Abstract
To evaluate the hypothalamic contribution to the development of anterior pituitary (AP) cells we surgically disconnected the hypothalamus from the pituitary (hypothalamo-pituitary disconnection, HPD) in fetal sheep and collected pituitaries 31 days later. Pituitaries (n = 6 per group) were obtained from fetal sheep (term = 147 ± 3 days) at 110 days (unoperated group) of gestation and at 141 days from animals that had undergone HPD or sham surgery at 110 days. Cells were identified by labelling pituitary sections with antisera against the six AP hormones. Additionally, we investigated the colocalization of glycoprotein hormones. The proportions of somatotrophs and corticotrophs were unchanged by age or HPD. Lactotrophs increased 80% over time, but the proportion was unaffected by HPD. Thyrotrophs, which were unaffected by age, increased 70% following HPD. Gonadotrophs increased with gestational age (LH+ cells 55%; FSH+ cells 19-fold), but this was severely attenuated by HPD. We investigated the possible existence of a reciprocal effect of HPD on multipotential glycoprotein-expressing cells. Co-expression of LH and TSH was extremely rare (< 1%) and unchanged over the last month of gestation or HPD. The increase of gonadotrophs expressing FSH only or LH and FSH was attenuated by HPD. Therefore, the proportions of somatotrophs, lactotrophs and corticotrophs are regulated independently of hypothalamic input in the late gestation fetal pituitary. In marked contrast, the determination of the thyrotroph and gonadotroph lineages over the same time period is subject to complex mechanisms involving hypothalamic factors, which inhibit differentiation and/or proliferation of thyrotrophs, but stimulate gonadotrophs down the FSH lineage. Development of a distinct population of gonadotrophs, expressing only LH, appears to be subject to alternative mechanisms.
The anterior pituitary is a highly heterogeneous organ composed of a variety of cells located throughout the gland. The process by which the individual secretory cells differentiate and proliferate during development is largely unknown. Considerable progress has been made in understanding the role played by transcription factors, but we still know relatively little about the input of the fetal hypothalamus in pituitary cell development. After birth, hypothalamic factors are critical in regulating anterior pituitary (AP) function, but the role played before birth remains largely unknown.
Recent studies suggest that the timely expression of various transcription factors is wholly responsible for the composition of the AP, independent of the hypothalamus (Dasen & Rosenfeld, 1999, 2001; Melmed, 2003). A number of experiments in genetically engineered mice are consistent with this view. For example, corticotrophin-releasing hormone (CRH) receptor knockout mice (Timpl et al. 1998) and thyrotrophin-releasing hormone (TRH) knockout mice have a normal complement of all pituitary cells (Shibusawa et al. 2000), clearly suggesting that these two hypothalamic factors are not required for normal development of the AP.
On the other hand, numerous other studies of the actions of various hypothalamic factors, including TRH, on differentiation and proliferation of AP cells would clearly suggest a potential role in AP development. For example, in cultured adult rat somatotrophs, GH-releasing hormone (GHRH) stimulates somatotroph proliferation (Billestrup et al. 1986). Similarly, in cultured fetal cells, TRH induces thyrotroph differentiation (Heritier & Dubois, 1993) and in pituitary primordia, gonadotropin-releasing hormone (GnRH) effectively stimulates the responsiveness of gonadotrophs during early pituitary differentiation (Kudo et al. 1994). Together, these studies suggest a high likelihood that hypothalamic trophic factors are important for determining the development of pituitary cells during fetal life.
The present study was therefore aimed at assessing the contribution of hypothalamic input on the development of pituitary cells in vivo by measuring the effects of total removal of hypothalamic input through the functional disconnection of the pituitary from the hypothalamus by surgery in late gestational fetal sheep. Hypothalamo-pituitary disconnection (HPD) was performed at 110 days gestation, representing a time many hypothalamic factors are expressed and there is a patent hypophysial portal system (Levidiotis et al. 1991). The well established HPD procedure (Antolovich et al. 1990, 1991; Canny et al. 1998; Clarke et al. 1983; Poore et al. 1999) isolates the pituitary from hypothalamic influence by severing the neural connections to the median eminence while maintaining afferent and efferent blood flow to the pituitary.
Methods
Animals and surgery
All experimental procedures were performed with prior approval from the University of Adelaide Standing Committee on Ethics in Animal Experimentation. Fixed fetal pituitary tissue from Merino ewes (singleton n = 7 and twin pregnancies n = 11) of known gestational age (110 or 141 days gestation; term = 147 ± 3 days gestation) (Houghton et al. 1997). A total of 18 fetal sheep pituitaries were collected.
HPD of fetal sheep (n = 6) was performed at 110 days gestation, as previously described (Clarke et al. 1983; Antolovich et al. 1990; Houghton et al. 1997). All surgery was performed under general anaesthesia using halothane (0.5–4.0%) and N2O : O2 (50 : 50 v/v). Aseptic procedures were followed and halothane concentrations adjusted to maintain the appropriate level of anaesthesia. The sham surgery, also performed at 110 days gestation, involved identical procedures to those for HPD except that no neural tissue was removed and the hypothalamus–pituitary connection remained intact.
Pituitaries were also collected from intact unoperated fetal sheep at 110 days gestation (n = 6; one fetus from twin pregnancies) for use as a gestational age control. All ewes were killed using an overdose of sodium pentobarbitone (200 mg kg−1; Lethobarb; Virbac Pty Ltd, New South Wales, Australia) at either 110 days gestation or 141 days gestation when pituitaries were collected. All fetal sheep were delivered via laparotomy, weighed and killed by decapitation.
Confirmation of HPD
The completeness of the disconnection of the hypothalamus from the pituitary in the tissues used for this study was confirmed in all HPD fetal sheep on the basis of plasma prolactin (PRL) responses to the administration of chlorpromazine (12.5 mg i.v.) and TRH (50 μg i.v.). Basal plasma PRL concentrations were significantly lower in HPD than sham operated fetuses. HPD was further confirmed by the absence of a PRL response to chlorpromazine, and pituitary viability was confirmed by an intact PRL response to TRH. Additionally, macroscopic examination of the lesion site post mortem was used to confirm accurate and complete anatomical disconnection (Houghton et al. 1997).
Immunohistochemistry
All pituitaries were rapidly removed post mortem and immediately fixed in ice-cold 4% formaldehyde in phosphate buffered saline (PBS; 0.01 m, pH 7.4) for 24 h, and embedded in paraffin. Whole pituitary glands were bisected along the coronal plane with the neurointermediate lobe kept intact. Thus, the pars intermedia and pars nervosa served as a positive control for ACTH and negative control tissue for all hormones, respectively. Sets of serial sections were cut at 5 μm thickness, with adjacent sets separated by at least 100 μm. Individual sections were mounted onto poly l-ornithine-coated (Sigma, St Louis, MO, USA) glass slides. Each section-mounted slide underwent a photo-bleaching process under a 50 W halogen light for a minimum of 24 h to reduce auto-fluorescence (Farrand et al. 2006). Each section was immuno-stained for expression of one of the six pituitary hormones.
Antigen retrieval was employed to normalize staining (to be either clearly negative or positive) across sections, thereby enabling the automated quantification described below. Following comparison of the results of several antigen retrieval trial results, Tris-HCl (0.1 m, pH 6.6) was used as the antigen retrieval buffer with optimal results for single-labelled immuno-staining of ACTH, PRL and GH. Tris-base (10 mm, pH 9.0) was used as the antigen retrieval buffer with optimal results for single-labelled immuno-staining of TSH, FSH and LH, and for double-labelled immuno-staining of TSH with LH (TSH–LH). For double-labelled immuno-staining of FSH with LH (FSH–LH), the optimal antigen retrieval buffer was PBS (0.01 m, pH 7.4).
The immuno-staining process began with the rehydration of sections in successive 2 min Histolene™ (Fronine, New South Wales, Australia) and ethanol rinses (100, 90 and 70%) followed by washing in PBS (0.01 m, pH 7.4) three times for 5 min each. For the double-labelling of FSH–LH, heat-induced antigen retrieval was performed in the designated antigen retrieval buffer for 10 min at 121°C. Sections were incubated in blocking solution (PBS, 0.01 m, pH 7.4), 0.01% azide, 10% normal donkey serum (Sigma-Aldrich, Inc., Saint Louis, MO, USA) at room temperature in a humidified chamber (30 min). Single-labelled immunofluorescence was performed by incubating the sections for 24 h at 4°C with specific primary antibodies as indicated in Table 1. Double-labelled immunofluorescence was performed by incubating the sections for 24 h at 4°C with a primary antibody cocktail containing antisera against LH and FSH, as noted in Table 1, to (co)localize gonadotrophins or against LH and TSH to (co)localize LHβ and TSHβ. Following primary antibody incubation, sections were washed in PBS (0.01 m, pH 7.4) and incubated with the appropriate fluorophore-conjugated secondary antibody: Cy3-conjugated donkey anti-mouse IgG, Cy3-conjugated donkey anti-rabbit IgG, Cy3-conjugated donkey anti-guinea pig IgG, and Cy2-conjugated donkey anti-rabbit IgG (1 : 200, Jackson ImmunoReseach, West Grove, PA, USA). Sections were washed in PBS (0.01 m, pH 7.4) and incubated with 3 μm 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI; Molecular Probes Inc., Eugene, OR, USA). The immunostained sections were washed in PBS (0.01 m, pH 7.4) and then mounted in antifade Fluorescent Mounting Medium (Dako, Carpentaria, CA, USA).
Table 1.
Primary antibodies for immunohistochemistry
| Antibody | Dilution | Antigen used to raise antibody | Host species |
|---|---|---|---|
| ACTH | 1 : 50 | Human ACTH (24–39) | Mouse |
| Prolactin | 1 : 100 | Ovine prolactin | Rabbit |
| GH | 1 : 1000 | Ovine GH | Rabbit |
| TSH | 1 : 500 | Ovine TSHβ | Guinea pig |
| LH | 1 : 500 | Ovine LHβ | Rabbit |
| FSH | 1 : 200 | Ovine FSHβ | Guinea pig |
Antibody for ACTH was from Dako, and the other antibodies were from the NIH National Hormone and Peptide Program.
Specificity and cellular localization of immunostaining
Although the specificity of the NIDDK polyclonal anti-ovine antibodies has been well established (e.g. Sheng et al. 1998; Liu et al. 2005), further assessment of the specificity of immunostaining of these pituitary hormones in the present system was accomplished with additional control procedures. These included omission of primary antibodies, replacement of the primary antibodies with 10% normal donkey serum, or incubations with primary antibodies followed by secondary antibodies produced in an inappropriate host species. We observed no immunoreactivity following any of these control procedures. We have previously confirmed specificity of immunostaining for oPRL, oTSHβ and oLHβ (Schwartz et al. 1999) and have validated the use of the mouse monoclonal antihuman ACTH antibody in ovine pituitaries, confirming the specificity of ACTH immunostaining (Farrand et al. 2006). Furthermore, to confirm the specificity of the fluorophore-conjugated secondary antibodies, separate sections incubated with each primary antibody were then incubated with inappropriate secondary antibodies to detect any bound fluorescence.
Epifluorescent imaging of pituitary cells
Immunohistochemical staining was visualized using an AX70 epifluorescence microscope (Olympus, Tokyo, Japan) connected to a digital camera (Photometrics CoolSNAPfx, Roper Scientific, Tucson, AZ, USA). Cy2, Cy3 and DAPI signals were individually imaged using excitation/emission wavelengths of 492/510 nm, 550/570 nm and 358/461 nm. The primary antisera used did not cause any fluorescence signals that would have indicated inappropriate cross-reactions with secondary antisera or spectral bleed-through of signals through microscope filters.
For each fetal pituitary, in each of the three to five sets of sections, spanning anterior to posterior regions, 10–20 randomly selected, non-overlapping fields (medial to lateral and inferior to superior) were photographed for each hormone or combination of hormones targeted on a section at 400× magnification (with oil immersion), using V++ image capture software (Total Turnkey Solutions, New South Wales, Australia). Greyscale images were acquired (1300 × 1030 pixel resolution) for each fluorescent label resulting in two images per field (single-labelled immunostaining) and three images per field (double-labelled immunostaining). All images were saved in tagged image file format.
Quantification
Extensive quantification (more than 120 000 cells per animal group) was determined using the image analysis software, analySIS (Soft Imaging Systems, Munster, Germany) (Farrand et al. 2006). For each photographed field, the total number of nuclei, identified by DAPI, and the number of cells immunopositive for each antigen were identified using a mechanical threshold function that establishes a window of optical density values equating to unequivocal positive staining. The number of nuclei and immunopositive cells in all sections were totalled for each animal per treatment. Results from the six animals per treatment group were compared as described below. For each antigen, the accuracy of the automated quantification method was validated against manual cell counts, whereby the total number of nuclei and immunopositive cells in five non-overlapping fields were assessed.
Statistical analysis
Corticotrophs, somatotrophs, lactotrophs, thyrotrophs and gonadotrophs were defined as any pituitary cell immunopositive for ACTH, GH, PRL, TSH and LH (or FSH), respectively. Double-labelled cells were assigned to population categories based on the phenotypic expression of TSH–LH or FSH–LH. Cell populations were represented as a proportion of the total number of pituitary cells. Analysis was performed on the total number of animals within each treatment group expressing the various hormones. The group proportion of pituitary cells expressing ACTH, GH, PRL, TSH, LH or FSH or combinations of TSH–LH or FSH–LH was calculated. There was an uneven distribution of male and female pituitaries among treatment groups (110 days gestation: 5 male/1 female; Sham: 3 male/3 female; HPD: 5 male/1 female). In the sham treatment group, where there were three males and females, there was no difference in the proportion of AP cells between sexes (data not shown) and therefore data from fetuses of both sexes were pooled in all analyses. Thus, for each pituitary cell type we determined (a) the ontogenic change over late gestation and (b) the effect of HPD on the proportion of pituitary cells in each phenotypic population. Each pituitary cell type is presented as a proportion of pituitary cells. In all cases, results are expressed as the mean (n = 6 per group) ±s.e.m. Data sets were determined to be normally distributed by the Kolmogorov–Smirnov test, after which analyses for significance of effects by age and HPD were assessed by one-way ANOVA. Where ANOVA indicated a significant effect, differences among groups were assessed using Tukey/s test post hoc (Dythan, 2003). Differences were considered statistically significant at P-values less than 0.05.
Results
Specificity of immunostaining
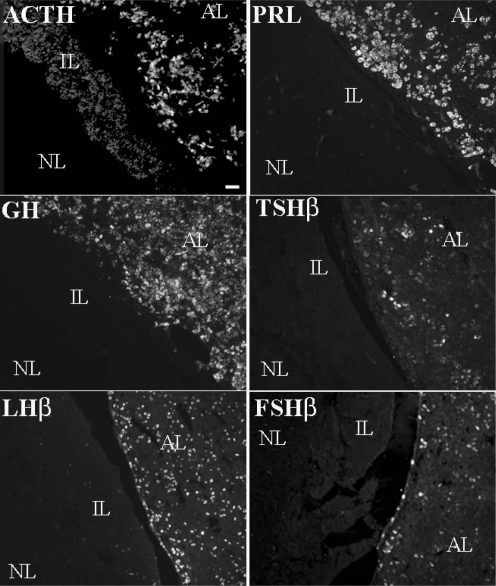
The immunohistochemistry provided clear and specific staining of cells for the various AP hormones and nuclei. ACTH, PRL, GH, TSHβ, LHβ and FSHβ were localized to the cytoplasm of specific pituitary cells (Fig. 1). Staining was not observed for any of these peptides in the neural lobe. Cells within the intermediate lobe stained positively for ACTH in the same proportion as found in an earlier study (Farrand et al. 2006). Preabsorption of ACTH, GH, PRL, TSHβ and LHβ antisera with corresponding peptides completely abolished immunostaining, as previously shown (Schwartz et al. 1999; Farrand et al. 2006). No staining was present when normal donkey serum was substituted for each of the primary antibodies (data not shown). Positive signals were only observed when the optic cube appropriate for each fluorophore was used, and no spectral bleed-through was evident. Additionally, there were no readily identifiable contiguous concentrations of the various cell types.
Figure 1. Single-labelled immunofluorescent staining for ACTH, GH, PRL, TSH, LH and FSH.
Photomicrographs from a 141 days gestation sham-operated fetal sheep pituitary demonstrating single-labelled immunofluorescent staining for ACTH, GH, PRL, TSH, LH and FSH in the anterior lobe (AL), intermediate lobe (IL) and neural lobe (NL). Scale bar = 25 μm.
Effects of ontogeny and HPD on pituitary cell development
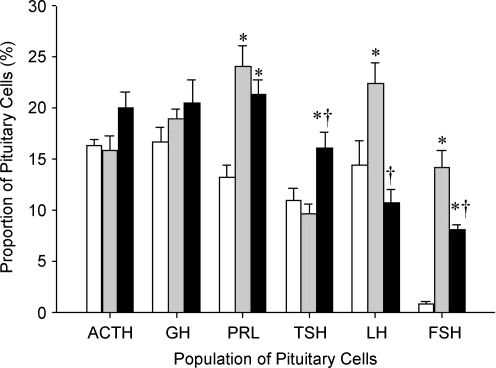
Pituitary cells identified as corticotrophs, somatotrophs and lactotrophs could be all detected at 110–141 days gestation. Despite the likely increase in the total number of AP cells over this period, there was no significant effect of gestational age on the proportion of corticotrophs and somatotrophs (Fig. 2). In contrast, the proportion of lactotrophs effectively doubled between 110 and 141 days gestation (Fig. 2). Thus, from 110 to 141 days gestation corticotrophs and somatotrophs increased in proportion to overall pituitary growth, whereas the proportion of lactotrophs increased disproportionately.
Figure 2. Numbers of AP cells immunopositive for ACTH, GH, PRL, TSH, LH and FSH.
Numbers of AP cells immunopositive for ACTH, GH, PRL, TSH, LH and FSH in 110 days gestation (white), 141 days gestation sham-operated (grey) and 141 days gestation HPD-operated (black) fetal sheep as a percentage of the total number of cells. Values represented as means ±s.e.m.*P < 0.05 compared with 110 days gestation control. †P < 0.05 compared with 141 days gestation sham-operated.
Pituitary cells identified as thyrotrophs and gonadotrophs could be all detected at 110 and 141 days gestation. There was no significant change in the relative proportion of thyrotrophs between 110 and 141 days gestation (Figs. 2, 3). In contrast, there was a marked increase (P < 0.05) in the proportion of gonadotrophs, defined as expressing either LH or FSH. The proportion of cells expressing LH increased by approximately half between 110 and 141 days gestation (Fig. 2). The increase in the proportion of cells staining positive for FSH over the same period is even more dramatic (Fig. 2).
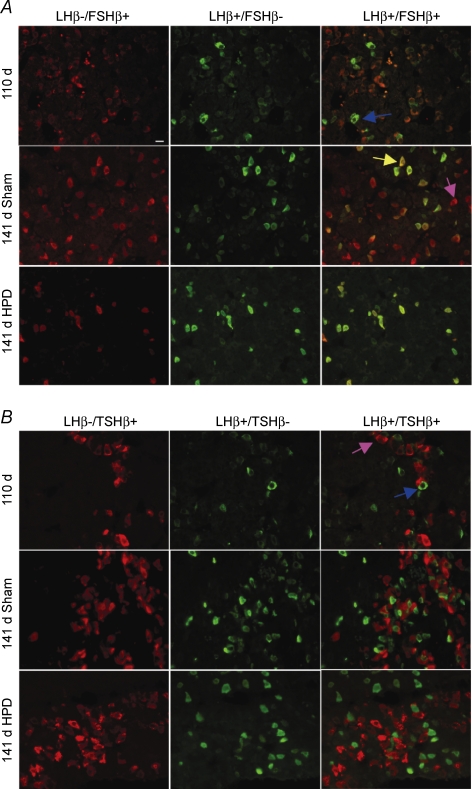
Figure 3. Photomicrographs of fetal sheep pituitary glands from 110 and 141 days gestation sham- and HPD-operated groups showing double-labelled immunofluorescence.
A, LH and FSH (co)expression: LH was found to be coexpressed with FSH in greater proportions of cells at 141 days gestation than at 110 days gestation. As a result of HPD there are visibly fewer cells per field. FSH–LH cells are evident as yellow/orange cells. Very few cells expressing FSH-only (red) are seen. B, LH and TSH (co)expression: TSH–LH double-labelled cells are very rare in all groups. Cells expressing TSH-only (red) or LH-only (green) are seen. Purple arrow, cell stained positive for FSH-only (A) or TSH-only (B). Blue arrow, cell stained positive for LH-only. Yellow arrow, cell stained positive for FSH–LH or TSH–LH. Scale bar applies to all images: 10 μm
There was no significant difference in the proportion of corticotrophs, somatotrophs or lactotrophs at 141 days gestation as a result of disconnection of the fetal pituitary from the hypothalamus.
In contrast to the absence of a gestational effect in the proportion of thyrotrophs in pituitaries from intact fetuses, disconnection of the fetal pituitary from the hypothalamus resulted in a significant increase in TSH-positive cells between 110 and 141 days gestation (P < 0.05). Importantly, compared to sham pituitaries, HPD was also associated with a relative decrease (P < 0.05) in the proportions of cells expressing either LH or FSH at 141 days gestation (Fig. 2).
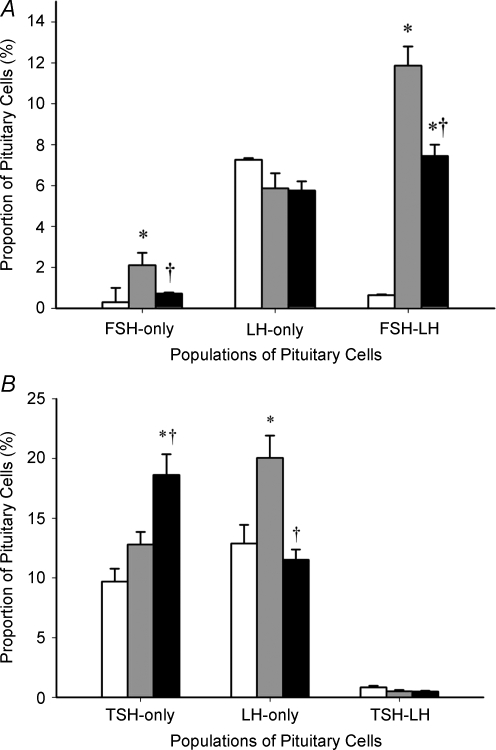
In the dedicated colocalization experiments, we identified cells that express either LH-only (LH+/FSH–); FSH-only (FSH+/LH–); or both FSH and LH (FSH–LH) (Figs. 3A, 4A). Significantly, the proportion of cells expressing LH-only was not affected by either gestational age or HPD. In marked contrast, between 110 and 141 days gestation there was an increase (P < 0.05) in the proportion of the population of cells that express both hormones (FSH–LH). Consistent with the effect observed in single-labelled gonadotrophs, HPD markedly attenuated the normal increase between 110 and 140 days gestation (P < 0.05) of cells coexpressing FSH and LH. Cells expressing FSH-only were present far less frequently than those coexpressing FSH and LH. The qualitative pattern of effects of gestation (increase) and HPD (attenuation of the increase) on FSH-only cells parallels that of the coexpressing cells. The proportional increase over gestation in total LH cells or total FSH cells, as observed in single labelled immunostaining, can thus be attributed to the appearance of the population of cells that coexpress FSH and LH, rather than increases in the respective LH-only and FSH-only cells.
Figure 4. Percentage of pituitary cells expressing FSH-only, LH-only and FSH–LH (A), and TSH-only, LH-only and TSH–LH (B).
Percentage of pituitary cells expressing FSH-only (FSH+/LH–), LH-only (LH+/FSH–), and FSH–LH (A), and TSH-only (TSH+/LH–), LH-only (LH+/TSH–), and TSH–LH (B), in 110 days gestation (white), 141 days gestation sham-operated (grey) and 141 days gestation HPD-operated (black) fetal sheep anterior pituitaries. *P < 0.05 compared with 110 days gestation, †P < 0.05 compared with 141 days gestation sham-operated. NB: for comparison of B with A, the numbers of cells represented by LH-only bars in B include all LH-positive cells that do not coexpress TSH; so these include cells immunopositive for LH+/FSH– as ell as FSH–LH.
Our single-label immunostaining showed an opposite effect of HPD on the proportion of LH and TSH expressing cells, suggesting a reciprocal hypothalamic influence on the expression of glycoprotein hormones. Accordingly, we hypothesized that there might be a population of cells that express both LH and TSH, with the potential to favour one over the other depending on signals from the hypothalamus. However, colocalization of TSH and LH was extremely rare (Figs. 3B, 4B), with fewer than 1% accounted for in the fetal pituitary at 110 days and 141 days gestation. The majority of cells exclusively expressed LH-only (LH+/TSH–) or TSH-only (TSH+/LH–). Note that for comparison of Fig. 4B with Fig. 4A, the numbers of cells represented by LH-only bars in Fig. 4B include all LH-positive cells that do not coexpress TSH; so these include cells immunopositive for LH+/FSH– as well as FSH–LH. Thus, we have identified that cells expressing LH and TSH are restricted to individual pituitary cell types and changes that occur in the proportions of these cells are likely to be attributable to independent changes that occur in different cells.
Discussion
In this study we successfully identified the extent of hypothalamic influence on the relative proportions of pituitary cells over late gestation in fetal sheep. Furthermore, we determined the ontogenic profile of these cells over the last quarter of gestation, as quantitative descriptions are scant. In intact fetuses we found that the proportion of corticotrophs, somatotrophs and thyrotrophs increased in step with the growth of the AP, whereas the proportions of lactotrophs and gonadotrophs increased disproportionately during late gestation.
Surgical removal of hypothalamic input from the pituitary increased the proportion of thyrotrophs and significantly attenuated the increase in proportion of gonadotrophs expressing FSH or LH. Interestingly, removal of hypothalamic input from the pituitary did not alter the proportion of corticotrophs, somatotrophs or lactotrophs. As a result of observing an opposite effect of comparable magnitude of HPD on the proportions of TSH and LH expressing cells, we examined hypothalamic influence on their colocalization. We observed no dual-labelled TSH–LH cells at any of the ontogenic stages studied. Thus, disconnection of the fetal pituitary from the hypothalamus significantly increased the proportion of cells expressing TSH, and decreased the proportion of cells expressing FSH or LH independently of each other. Together, these results suggest that late in gestation the hypothalamus exerts a net negative influence on the differentiation or proliferation of thyrotrophs and a net positive influence on gonadotrophs. It appears to have no net effect on corticotrophs, somatotrophs and lactotrophs.
Together, these results suggest that a normal complement of the most numerous components of the sheep anterior pituitary, lactotrophs and somatotrophs, is established in late gestation independently of hypothalamic input. In addition corticotrophs are unaffected by hypothalamic disconnection, as are a population of gonadotrophs that express only LH. In marked contrast, the increase in FSH cells, but also LH–FSH cells, is severely curtailed by hypothalamic disconnection, and the hypothalamus exerts a tonic inhibitory effect on cells of the thyrotroph lineage. These surprising results show that one or more of the hypothalamo-hypophysial factors inhibit differentiation and/or proliferation of thyrotrophs, but stimulate the FSH gonadotroph lineage.
Between 110 and 140 days gestation, the AP grows. Although weights or volumes of the anterior pituitary were not specifically measured in the present study, it is possible to estimate the growth of the gland over this period at approximately 60% (Smith et al. 1993). Accordingly, while it is also possible to expect that proportional changes in the composition of the anterior pituitary translate into numbers of cells that reflect a 60% increase, it must be borne in mind that changes in weight are not necessarily reflected 1 : 1 with changes in total numbers of cells and that the anterior pituitary also includes hormone-negative cells. In studies in which the effects of HPD on pituitary volume were measured there was no significant change as a result of HPD, and so comparisons between sham and HPD pituitaries are likely to represent the same number (Antolovich et al. 1990).
The proportion of pituitary cells expressing each hormone was not specifically totalled in this study because during development pituitary cells store, as well as release, more than one AP hormone (Villalobos et al. 1997, 2004; Denef et al. 2005). However, rough estimates of the totals at 110 days gestation (71%) compared with 141 days gestation (93% sham; 89% HPD) suggest that a lower proportion of cells are differentiated at 110 days gestation.
The increase in the proportion of lactotrophs represents a maturational change in late gestation. Lactotrophs are the last cells to appear; thus an increase in the proportion of lactotrophs in late gestation was expected. The lack of effect of pituitary disconnection suggests there is negligible hypothalamic influence on development of these cells over this time period. It is noteworthy that the proportion of lactotrophs, even at 141 days gestation, is not as high as usually reported. This is likely to reflect the immature state of the pituitary. The increase in lactotrophs between 110 days and 141 days gestation would suggest, however, that lactotrophs are on a trajectory to become the predominant cell soon after birth.
Removal of hypothalamic input from the pituitary
The greater proportion of thyrotrophs observed in HPD fetal pituitaries at 141 days gestation suggests that hypothalamic input exerts a modulatory influence on thyrotroph development in the fetus during late gestation. Previous findings in TRH-null mice (Shibusawa et al. 2000) suggest that TRH is not required for thyrotroph development, and the present results would support the concept that positive influence from the hypothalamus is not required for increases in TSH-containing cells. HPD removes all hypothalamic factors from the pituitary and the present data are consistent with net inhibitory hypothalamic influence on TSH cells during development. A study in another model of development, fetal rats following encephalectomy, resulted in no change in numbers of thyrotrophs. (Begeot et al. 1981). Although the reasons for discrepant results between HPD and encephalectomy studies are unknown, encephalectomy would clearly involve gestational adaptation and not simply isolation of the AP from the hypothalamus. With respect to HPD, it should also be remembered that this procedure might also be expected to alter feedback on the AP from peripheral target organs. In the case of the hypothalamo-pituitary-thyroid axis, diminished TRH drive would ultimately be expected to result in decreased secretion of thyroid hormones. It is possible that lower feedback inhibition at the pituitary might influence the differentiation/proliferation of thyrotrophs, and this possibility cannot be ignored.
It has been proposed that there is an absolute requirement for gonadotropin-releasing hormone input to gonadotrophs for the maintenance of gonadotrophs, at least in the adult animal (Childs, 1997). On that basis, one would expect there to be a reduction in LH and FSH immunostaining cells in HPD fetal pituitaries and our results clearly support the literature. Remarkably, our results show that the proportion of LH-staining cells falls by only 12%. Although subtle, the decrease in gonadotrophs following HPD suggests development of these cells is dependent on positive hypothalamic input. It has been shown (Pilavdzic et al. 1997) that gonadotrophs expressing LH or FSH are almost entirely absent (< 2%) at 32 weeks gestation in anencephalic human fetuses. This is interesting because both LH and FSH expressing cells are absent and there is clearly a greater impact than we obtained by the simple loss of hypothalamic input. Perhaps additional factors originating outside the hypothalamus may be influencing the development of LH-containing cells. Alternatively, there may be paracrine or autocrine roles within the pituitary cell population.
In the present study, when we examined colocalization of FSH and LH (i.e. FSH–LH cells) we found that FSH and LH are coexpressed in many cells. This is consistent with earlier reports of coexpressing gonadotrophs such as observed in the juvenile and adult male rhesus monkey (Meeran et al. 2003). We showed that there is a substantial, persistent population of gonadotrophs that express LH-only (i.e. cells LH+/FSH–) and, in contrast to other gonadotrophs, these cells are unaltered by HPD. Together, these data suggest that although some gonadotrophs may develop in the absence of hypothalamic input, achieving a full complement of gonadotrophs that coexpress both gonadotrophins requires hypothalamic signalling. Thus, it is possible that during late gestation, gonadotroph development may be, in part, dependent on hypothalamic input, or at least hypothalamic influence may be maintaining this cell type.
Another unexpected finding is that the substantial proportion of gonadotrophs that only express LH also appear to be unaltered by age, unlike FSH–LH cells. This suggests independent regulation of subpopulations of LH-expressing cells and warrants further investigation.
As our single-label immunostaining clearly showed an opposite effect of comparable magnitude of HPD on the proportion of LH and TSH expressing cells, we examined hypothalamic influence on their colocalization. We hypothesized that during fetal development there might be a population of cells capable of producing glycoprotein hormones, the determination of which is produced being dictated by the hypothalamus. We found that dual-labelled TSH–LH immunopositive cells were extremely rare (< 1%) in all groups we studied. In contrast to the present study in fetal sheep, previous work has shown colocalization of TSH with LH in the mouse (Nunez et al. 2003; Villalobos et al. 2004), and sexually mature ewe (Mignot & Skinner, 2005); explanations for the apparent discrepancy, other than the differences in stage of life are not readily apparent.
The relative proportions of corticotrophs were altered neither by age nor HPD, suggesting either independence from hypothalamic input (Timpl et al. 1998) or that it is required before 110 days gestation. In support of this, it has been shown that expression of ACTH is apparent before the formation of Rathke/s pouch (Kouki et al. 2001). The absence of any significant change in the proportion of corticotrophs over the time period of the present study is consistent with some previous observations (Matthews et al. 1994), but not others in which increases (Braems et al. 1996) or decreases (Antolovich et al. 1989; Perez et al. 1997) were reported. The reason for the inconsistency is not readily apparent, except that a variety of age windows methods and anti-ACTH antibodies were associated with the reports. In any event, the proportion of corticotrophs at 110 days and 140 days in the present study is comfortably within the range of values (10% to 21%) reported across all the aforementioned studies. The presence of the same proportions of somatotrophs and lactotrophs following HPD as in age-matched intact fetuses suggests that these cells develop normally in the absence of hypothalamic input. This is consistent with somatotroph and lactotroph cell numbers in anencephalic human fetuses (Begeot et al. 1984; Pilavdzik et al. 1997).
The differential staining of individual and coexpressing AP cells provided clear and specific cytoplasmic immunostaining. Since fixation in formalin can impair binding of antibodies we included an antigen retrieval step (Shi et al. 2001), using suitable buffers, at 121°C for 10 min. This treatment evened out binding across all sections and enhanced only the specific binding.
To our knowledge this is the first time the late gestational developmental change and hypothalamic influence on the separate expression of all pituitary hormones in the fetal sheep pituitary have been assessed in a single study. We have demonstrated an overall increase in thyrotrophs following HPD, possibly due to removal of a modulatory hypothalamic influence. In contrast, we have shown that HPD decreases the proportion of gonadotrophs expressing FSH–LH or only FSH, suggesting hypothalamic input is essential for maintenance of these gonadotrophs. Cells expressing only LH appear to be independently regulated during late gestation. In addition, we found colocalization of LH and TSH extremely rare indicating that changes in numbers of cells expressing TSH or LH occur independently and that this is restricted to individual pituitary cell types during late gestation. With respect to lactotroph, somatotroph and corticotroph development, maintenance of these cell types during late gestation appears to be independent of hypothalamic input. The findings from our study raise interesting questions regarding identification of non-hypothalamic and/or intrapituitary factors that may influence pituitary cell development during late gestation. Continued research will be required to further investigate the factors that regulate the development of pituitary cell populations and to assess their physiological importance.
Acknowledgments
This work was partially supported by the National Health and Medical Research Council of Australia. Antibodies were obtained from Dr AF Parlow via the NIH National Hormone and Peptide Program. The authors gratefully acknowledge the suggestions of Prof A Levy, Bristol, UK, in the preparation of the manuscript and Dr Michael Davies, University of Adelaide, for his assistance with data analysis.
References
- Antolovich GC, Clarke IJ, McMillen IC, Perry RA, Robinson PM, Silver M, Young R. Hypothalamo-pituitary disconnection in the fetal sheep. Neuroendocrinology. 1990;51:1–9. doi: 10.1159/000125308. [DOI] [PubMed] [Google Scholar]
- Antolovich GC, McMillen IC, Robinson PM, Silver M, Young IR, Perry RA. The effect of hypothalamo-pituitary disconnection on the functional and morphologic development of the pituitary-adrenal axis in the fetal sheep in the last third of gestation. Neuroendocrinology. 1991;54:254–261. doi: 10.1159/000125883. [DOI] [PubMed] [Google Scholar]
- Antolovich GC, Perry RA, Trahair JF, Silver M, Robinson PM. The development of corticotrophs in the fetal sheep pars distalis: the effect of adrenalectomy or cortisol infusion. Endocrinology. 1989;124:1333–1339. doi: 10.1210/endo-124-3-1333. [DOI] [PubMed] [Google Scholar]
- Begeot M, Dubois MP, Dubois PM. Evolution of lactotropes in normal and anencephalic human fetuses. J Clin Endocrinol Metab. 1984;58:726–730. doi: 10.1210/jcem-58-4-726. [DOI] [PubMed] [Google Scholar]
- Begeot M, Dupouy JP, Dubois MP, Dubois PM. Immunocytological determination of gonadotropic and thyrotropic cells in fetal rat anterior pituitary during normal development and under experimental conditions. Neuroendocrinology. 1981;32:285–294. doi: 10.1159/000123174. [DOI] [PubMed] [Google Scholar]
- Billestrup N, Swanson LW, Vale W. Growth hormone-releasing factor stimulates proliferation of somatotrophs in vitro. Proc Natl Acad Sci U S A. 1986;83:6854–6857. doi: 10.1073/pnas.83.18.6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braems GA, Matthews SG, Challis JR. Differential regulation of proopiomelanocortin messenger ribonucleic acid in the pars distalis and pars intermedia of the pituitary gland after prolonged hypoxemia in fetal sheep. Endocrinology. 1996;137:2731–2738. doi: 10.1210/endo.137.7.8770892. [DOI] [PubMed] [Google Scholar]
- Canny BJ, Young IR, Veldhuis JD. Hypothalamo-pituitary disconnection of the late-gestation ovine fetus results in profound changes in cortisol secretion that are not reflected in commensurate changes in adrenocorticotropin secretion. Endocrinology. 1998;139:3210–3219. doi: 10.1210/endo.139.7.6086. [DOI] [PubMed] [Google Scholar]
- Childs GV. Cytochemical studies of multifunctional gonadotropes. Microsc Res Tech. 1997;39:114–130. doi: 10.1002/(SICI)1097-0029(19971015)39:2<114::AID-JEMT3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Clarke IJ, Cummins JT, de Kretser DM. Pituitary gland function after disconnection from direct hypothalamic influences in the sheep. Neuroendocrinology. 1983;36:376–384. doi: 10.1159/000123484. [DOI] [PubMed] [Google Scholar]
- Dasen JS, Rosenfeld MG. Signaling mechanisms in pituitary morphogenesis and cell fate determination. Curr Opin Cell Biol. 1999;11:669–677. doi: 10.1016/s0955-0674(99)00034-4. [DOI] [PubMed] [Google Scholar]
- Dasen JS, Rosenfeld MG. Signaling and transcriptional mechanisms in pituitary development. Annu Rev Neurosci. 2001;24:327–355. doi: 10.1146/annurev.neuro.24.1.327. [DOI] [PubMed] [Google Scholar]
- Denef C, Pals K, Hauspie A, Vankelecom H, Seuntjens E. Combinatorial expression of phenotypes of different cell lineages in the rat and mouse pituitary. Ann N Y Acad Sci. 2005;1040:84–88. doi: 10.1196/annals.1327.010. [DOI] [PubMed] [Google Scholar]
- Dythan C. Choosing and Using Statistics: A Biologist/s Guide. 2. Malden, Massachusetts, USA: Blackwell Publishing; 2003. [Google Scholar]
- Farrand K, McMillen IC, Tanaka S, Schwartz J. Subpopulations of corticotrophs in the sheep pituitary during late gestation: effects of development and placental restriction. Endocrinology. 2006;147:4762–4771. doi: 10.1210/en.2005-1522. [DOI] [PubMed] [Google Scholar]
- Heritier AG, Dubois PM. Influence of thyroliberin on the rat pituitary cell type differentiation: an in vitro study. Endocrinology. 1993;132:634–639. doi: 10.1210/endo.132.2.7678797. [DOI] [PubMed] [Google Scholar]
- Houghton DC, Young IR, McMillen IC. Photoperiodic history and hypothalamic control of prolactin secretion before birth. Endocrinology. 1997;138:1506–1511. doi: 10.1210/endo.138.4.5041. [DOI] [PubMed] [Google Scholar]
- Kouki T, Imai H, Aoto K, Eto K, Shioda S, Kawamura K, Kikuyama S. Developmental origin of the rat adenohypophysis prior to the formation of Rathke/s pouch. Development. 2001;128:959–963. doi: 10.1242/dev.128.6.959. [DOI] [PubMed] [Google Scholar]
- Kudo A, Park MK, Kawashima S. Effects of gonadotropin-releasing hormone (GnRH) on the cytodifferentiation of gonadotropes in rat adenohypophysial primordia in organ culture. Cell Tissue Res. 1994;276:35–43. doi: 10.1007/BF00354782. [DOI] [PubMed] [Google Scholar]
- Levidiotis M, Perry RA, Wintour EM, Oldfield BJ. Analysis of the appearance of fenestrations in the blood vessels of the fetal sheep pituitary. Neuroendocrinology. 1991;53:222–228. doi: 10.1159/000125722. [DOI] [PubMed] [Google Scholar]
- Liu J, Liu Z, Yi S, Cui S. Islet-1 expression and its colocalization with luteinising hormone, thyroid-stimulating hormone and oestrogen receptor alpha in the developing pituitary gland of the sheep foetus. J Neuroendocrinol. 2005;17:773–780. doi: 10.1111/j.1365-2826.2005.01364.x. [DOI] [PubMed] [Google Scholar]
- Matthews SG, Han X, Lu F, Challis JR. Developmental changes in the distribution of pro-opiomelanocortin and prolactin mRNA in the pituitary of the ovine fetus and lamb. J Mol Endocrinol. 1994;13:175–185. doi: 10.1677/jme.0.0130175. [DOI] [PubMed] [Google Scholar]
- Meeran D, Urbanski HF, Gregory SJ, Townsend J, Tortonese DJ. Developmental changes in the hormonal identity of gonadotroph cells in the rhesus monkey pituitary gland. J Clin Endocrinol Metab. 2003;88:2934–2942. doi: 10.1210/jc.2002-021001. [DOI] [PubMed] [Google Scholar]
- Melmed S. Mechanisms for pituitary tumorigenesis: the plastic pituitary. J Clin Invest. 2003;112:1603–1618. doi: 10.1172/JCI20401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignot M, Skinner DC. Colocalization of GH, TSH and prolactin, but not ACTH, with bLH-immunoreactivity: evidence for pluripotential cells in the ovine pituitary. Cell Tissue Res. 2005;319:413–421. doi: 10.1007/s00441-004-1009-0. [DOI] [PubMed] [Google Scholar]
- Nunez L, Villalobos C, Senovilla L, Garcia-Sancho J. Multifunctional cells of mouse anterior pituitary reveal a striking sexual dimorphism. J Physiol. 2003;549:835–843. doi: 10.1113/jphysiol.2003.040758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez FM, Schwartz J, Rose JC. Developmental changes in ovine corticotrophs in vitro. Endocrinology. 1997;138:916–921. doi: 10.1210/endo.138.3.4972. [DOI] [PubMed] [Google Scholar]
- Pilavdzic D, Kovacs K, Asa SL. Pituitary morphology in anencephalic human fetuses. Neuroendocrinology. 1997;65:164–172. doi: 10.1159/000127177. [DOI] [PubMed] [Google Scholar]
- Poore KR, Canny BJ, Young IR. Adrenal responsiveness and the timing of parturition in hypothalamo-pituitary disconnected ovine foetuses with and without constant adrenocorticotrophin infusion. J Neuroendocrinol. 1999;11:343–349. doi: 10.1046/j.1365-2826.1999.00340.x. [DOI] [PubMed] [Google Scholar]
- Schwartz J, Ray DW, Perez FM. Leukemia inhibitory factor as an intrapituitary mediator of ACTH secretion. Neuroendocrinology. 1999;69:34–43. doi: 10.1159/000054401. [DOI] [PubMed] [Google Scholar]
- Sheng C, McNeilly AS, Brooks AN. Immunohistochemical distribution of oestrogen receptor and luteinizing hormone B subunit in the ovine pituitary gland during foetal development. J Neuroendocrinol. 1998;10:713–718. doi: 10.1046/j.1365-2826.1998.00255.x. [DOI] [PubMed] [Google Scholar]
- Shi SR, Cote RJ, Taylor CR. Antigen retrieval techniques: current perspectives. J Histochem Cytochem. 2001;49:931–937. doi: 10.1177/002215540104900801. [DOI] [PubMed] [Google Scholar]
- Shibusawa N, Yamada M, Hirato J, Monden T, Satoh T, Mori M. Requirement of thyrotropin-releasing hormone for the postnatal functions of pituitary thyrotrophs: ontogeny study of congenital tertiary hypothyroidism in mice. Mol Endocrinol. 2000;14:137–146. doi: 10.1210/mend.14.1.0404. [DOI] [PubMed] [Google Scholar]
- Smith P, O WS, Hudson NL, Shaw L, Heath DA, Condell L, Phillips DJ, McNatty KP. Effects of the Booroola gene (FecB) on body weight, ovarian development and hormone concentrations during fetal life. J Reprod Fertil. 1993;98:41–54. doi: 10.1530/jrf.0.0980041. [DOI] [PubMed] [Google Scholar]
- Timpl P, Spanagel R, Sillaber I, Kresse A, Reul JM, Stalla GK, Blanquet V, Steckler T, Holsboer F, Wurst W. Impaired stress response and reduced anxiety in mice lacking a functional corticotropin-releasing hormone receptor 1. Nat Genet. 1998;19:162–166. doi: 10.1038/520. [DOI] [PubMed] [Google Scholar]
- Villalobos C, Nunez L, Frawley LS, Garcia-Sancho J, Sanchez A. Multi-responsiveness of single anterior pituitary cells to hypothalamic-releasing hormones: a cellular basis for paradoxical secretion. Proc Natl Acad Sci U S A. 1997;94:14132–14137. doi: 10.1073/pnas.94.25.14132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalobos C, Nunez L, Garcia-Sancho J. Anterior pituitary thyrotropes are multifunctional cells. Am J Physiol Endocrinol Metab. 2004;287:E1166–E1170. doi: 10.1152/ajpendo.00194.2004. [DOI] [PubMed] [Google Scholar]