMed] [Google Scholar]
- CROWTHER D., MELNICK J. L. Studies of the inhibitory action of guanidine on poliovirus multiplication in cell cultures. Virology. 1961 Sep;15:65–74. doi: 10.1016/0042-6822(61)90078-2. [DOI] [PubMed] [Google Scholar]
- CROWTHER D., MELNICK J. L. The incorporation of neutral red and acridine orange into developing poliovirus particles making them photosensitive. Virology. 1961 May;14:11–21. doi: 10.1016/0042-6822(61)90127-1. [DOI] [PubMed] [Google Scholar]
- CUTCHINS E. C., DAYHUFF T. R. Photoinactivation of measles virus. Virology. 1962 Jul;17:420–425. doi: 10.1016/0042-6822(62)90137-x. [DOI] [PubMed] [Google Scholar]
- Chang C. T., Zweerink H. J. Fate of parental reovirus in infected cell. Virology. 1971 Dec;46(3):544–555. doi: 10.1016/0042-6822(71)90058-4. [DOI] [PubMed] [Google Scholar]
- Crumpacker C. S., Schnipper L. E., Marlowe S. I., Kowalsky P. N., Hershey B. J., Levin M. J. Resistance to antiviral drugs of herpes simplex virus isolated from a patient treated with acyclovir. N Engl J Med. 1982 Feb 11;306(6):343–346. doi: 10.1056/NEJM198202113060606. [DOI] [PubMed] [Google Scholar]
- Drayna D., Fields B. N. Activation and characterization of the reovirus transcriptase: genetic analysis. J Virol. 1982 Jan;41(1):110–118. doi: 10.1128/jvi.41.1.110-118.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drayna D., Fields B. N. Biochemical studies on the mechanism of chemical and physical inactivation of reovirus. J Gen Virol. 1982 Nov;63(Pt 1):161–170. doi: 10.1099/0022-1317-63-1-161. [DOI] [PubMed] [Google Scholar]
- Drayna D., Fields B. N. Genetic studies on the mechanism of chemical and physical inactivation of reovirus. J Gen Virol. 1982 Nov;63(Pt 1):149–159. doi: 10.1099/0022-1317-63-1-149. [DOI] [PubMed] [Google Scholar]
- Ewing D. D., Sargent M. D., Borsa J. Switch-on of transcriptase function in reovirus: analysis of polypeptide changes using 2-D gels. Virology. 1985 Jul 30;144(2):448–456. doi: 10.1016/0042-6822(85)90285-5. [DOI] [PubMed] [Google Scholar]
- Furlong D. B., Nibert M. L., Fields B. N. Sigma 1 protein of mammalian reoviruses extends from the surfaces of viral particles. J Virol. 1988 Jan;62(1):246–256. doi: 10.1128/jvi.62.1.246-256.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIATT C. W., KAUFMAN E., HELPRIN J. J., BARON S. Inactivation of viruses by the photodynamic action of toluidine blue. J Immunol. 1960 May;84:480–484. [PubMed] [Google Scholar]
- Hrdy D. B., Rubin D. H., Fields B. N. Molecular basis of reovirus neurovirulence: role of the M2 gene in avirulence. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1298–1302. doi: 10.1073/pnas.79.4.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huismans H., Joklik W. K. Reovirus-coded polypeptides in infected cells: isolation of two native monomeric polypeptides with affinity for single-stranded and double-stranded RNA, respectively. Virology. 1976 Apr;70(2):411–424. doi: 10.1016/0042-6822(76)90282-8. [DOI] [PubMed] [Google Scholar]
- Jayasuriya A. K., Nibert M. L., Fields B. N. Complete nucleotide sequence of the M2 gene segment of reovirus type 3 dearing and analysis of its protein product mu 1. Virology. 1988 Apr;163(2):591–602. doi: 10.1016/0042-6822(88)90300-5. [DOI] [PubMed] [Google Scholar]
- Joklik W. K. Studies on the effect of chymotrypsin on reovirions. Virology. 1972 Sep;49(3):700–715. doi: 10.1016/0042-6822(72)90527-2. [DOI] [PubMed] [Google Scholar]
- Lee P. W., Hayes E. C., Joklik W. K. Characterization of anti-reovirus immunoglobulins secreted by cloned hybridoma cell lines. Virology. 1981 Jan 15;108(1):134–146. doi: 10.1016/0042-6822(81)90533-x. [DOI] [PubMed] [Google Scholar]
- McCrae M. A., Joklik W. K. The nature of the polypeptide encoded by each of the 10 double-stranded RNA segments of reovirus type 3. Virology. 1978 Sep;89(2):578–593. doi: 10.1016/0042-6822(78)90199-x. [DOI] [PubMed] [Google Scholar]
- Mustoe T. A., Ramig R. F., Sharpe A. H., Fields B. N. A genetic map of reovirus. III. Assignment of the double-stranded RNA-positive mutant groups A, B, and G to genome segments. Virology. 1978 Apr;85(2):545–556. doi: 10.1016/0042-6822(78)90460-9. [DOI] [PubMed] [Google Scholar]
- Mustoe T. A., Ramig R. F., Sharpe A. H., Fields B. N. Genetics of reovirus: identification of the ds RNA segments encoding the polypeptides of the mu and sigma size classes. Virology. 1978 Sep;89(2):594–604. doi: 10.1016/0042-6822(78)90200-3. [DOI] [PubMed] [Google Scholar]
- Nibert M. L., Fields B. N. A carboxy-terminal fragment of protein mu 1/mu 1C is present in infectious subvirion particles of mammalian reoviruses and is proposed to have a role in penetration. J Virol. 1992 Nov;66(11):6408–6418. doi: 10.1128/jvi.66.11.6408-6418.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibert M. L., Schiff L. A., Fields B. N. Mammalian reoviruses contain a myristoylated structural protein. J Virol. 1991 Apr;65(4):1960–1967. doi: 10.1128/jvi.65.4.1960-1967.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxford J. S., Logan I. S., Potter C. W. In vivo selection of an influenza A2 strain resistant to amantadine. Nature. 1970 Apr 4;226(5240):82–83. doi: 10.1038/226082a0. [DOI] [PubMed] [Google Scholar]
- Rubin D. H., Fields B. N. Molecular basis of reovirus virulence. Role of the M2 gene. J Exp Med. 1980 Oct 1;152(4):853–868. doi: 10.1084/jem.152.4.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SKINNER H. H., BRADISH C. J. Exposure to light as a source of error in the estimation of the infectivity of virus suspensions. J Gen Microbiol. 1954 Jun;10(3):377–397. doi: 10.1099/00221287-10-3-377. [DOI] [PubMed] [Google Scholar]
- Shatkin A. J., LaFiandra A. J. Transcription by infectious subviral particles of reovirus. J Virol. 1972 Oct;10(4):698–706. doi: 10.1128/jvi.10.4.698-706.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein S. C., Astell C., Levin D. H., Schonberg M., Acs G. The mechanisms of reovirus uncoating and gene activation in vivo. Virology. 1972 Mar;47(3):797–806. doi: 10.1016/0042-6822(72)90571-5. [DOI] [PubMed] [Google Scholar]
- Smith R. E., Zweerink H. J., Joklik W. K. Polypeptide components of virions, top component and cores of reovirus type 3. Virology. 1969 Dec;39(4):791–810. doi: 10.1016/0042-6822(69)90017-8. [DOI] [PubMed] [Google Scholar]
- Sturzenbecker L. J., Nibert M., Furlong D., Fields B. N. Intracellular digestion of reovirus particles requires a low pH and is an essential step in the viral infectious cycle. J Virol. 1987 Aug;61(8):2351–2361. doi: 10.1128/jvi.61.8.2351-2361.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarlow O., McCorquodale J. G., McCrae M. A. Molecular cloning and sequencing of the gene (M2) encoding the major virion structural protein (mu 1-mu 1C) of serotypes 1 and 3 of mammalian reovirus. Virology. 1988 May;164(1):141–146. doi: 10.1016/0042-6822(88)90629-0. [DOI] [PubMed] [Google Scholar]
- WEIDENKOPF S. J. Inactivation of type 1, poliomyelitis virus with chlorine. Virology. 1958 Feb;5(1):56–67. doi: 10.1016/0042-6822(58)90005-9. [DOI] [PubMed] [Google Scholar]
- Wiener J. R., Joklik W. K. Evolution of reovirus genes: a comparison of serotype 1, 2, and 3 M2 genome segments, which encode the major structural capsid protein mu 1C. Virology. 1988 Apr;163(2):603–613. doi: 10.1016/0042-6822(88)90301-7. [DOI] [PubMed] [Google Scholar]
- Zweerink H. J., Joklik W. K. Studies on the intracellular synthesis of reovirus-specified proteins. Virology. 1970 Jul;41(3):501–518. doi: 10.1016/0042-6822(70)90171-6. [DOI] [PubMed] [Google Scholar]


