Abstract
Lung vagal sensory fibres are broadly categorized as C fibres (nociceptors) and A fibres (non-nociceptive; rapidly and slowly adapting low-threshold stretch receptors). These afferent fibre types differ in degree of myelination, conduction velocity, neuropeptide content, sensitivity to chemical and mechanical stimuli, as well as evoked reflex responses. Recent studies in nociceptive fibres of the somatosensory system indicated that the tetrodotoxin-resistant (TTX-R) voltage-gated sodium channels (VGSC) are preferentially expressed in the nociceptive fibres of the somatosensory system (dorsal root ganglia). Whereas TTX-R sodium currents have been documented in lung vagal sensory nerves fibres, a rigorous comparison of their expression in nociceptive versus non-nociceptive vagal sensory neurons has not been carried out. Using multiple approaches including patch clamp electrophysiology, immunohistochemistry, and single-cell gene expression analysis in the guinea pig, we obtained data supporting the hypothesis that the TTX-R sodium currents are similarly distributed between nodose ganglion A-fibres and C-fibres innervating the lung. Moreover, mRNA and immunoreactivity for the TTX-R VGSC molecules NaV1.8 and NaV1.9 were present in nearly all neurons. We conclude that contrary to findings in the somatosensory neurons, TTX-R VGSCs are not preferentially expressed in the nociceptive C-fibre population innervating the lungs.
Sensitivity to tetrodotoxin (TTX) has long been used to pharmacologically differentiate among different types of voltage-gated sodium channels. More recently, molecular studies have identified nine specific voltage-gated sodium channel α chain subtypes (NaV1.1 to NaV1.9). Among these only NaV1.5, NaV1.8 and NaV1.9 are TTX resistant (TTX-R) (Catterall et al. 2005). The TTX-R sodium channels are of particular interest in the somatosensory system because they appear to be selectively expressed in nociceptive type neurons (i.e. small diameter capsaicin-sensitive neurons). Virtually all nociceptive neurons express both TTX-sensitive (TTX-S) and TTX-R currents, whereas the vast majority of large diameter capsaicin-insensitive low-threshold mechano-sensory neurons in the dorsal root ganglia express a single TTX-S current (Caffrey et al. 1992; Arbuckle & Docherty, 1995; Tate et al. 1998). Molecular, biophysical and immunohistochemical studies have revealed that the TTX-R current in somatosensory nociceptors includes both NaV 1.8 and NaV 1.9 channels (Akopian et al. 1996; Sangameswaran et al. 1996; Dib-Hajj et al. 1998; Cummins et al. 1999; Benn et al. 2001). Ablation of Runx1, which encodes a transcription factor controlling the expression of multiple receptor and ion channels relevant to nociception, virtually eliminates the expression of TRPV1 and NaV1.9 in mouse dorsal root ganglia neurons (Chen et al. 2006). Inflammatory mediators that increase pain sensitivity can increase current through TTX-R sodium channels (Gold et al. 1996), and genetic deletion of TTX-R INa channels can decrease inflammation induced hyperalgesia (Amaya et al. 2006). Based on this, there has been a large effort in developing blockers of TTX-R INa in an attempt to selectively decrease the activity of nociceptors inflammatory and neuropathic pain (Lai et al. 2004).
There has been less attention given to the relative expression of sodium channels in vagal afferent nerves innervating visceral tissues. In guinea pigs (and other mammals) a substantial number of nodose ganglion neurons (in addition to jugular and a small percentage of dorsal root ganglion neurons) project capsaicin-sensitive nociceptive C-fibres to the lungs. Like somatosensory nociceptors these nerves are activated by capsaicin, various inflammatory mediators, and noxious physical stimuli. Unlike the somatosensory nociceptors, pulmonary nociceptors do not transmit pain. Rather their activation can lead to parasympathetic reflex bronchospasm and mucus secretion, changes in breathing patterns, dyspnoeic sensations, and urge to cough sensations. In airway inflammatory disorders, the activity of nociceptors may be inappropriate thereby contributing to the signs and symptoms of the disease. Accordingly, there is interest at finding mechanisms that can selectively inhibit respiratory nociceptor activity. There are also, however, an approximately equal number of nodose neurons that project non-nociceptive capsaicin-insensitive low-threshold mechanosensors to the lungs. The low-threshold stretch receptors comprise rapidly and slowly adapting receptors (RARs and SARs) that conduct action potentials at greater than 10 m s−1 in the guinea pig (Bergren & Sampson, 1982; Bergren & Peterson, 1993). In addition to the nociceptive C-fibres and the stretch receptors, a third subpopulation of nodose neurons also project touch-sensitive cough evoking fibres to the larynx, trachea and extrapulmonary bronchi that conduct action potentials at ∼3–5 m s−1, which is 3–5 times faster than C-fibres and 3–4 times slower than the vagal stretch receptors (Canning et al. 2004).
In the present study, experiments were designed to evaluate and compare the sodium currents among three major afferent nodose phenotypes innervating the adult guinea pig lung: nociceptive C-fibre neurons, capsaicin-insensitive pulmonary stretch receptor neurons, and tracheal touch-sensitive fibres. The data support the hypothesis that the TTX-R expression in nodose vagal afferent neurons innervating the respiratory tract is independent of nerve phenotype.
Methods
Retrograde tracing of neurons innervating the lungs and trachea
Surgical procedures were approved by the Johns Hopkins University Animal Care and Use Committee. Young adult male Hartley guinea pigs were obtained from Hilltop Laboratory Animals, Inc. (Scottdale, PA, USA). Animals were anaesthetized with ketamine (50 mg ml−1) and xylazine (2.5 mg ml−1) dissolved in phosphate buffered saline (PBS). Supplemental anaesthesia was given as needed to abolish the hindpaw-pinch reflex. A tracheotomy was performed. For lung-labelling experiments, the head and thorax of the animal were elevated for delivery of the fluorescent tracer DiI (2% in DMSO then diluted 1 : 10 in saline; Invitrogen Corp., Carlsbad, CA, USA). The dye was injected into the lumen of the trachea using a bent syringe needle angled caudally such that the tip was positioned just rostral to the carina. For trachea-labelling experiments, DiI (1% in ethanol) was injected into the submucosa at multiple sites along the trachea. The animals were sutured and allowed to recover for approximately 2 weeks for sufficient labelling of cell bodies in the vagal ganglia. All animals were closely monitored on an hourly basis for several hours postoperatively and twice daily thereafter. Any animal that displayed behaviours indicating excessive pain or infection was killed immediately by overdose of CO2.
Patch clamp recordings from lung-specific nodose ganglion neurons with attached vagus nerve
The tissue preparation was similar to that reported by Li & Schild (2002). Animals were killed using CO2 asphyxiation. The nodose ganglion with ≥ 15 mm of the vagus nerve attached was removed from an adult guinea pig that had been lung-labelled with DiI. The sheath-like covering surrounding the ganglia was carefully removed using a scalpel. A small slice was made by hand parallel to the vagus nerve axis on the side of the ganglion where cell soma are concentrated (opposite to the fibres of passage). The ganglion was anchored into a Plexiglas recording chamber filled with Sylgard using fine metal pins. The ganglion was immersed in an enzymatic cocktail of dispase (2 mg ml−1) and collagenase (2 mg ml−1) dissolved in Hanks' balanced salt solution (HBSS) without calcium or magnesium at 37°C for ∼20 min. The enzymatic solution was replaced with Locke's solution (see below). Additional focal application of the enzymatic cocktail was applied via a large-diameter patch pipette when necessary.
Somatic action potentials were recorded using a conventional patch clamp recording in current clamp mode. Nodose afferent fibres were stimulated using a suction electrode attached to the cut end of the vagus nerve. Fibre conduction velocity was calculated by dividing the distance from the stimulating electrode to the recording electrode by the interval from the initiation of the shock artifact to the initiation of the action potential wave form. Recordings were made at 37°C.
Cell culture
The cell culture is a modification of the protocol performed previously (Lee et al. 2005). Briefly, animals were killed using CO2 asphyxiation. The nodose ganglia were harvested and each ganglion was cut into two or three pieces and incubated in an enzymatic cocktail of dispase (2 mg ml−1) and collagenase (2 mg ml−1) dissolved in HBSS without calcium or magnesium at 37°C for 30 min. The cells were gently triturated with a large-bore fire-polished Pasteur pipette. Two additional iterations of enzyme digest (37°C for 15 min) followed by progressively smaller bore pipette triturations were performed. The cells were washed three times in L-15 medium supplemented with 10% fetal bovine serum and centrifuged at ∼600 g for 2 min. To increase the density of culture, the pellet was resuspended in a small volume of L-15 medium supplemented with 10% fetal bovine serum, pipetted (25 μl) onto the centre coverglass treated with poly d-lysine (0.1 mg ml−1) and laminin (0.005 mg ml−1), and incubated at 37°C for 2 h. The cells were flooded with additional L-15 supplemented with 10% fetal bovine serum and incubated at 37°C. Most recordings were made within 12 h to 24 h. In one set of experiments, recordings were made 2 h to 6 h after culture.
Patch clamp electrophysiology
Voltage and current clamp recordings were made using standard whole cell patch clamp techniques. Patch pipettes (1–3 MΩ) were fabricated from standard thickness capillary tube with filament (WPI, Sarasota, FL, USA) using a Flaming–Brown pipette puller (Sutter Instrument, Novato, CA, USA). The electrode tips were wrapped with strips of Parafilm to reduce pipette capacitance. The intracellular solution was composed of (mm): 100 CsCl, 40 tetraethylammonium-Cl, 10 NaCl, 1 CaCl2, 10 MgCl2, 10 Hepes, 11 EGTA and 2 Mg-ATP, pH 7.2, 334 mosmol l−1. Reduced sodium solutions were used to improve recording of sodium currents. For the recording of sodium currents, the bath solution was composed of (mm): 35 NaCl, 10 CsCl, 65 choline-Cl, 0.1 CdCl2, 1 CaCl2, 4 MgCl2, 35 tetraethylammonium-Cl, 10 Hepes and 10 dextrose, pH 7.4, 325 mosmol l−1. Recordings were made at room temperature. The bath solution for recording of capsaicin- and α,β-methylene ATP-activated currents was Locke's solution, which was composed of (mm): 136 NaCl, 5.6 KCl, 2.2 CaCl2, 1.2 MgCl2, 14.3 NaHCO3, 1.2 sodium phosphate and 10 dextrose, pH 7.4, 336 mosmol l−1, bubbled with a 95% O2 and 5% CO2 mixture. Electrophysiological recordings were made using a 700-A Multiclamp amplifier and Digidata 1320A (Molecular Devices, Sunnyvale, CA, USA). Series resistance compensation was set at ≥ 70%. Sodium channel current signals were sampled at 50 kHz and filtered at 10 kHz. Capsaicin- and α,β-methylene ATP-activated current signals were sampled at 50 kHz and filtered at 2 kHz or 10 kHz. Current clamp signals were sampled at 100 kHz and filtered at 10 kHz.
Steady-state inactivation curves were generated by stepping from a series of prepulse potentials from −120 mV to 0 mV for 1 s to a test pulse of −10 mV. Sweep-to-sweep interval was 10 s. TTX-R INa was obtained by recording in the presence of TTX (0.3 μm). Digital subtraction of the TTX-R INa from the total INa revealed the TTX-S component. Peak currents evoked at the test potential were plotted against prepulse potential and normalized to maximal current.
The voltage-clamp protocol used to isolate NaV1.8 from NaV1.9 was similar to that used by Cummins et al. (1999). Time dependency has been shown for the NaV1.9 current; therefore, all recordings were made within 5 min after membrane rupture (Coste et al. 2004; Maruyama et al. 2004). Recordings were performed with fluoride as the primary anion in the pipette solution to improve resolution of the NaV1.9 current; however, fluoride also shifts voltage dependence in a hyperpolarizing direction (Coste et al. 2004). Intracellular recording solution was composed of (mm): 140 CsF, 10 NaCl, 10 Hepes, 10 EGTA and 2 Mg-ATP, pH 7.2, 315 mosmol l−1. The bath solution was composed of (mm): 65 choline-Cl, 35 NaCl, 35 tetraethylammonium-Cl, 10 CsCl, 10 Hepes, 10 dextrose, 4 MgCl2, 1 CaCl2 and 0.1 CdCl2, pH 7.4, 325 mosmol l−1. Total TTX-R INa was generated with 100 ms voltage steps from −90 mV to 30 mV from a resting potential of −120 mV in the presence of 0.3 μm TTX. Sweep-to-sweep interval was 10 s. To isolate the NaV1.8 current, the same voltage protocol was used but with a prepulse of −40 mV for 500 ms prior to the voltage steps. Subtraction of the NaV1.8 current from the total TTX-R INa revealed the NaV1.9 current.
Chord conductance was obtained by dividing peak current by the driving force in the form:
where G is conductance, Vm is the command potential, and Erev is the reversal potential, as determined by extrapolation or interpolation of the linear portion of the I–V curve through 0 current. Activation and steady state inactivation curves were fitted using a Boltzmann function in the form
where Gmax is the fitted maximal conductance, V0.5 is half activation potential or availability, Vm is the command potential, and k is the slope factor.
Cells were visualized for recording using an upright microscope (Carl Zeiss Corp., Jena, Germany). DiI fluorescence was visualized with a rhodamine optical filter set. A gravity-feed perfusion system was used to change bath solution and to deliver drugs (∼8 ml min−1). A complete solution change in the recording chamber was estimated to be < 1 min. An in-line heating system was used for experiments in which the temperature was set to physiological levels.
Cells were categorized as sensitive to capsaicin if the evoked current magnitude was > −2 pA pF−1 (−100 pA for an average cell) and displayed an appropriate time course for onset and offset of the response.
Real time PCR primers and probes design
Sequences for guinea pig NaV1.1 to NaV1.9 α subunits were identified using the reciprocal-best-blast-hit (RBH) approach (Hirsh & Fraser, 2001). The putative guinea pig NaV1.1 to NaV1.9 α subunit sequences were identified from a search of the Cavia porcellus whole genome shotgun sequences (GenBank accession AAKN00000000) and Cavia porcellus cDNA sequences from GenBank (Table 1). Searches based on either human or mouse individual NaV subtype sequences identified the same guinea pig putative NaV subtype sequence. Primers and probes were designed using Primer3 (Rozen & Skaletsky, 2000) from coding regions contained within the genomic sequences (Table 1).
Table 1.
Probe sequences and accession numbers
| Gene | Probe Sequence | Accession number |
|---|---|---|
| NaV1.1 | AY280243 | |
| Forward primers | TGAATGCCCTTTTAGGAGCAAT | |
| Reverse primers | TGATGCTGAAAATTAGCCAGAATATAAG | |
| Probe sequence | CCATCCATCATGAATGTGCTTCTGGTTTG | |
| NaV1.2 | AAKN01359644 | |
| Forward primers | GGACCTGACAGCTTCCGCTT | |
| Reverse primers | GCGCTCCTGTTTGGGTCTCT | |
| Probe sequence | CCCTTGCTGCTATTGAACAACGCATTGC | |
| NaV1.3 | AAKN01597291 | |
| Forward primers | AGCTTCCAGGGTGGATTGC | |
| Reverse primers | CTGCTTGCTCCCATTCTCAAC | |
| Probe sequence | TGCACCACCAGACTGCGACCCT | |
| NaV1.4 | AAKN01492966 | |
| Forward primers | CGTGTGTAAGATCGCCTCGG | |
| Reverse primers | CCACACAGGATGCGGAAGAC | |
| Probe sequence | TGGCACATGCACGATTTCTTCCACTCC | |
| NaV1.5 | AY263354 | |
| Forward primers | TCTCTGATGGCCCAGAGCAC | |
| Reverse primers | CGCCAGTTTGCCTGAGACAC | |
| Probe sequence | ACCACCACCTCTGAGGCTGAGACTGGG | |
| NaV1.6 | AAKN01442630 | |
| Forward primers | TGGGCAGGTGGTTACTGCAT | |
| Reverse primers | CGGCAGCCTCTCCTCTGTTT | |
| Probe sequence | TCCACATTCAGTCAATGCAACTTAGAACA | |
| NaV1.7 | AAKN01307579 | |
| Forward primers | TTTTGCGGCTGCCCTAGA | |
| Reverse primers | CATGGCAATGAGCTGGACTTT | |
| Probe sequence | CCCCCTCTTCTCATAGCAAAACCTAA | |
| NaV1.8 | CQ891331 | |
| Forward primers | CTCTTCCGAGTCATCCGCCT | |
| Reverse primers | GCCCGATGTTGAAGAGAGCA | |
| Probe sequence | CACGCTGCTCTTTGCCCTCATGATGTC | |
| NaV1.9 | AAKN01691927 | |
| Forward primers | CACCAGGGTCCTCGGAGAGT | |
| Reverse primers | TTGGTGGTGGTGACTATGGG | |
| Probe sequence | CCAGTGGCCTGGACACTATGAAAGCAA |
Primer/probe sets were tested for efficiency and optimized with universal PCR conditions by generating standard curves using 10-fold dilutions of guinea pig genomic DNA and a guinea pig reference mixture. The reference mixture contained cDNA synthesized from RNA extracted from various guinea pig tissues representative of major organs and tissues. All slopes fell well within the acceptable range between −3.2 and −3.5, with an ideal slope of −3.3 for 100% efficiency.
Gene expression using quantitative real-time PCR (qRT-PCR)
Guinea pig jugular, nodose, and dorsal root ganglia were pooled separately and homogenized in 800 μl Trizol Reagent (Invitrogen) using a glass dounce homogenizer. Control tissues heart, skeletal muscle and brain were homogenized separately in Trizol Reagent (Invitrogen) using a single probe homogenizer. After phase separation with chloroform, total RNA was extracted using the RNeasy Mini Kit (Qiagen, Valencia, CA, USA) following the manufacturer's instructions. Any genomic DNA contamination was removed using DNase I (Ambion). RNA samples were judged to be free of genomic DNA contamination by the absence of amplification in a standard TaqMan assay using 10 ng of RNA and ACTB primer/probe oligonucleotides. The RNA was quantified using Ribogreen RNA quantification reagent (Molecular Probes) and quality assessed using the Eukaryote Total RNA Nano chip on a Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA, USA). cDNA was synthesized by reverse transcription utilizing the High Capacity cDNA Archive Kit (Applied Biosystems).
The equivalent of 125 ng mRNA per well was arrayed into 384-well plates and quantitative RT-PCR was carried out using a 7900HT Sequence Detector System (Applied Biosystems) in a 12.5 μl total reaction volume. TaqMan Universal PCR Master Mix 2X (Applied Biosystems) and universal PCR conditions recommended by the manufacturer were followed. Each sample was assayed using three technical replicates.
The readout for qRT-PCR was taken as the number of PCR cycles needed to achieve a threshold level of fluorescence (CT). Raw abundance is calculated from the CT by applying the formula
To normalize the data, raw abundance values for the each of the nine NaVα subunit transcripts are scaled relative to each other by the geometric mean of the set of valid housekeeper (HK) data points for that sample, using the equation HK″w,g= HK′w,g/mean (HK′w,1, HK′w,2, …), i.e. scaled abundance adjusted by the mean of all housekeeper scaled abundances with HKw,g= abundance for well w and housekeeper gene g. Each data point is expressed as the ratio of the HK abundance in the sample to the average of that HK in all samples. A data point is marked invalid if it has statistically inconsistent behaviour with the other HKs in those samples with similar tissue types.
Single cell RT-PCR
Individual fluorescent cells were collected in microcentrifuge tubes that contained 1 μl of RNAse Out (Invitrogen) and immediately placed in a bed of pulverized dry ice. Cells were processed using Superscript III CellsDirect cDNA Synthesis Kit (Invitrogen) according to the manufacturer's instruction. First strand synthesis utilized 1 μl of oligo dT, and 1 μl of random hexamers (Roche Applied Science, Indianapolis, IN, USA) was used for each reverse transcriptase (RT) reaction. Each cell was split into two half-reactions yielding a RT product and a no-RT product. RT reaction conditions were 50°C for 50 min followed by 85°C for 5 min. PCR was used to amplify five targets per cell. HotStar Taq DNA Polymerase Kit (Qiagen) was used according to the manufacturer's instructions. Each PCR amplification utilized 20% of +RT-product. Final Mg2+ concentration was 2.0 mm. PCR reaction conditions were initial activation at 95°C for 15 min; denaturation at 94°C for 30 s, annealing at 60°C for 30 s, and extension at 72°C for 60 s for 45 cycles; and final extension at 72°C for 10 min. A parallel set of PCR reactions for each target was performed for each cell using the no-RT control. Electrophoresis of PCR products was run on 1.5% agarose gels.
Primers used for single cell RT-PCR were the same as those used for real-time RT-PCR in the whole ganglia with the exception of NaV1.7 because the alternative primer set yielded a larger PCR product for better spatial resolution when run on our agarose gels. Cells were selected for analysis based on the identical criteria for selecting cells for patch clamping: (1) individual cells were not attached to extraneous material, (2) cells possessed bright and uniform fluorescence, and (3) cells were firmly attached to the coverslip. As a negative control, a sample of bath solution adjacent to a collected cell was taken from each of the coverplates from which a cell was collected. Also, non-neuronal tissue from some of the coverplates was collected and processed using the identical protocol.
Immunohistochemistry
Nodose ganglia were removed from adult guinea pigs and fixed for 24 h in 4% paraformaldehyde, prior to conventional paraffin processing in wax blocks. Sections of ganglia were cut at a thickness of 4 μm and dried overnight at 37°C. Sections were dewaxed and rehydrated prior to antigen retrieval step involving microwaving (Milestone, Sorisole, Italy) at 100°C for 20 min in pH 6 citrate buffer followed by 20 min cooling. Slides were then rinsed in PBS and stained overnight at 4°C with rabbit primary antibodies to NaV1.7 (1 : 1500), NaV1.8 (1 : 3000), or NaV1.9 (1 : 1500), purchased from Alomone Laboratories Ltd (Jerusalem, Israel). Negative controls were incubated with rabbit isotype IgG at the same concentration and conditions as the primary antibodies. All sections were washed in PBS, incubated with a biotin labelled antirabbit antibody (BioGenex Laboratories, San Ramon, CA, USA) for 20 min, followed by further washing in PBS. A streptavidin–horseradish peroxidase complex (BioGenex Laboratories) was then applied for 20 min followed by further washing in PBS. The entire complex was then visualized using DAB substrate (BioGenex Laboratories) for 5 min. After extensive washing in tap water, sections were lightly counterstained in Mayer's haematoxylin and washed in tap water to visualize the nuclei. Sections were then dehydrated in graded alcohols, cleared in xylene and coverslipped using an automatic tape coverslipper (Sakura Tissue-Tek). The sections were examined using a Leica DM RB Research microscope and JVC 3 CCD camera. Representative images were captured and annotated using an Acquis software package (Synoptics Ltd, Cambridge, UK).
Quantification of IHC data was obtained by analysing tissue slices randomly selected from sectioned ganglia.
Isolated lung–nerve preparation
The ex vivo trachea/bronchus preparation was prepared for extracellular recording of action potential from nodose vagal afferent nerve fibres that had defined receptive fields in the airway wall, as previously described (Riccio et al. 1996b). The airway along with intact right-side extrinsic vagal innervation was removed and placed in a dissecting dish containing Krebs bicarbonate buffer solution (KBS) gassed with 95% O2–5% CO2 and composed of (mm): NaCl, 118; KCl, 5.4; NaH2PO4, 1.0; MgSO4, 1.2; CaCl2, 1.9; NaHCO3, 25.0; dextrose, 11.1. Connective tissue was trimmed away, leaving the trachea, larynx and right mainstem bronchus with intact nerves (vagus, superior laryngeal and recurrent laryngeal), including nodose ganglia. A longitudinal cut was made through the ventral surface of the larynx, trachea and bronchi and the airways were then pinned, mucosal side up, to a Sylgard-lined Perspex chamber. The right nodose ganglia, along with the rostral-most vagus and superior laryngeal nerves, were gently pulled through a small hole into an adjacent compartment of the same chamber for recording of single fibre activity. Both compartments were superfused with KBS and the temperature was maintained at 35°C with a flow rate of 6–8 ml min−1. Extracellular recordings were performed by manipulating a fine aluminosilicate glass microelectrode filled with 3 m sodium chloride near neuronal cell bodies in the nodose ganglion. Mechanically sensitive receptive fields were revealed when a burst of action potentials was recorded in response to von Frey filament stimulation of the airway mucosal surface. Conduction velocities were calculated by electrically stimulating the receptive field and measuring the distance travelled along the nerve pathway divided by the time between the shock artifact and the recorded action potential. Selective nerve crushes were performed at the end of the study to ascertain the extrinsic pathway of the nerve fibre under study (recurrent laryngeal or superior laryngeal nerve). Only one nerve was studied per tissue.
Statistics
For the electrophysiological studies, comparisons were made using paired or unpaired Student's t test, where appropriate. A P-value < 0.05 was considered significant. Data are expressed as means ±s.e.m.
All materials were purchased from Sigma unless stated otherwise.
Results
INa currents in guinea pig nodose neurons
Macroscopic voltage-gated sodium currents were measured in dissociated nodose ganglion neurons using traditional patch clamp techniques (Fig. 1). These currents reversed polarity at 37.0 ± 1.0 mV (n = 68), which is consistent with the predicted Nernst potential for sodium (32.2 mV) under the present recording conditions. Equimolar replacement of sodium with choline in the bath solution abolished inward currents (n = 4). These findings are consistent with the proposal that the measured currents were carried by sodium ions.
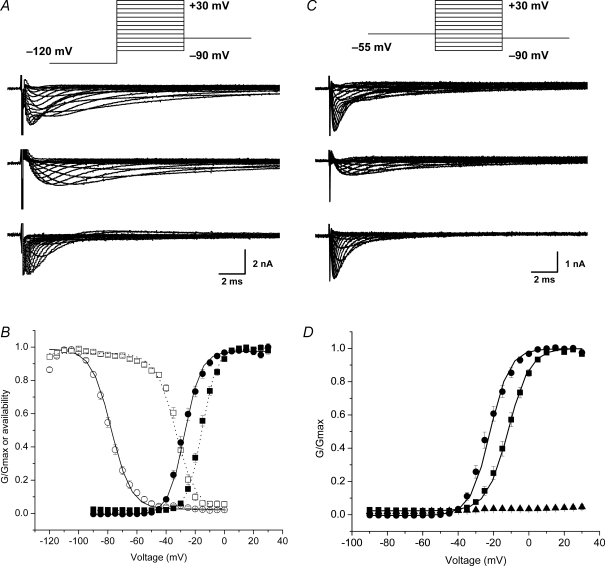
Figure 1. Voltage-gated sodium currents in lung-specific nodose ganglion neurons.
A, pharmacological method for isolating TTX-R INa. Families of currents evoked by 30 ms voltage steps from −90 mV to 30 mV in 5 mV increments from a holding potential of −120 mV. Inset: voltage protocol. Total INa was recorded in a reduced sodium solution (upper traces). Only 20 ms is depicted for clarity. TTX-R INa was recorded in the presence of 0.3 μm TTX (middle traces). Digital subtraction of the TTX-R INa from the total INa revealed the TTX-S component (lower traces). B, activation and steady-state inactivation curves for TTX-S INa (n = 33 and 29, respectively) and TTX-R INa (n = 39 and 31, respectively). Circles represent TTX-S INa. Squares represent TTX-R INa. Filled symbols, activation. Open symbols, steady-state inactivation. Continuous and dotted lines represent Boltzmann fits for TTX-S INa and TTX-R INa, respectively. G is conductance. C, prepulse inactivation method for isolating TTX-R INa. In a separate neuron, total INa was recorded in a reduced sodium solution using the identical voltage protocol in A (upper traces). Middle traces: TTX-R-like INa was generated using a prepulse inactivation step to −55 mV for 500 ms followed by a series of 30 ms steps from −90 mV to 30 mV in 5 mV increments (inset). Digital subtraction of the TTX-R INa from the total INa revealed the TTX-S component (lower traces). D, activation curves for TTX-S INa (n = 23) and TTX-R INa (n = 23). •, TTX-S-like INa. ▪, TTX-R-like INa. ▴, recordings in zero-concentration sodium (n = 4). Data are means ±s.e.m.
Total sodium current (Fig. 1A and C upper panels) was obtained by holding the cell at −60 mV and then stepping to a prepulse potential of −120 mV for 1 s followed by 30 ms voltage steps from −90 mV to 30 mV in 5 mV increments (Fig. 1, inset). TTX-R sodium current was obtained in one of two ways, either by applying TTX (0.3 μm) to the bathing solution (Fig. 1A, middle panel) or by applying a prepulse potential of −55 mV for 500 ms to inactivate TTX-S-like INa (Fig. 1C, middle panel) followed by 30 ms voltage steps from −90 mV to 30 mV in 5 mV increments (Lancaster & Weinreich, 2001; Gold et al. 2002; Gold et al. 2003; Flake & Gold, 2005; Rush et al. 2005). Digital subtraction of the TTX-R-like current from the total INa revealed the TTX-S-like component (Fig. 1A and C bottom panels). As can be seen in Fig. 1B and D, either method produced equivalent results, consistent with findings in other studies. For most of the studies presented below, the TTX-R current was evaluated using the prepulse inactivation method. Biophysical parameters for the TTX-R INa and TTX-S INa listed in Table 2 are consistent with that reported elsewhere (Catterall et al. 2005).
Table 2.
Biophysical properties of voltage-gated INa in vagal nodose ganglion neurons
| TTX method | Voltage method | |
|---|---|---|
| TTX-R INa | ||
| Activation | ||
| V1/2 | −15.47 ± 0.72 | −11.70 ± 1.21 |
| k | 4.91 ± 0.17 | 5.70 ± 0.20 |
| n | 39 | 23 |
| Steady-state inactivation | ||
| V1/2 | −32.82 ± 0.73 | — |
| k | 5.55 ± 0.33 | — |
| n | 31 | — |
| TTX-S INa | ||
| Activation | ||
| V1/2 | −26.99 ± 0.99 | −22.57 ± 1.22 |
| k | 5.98 ± 0.30 | 5.69 ± 0.29 |
| n | 33 | 23 |
| Steady-state inactivation | ||
| V1/2 | −77.83 ± 1.03 | — |
| k | 6.31 ± 0.34 | — |
| n | 29 | — |
| NaV1.8 | ||
| V1/2 | −12.6 ± 2.0 | — |
| k | 7.4 ± 0.7 | — |
| n | 17 | — |
| NaV1.9 | ||
| V1/2 | −54.8 ± 2.8 | — |
| k | 4.8 ± 0.7 | — |
| n | 13 | — |
TTX-R INa in intact lung-specific neurons recorded from the nodose ganglia
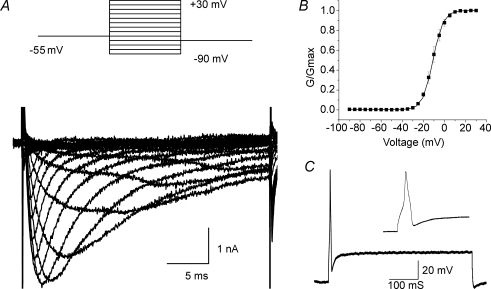
In order to ascertain the possibility that currents obtained in the cultured primary cells are different from those in the intact animal due to culturing artifacts, we made patch clamp measurements from intact neurons within the nodose ganglia. Voltage-gated sodium currents recorded from intact nodose ganglion neurons displayed both TTX-S and TTX-R. Under the recording conditions, measurement of the TTX-S component displayed significant voltage control issues (not shown) making it difficult to obtain the total current density. Nevertheless, the TTX-R INa studied in five neurons in situ displayed a magnitude and kinetics similar to that reported above in cultured neurons (Fig. 2B). The half-activation potential and slope factor of −10.9 ± 1.4 mV and 5.0 ± 0.4 mV, respectively (Fig. 2B; n = 5; 2 of which were capsaicin insensitive), are similar to reported values. We confirmed that TTX-R sodium channels participate in action potential generation in the intact capsaicin-sensitive and capsaicin-insensitive nodose neurons. Somal action potentials were routinely recorded in response to a suprathreshold current step in the presence of TTX (1.0 μm) (Fig. 2C).
Figure 2. TTX-R INa recorded from intact neurons in the nodose ganglion.
A, representative traces from a lung-specific non-nociceptive neuron showing a family of TTX-R INa evoked using a prepulse inactivation protocol (inset; see Fig. 1 legend). B, activation curves fitted using a Boltzmann function (n = 5). G is conductance. C, action potential elicited with a suprathreshold current step in the presence of TTX (1 μm). Inset depicts an expanded time scale. Data are means ±s.e.m.
TTX-R INa in defined neuronal phenotypes innervating the trachea- and lung-labelled nodose ganglion neurons
Lung-specific nodose afferent neurons
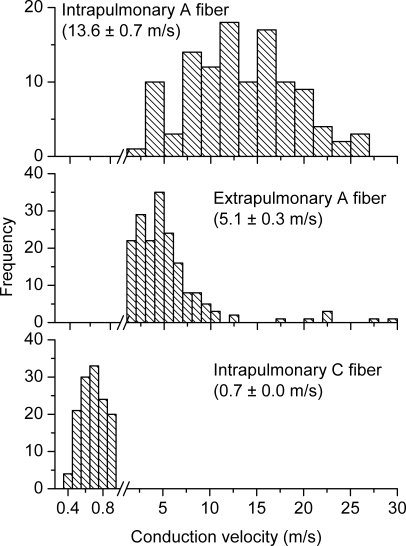
We have previously defined two distinct phenotypes of nodose afferent fibres innervating the guinea pig lungs (Canning et al. 2004; Undem et al. 2004). The nodose nerves innervating the lungs comprise both capsaicin-sensitive C-fibres and relatively fast conducting capsaicin-insensitive A fibres. The nodose lung C-fibres conduct action potentials < 1 m s−1 and are uniformly activated by capsaicin and several inflammatory mediators. The capsaicin-insensitive nodose fibres innervating the lungs conduct action potentials at ∼10–30 m s−1 and are low-threshold stretch-sensitive receptors, i.e. they can be stimulated by modest degrees of lung distension (Canning et al. 2004). They are relatively unresponsive to inflammatory mediators. The lung afferent A-fibres correspond to the classically defined rapidly and slowly adapting receptors (RARs and SARs). Using the isolated innervated guinea pig lung preparation we evaluated 263 nodose nerve fibres with receptive fields within the lung compartment. We found similar numbers of both C-fibre (Fig. 3, lower panel; n = 149; conduction velocity = 0.7 ± 0.0 m s−1) and A fibres (Fig. 3, upper panel; n = 114; conduction velocity = 13.6 ± 0.7 m s−1). We refer to these fibres as Aβ fibres as they can be easily distinguished from Aδ fibres innervating the trachea (see below).
Figure 3. Three distinct phenotypes of nodose fibres innervate airways and lungs of guinea pigs.
Histograms of conduction velocities were obtained from recordings in isolated innervated trachea or lung preparation (see methods in Riccio et al. 1996b; Undem et al. 2004). Nodose ganglion fibres innervating the intrapulmonary compartment segregate into two populations. Upper panel: the faster conducting group (n = 114) has a conduction velocity centred around 13.6 ± 0.7 m s−1, which is classified as Aβ. In general, this population possesses low-threshold mechanosensitivity and is often attributed as stretch receptors. Note the different frequency scale used. Lower panel: the slower conducting group (n = 149) has a conduction velocity centred around 0.7 ± 0.01 m s−1, which falls in the C fibre range. Hallmarks of this group include sensitivity to a variety of chemicals, including capsaicin, but relative insensitivity to mechanical stimulation. Middle panel: nodose nerves innervating the extrapulmonary airways (larynx, trachea and main bronchi) have an intermediate conduction velocity centred around 5.1 ± 0.3 m s−1, which is classified as Aδ (n = 190). This population is not activated by capsaicin, but is highly sensitive to punctate mechanical stimulation, which evokes cough in intact animals. Histograms in upper, middle, and lower panels were binned at 0.1, 1, and 2 m s−1, respectively. Data are means ±s.e.m.
Nodose neurons retrogradely labelled from the lung compartment should non-discriminately label both the capsaicin-sensitive C-fibre (nociceptor) population and the capsaicin-insensitive (non-nociceptor) stretch-receptor population of neurons. Consistent with this we found that that there were similar numbers of capsaicin-sensitive (n = 9) and capsaicin-insensitive (n = 14) lung-specific nodose neurons in our whole cell patch clamp studies. This is in approximate agreement with the analysis of our single fibre recordings.
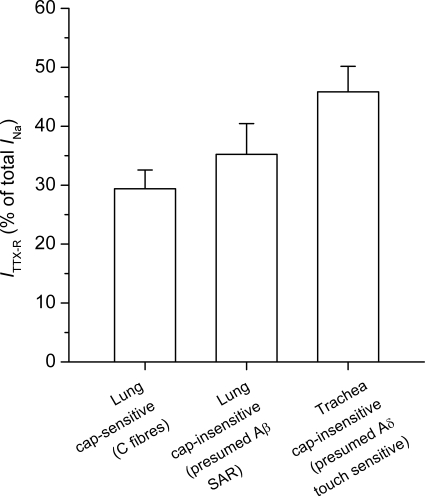
The contribution of TTX-R INa (the peak TTX-R INa as a percentage of the peak INa) was compared between capsaicin-sensitive and capsaicin-insensitive lung-labelled nodose neurons. There was no significant difference (P > 0.1) in the peak TTX-R current between the capsaicin-insensitive and capsaicin-sensitive populations. The peak TTX-R current averaged 35.2 ± 5.2% and 29.4 ± 3.2% of the total current in the capsaicin insensitive and capsaicin-sensitive populations, respectively (Fig. 4).
Figure 4. TTX-R INa in three distinct phenotypes of nodose ganglion neurons innervating the lungs and airways.
TTX-R INa as a percentage of total INa was evaluated in lung-specific capsaicin-sensitive (n = 9), lung-specific capsaicin-insensitive (n = 14), and trachea-specific capsaicin-insensitive (n = 13) neurons. Cap is capsaicin. Data are means ±s.e.m.
As mentioned above, TTX-R INa activated at more depolarized potentials than the total INa (−40 mV versus−50 mV) with a slower time course (e.g. Fig. 1). To address the possibility that the comparison of peak values of the TTX-R and total INa may be skewed by differences in kinetics, we re-analysed the same data by looking at the fitted maximal conductance (Gmax) and area under the curve. Both alternate analyses corroborated the original analysis using peak INa. As analysed by Gmax, the percentage TTX-R for capsaicin-insensitive and capsaicin-sensitive populations was 41.3 ± 4.9% and 33.2 ± 4.0%, respectively (P > 0.1). By analysing the total inward charge (area under the curve), the percentage TTX-R values for capsaicin-insensitive and capsaicin sensitive populations were nearly identical, 61.8 ± 12.3% and 61.6 ± 8.4%, respectively. We also did not detect a difference (P > 0.1) in current density values between capsaicin-insensitive and -sensitive neurons, which were −56.5 ± 10.9 pA pF−1 and −32.86 ± 4.9 pA pF−1, respectively.
Tracheal cough-evoking nodose fibres
The third distinct phenotype of nodose nerves innervating guinea pig airways and lungs is one that is limited to the larynx, trachea and main-bronchi. These nerves conduct action potentials in the Aδ range, about 3–4 times slower than the lung stretch receptors, but about 3–5 times faster than C-fibres (Canning et al. 2004). The tracheal Aδ fibres are not activated by capsaicin, or any inflammatory mediator so far tested, but are exquisitely sensitive to punctate mechanical stimulation, acid, and water. This nerve phenotype evokes cough when activated. In 190 tracheal nodose afferent nerves studied we found that 172 (91%) were Aδ fibres (conduction velocity = 5.6 ± 0.3 m s−1), and 18 (9%) were C-fibres (conduction velocity = 0.97 ± 0.05 m s−1). The overall conduction velocity was 5.1 ± 0.3 m s−1 (Fig. 3, middle panel).
Consistent with our analysis of extracellular recording of nodose tracheal afferent fibres, the vast majority of trachea-specific nodose neurons (13 of 16) were insensitive to capsaicin (1 μm). The peak TTX-R current in this nerve phenotype averaged 45.8 ± 4.3%, a value not statistically different from either of the two phenotypes found retrogradely labelled from the lungs described above (Fig. 4).
Voltage-gated sodium channel α subunit mRNA expression in the adult guinea pig
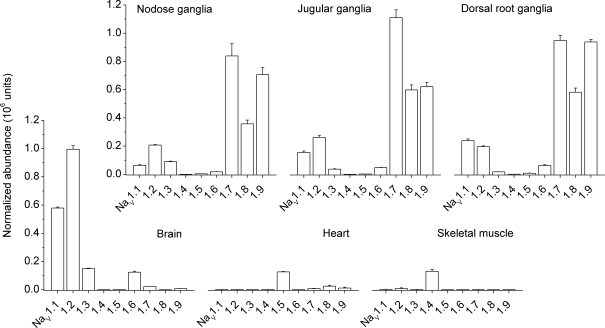
To begin to determine the molecular identity of sodium channels that contribute to the total INa in nodose ganglion neurons, we undertook an analysis using quantitative real-time RT-PCR to examine the mRNA expression of the nine known voltage gated sodium channel α subunits among a variety of guinea pig tissues. The α subunit is the primary functional unit of VGSCs. We detected distinct patterns of tissue expression for each of the sodium channels (Fig. 5) in brain, cardiac muscle, skeletal muscle, nodose ganglia, jugular ganglia and dorsal root ganglia. The tetrodotoxin-sensitive sodium channels NaV1.1 and NaV1.2 displayed wide distribution among neuronal tissues, with a slight preference for brain. NaV1.3 and NaV1.6 were widely and evenly distributed among neuronal tissues. NaV1.4 was found almost exclusively in skeletal muscle. NaV1.5, which is one of the three tetrodotoxin-resistant sodium channels, was found almost exclusively in cardiac tissue. NaV1.7, which is sensitive to tetrodotoxin, and two of the tetrodotoxin-resistant channels, NaV1.8 and NaV1.9, were expressed, as expected, almost exclusively in sensory ganglia.
Figure 5. Voltage-gated α subunit mRNA expression in guinea pig tissues.
Quantitative real-time RT-PCR was used to determine expression of 9 sodium channel α subunits in a variety of guinea pig tissues. Tissues studied were nodose ganglia (n = 3), jugular ganglia (n = 3), dorsal root ganglia (n = 3), brain tissue (n = 2), heart (n = 3), and skeletal muscle (n = 2). Note that relative amounts of mRNA within a given tissue type are approximate as the relative efficiencies of the RT step for each of the 9 NaVs is unknown. Data are means ±s.e.m.
NaV1.7, NaV1.8 and NaV1.9 mRNA expression profile in individual lung-specific nodose ganglion neurons
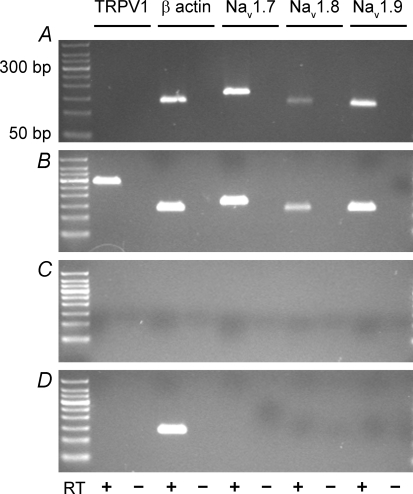
Due to the high level of expression of the NaV1.7, NaV1.8 and NaV1.9 α subunit mRNA in the nodose ganglia, we determined the expression of these three transcripts in individual cultured lung-labelled neurons using single-cell RT-PCR. We hypothesized, based on studies of whole cell currents, that the TTX-R channel proteins (NaV1.8 and NaV1.9 α subunits), would be expressed in lung-specific neurons that express TRPV1 (as a marker of capsaicin-sensitive neurons), as well as those that do not express TRPV1 (an indicator of capsaicin-insensitive neurons). Single cell RT-PCR was used to determine the mRNA expression profile of NaV1.7, NaV1.8 and NaV1.9 α subunits, TRPV1 and β-actin. In 4 of 4 lung-specific neurons that did not express TRPV1 mRNA, transcripts for the α subunit of NaV1.7, NaV1.8 and NaV1.9 were coexpressed (Fig. 6A). In 7 of 7 lung-specific neurons examined that expressed TRPV1 mRNA, transcripts for the α subunit of NaV1.7, NaV1.8 and NaV1.9 were coexpressed (Fig. 6B). For the negative bath controls, we failed to detect expression of β actin mRNA, TRPV1 and the three sodium channels (Fig. 6C; n = 7). Moreover, the identical single cell RT-PCR protocol applied to an equivalent volume of non-neuronal tissue surrounding the neurons revealed expression of β actin only (Fig. 6D; n = 4). Overall, we were unable to detect TRPV1-positive or TRPV1-negative neurons that did not display colocalized expression of mRNA messages for the three sodium channel α subunits.
Figure 6. Coexpression of NaV1.7, NaV1.8 and NaV1.9 α subunits in lung-specific nodose neurons.
Single-cell RT-PCR was used to evaluate coincident expression of α subunit of NaV1.7, NaV1.8 and NaV1.9 in TRPV1-positive (presumed capsaicin-sensitive) and TRPV1-negative (presumed capsaicin-insensitive) lung-specific nodose ganglion neurons. Examples of TRPV1-negative (A) and TRPV1-positive (B) neurons are depicted. C, no signals were detected in bath controls. D, non-neuronal material surrounding the cells was also evaluated. Message for β actin was routinely detected. PCR products were run on 1.5% agarose gels. Left-most lane represents a 50 bp ladder. Predicted sizes for TRPV1, β-actin, NaV1.7, NaV1.8 and NaV1.9 were 284 bp, 132 bp, 158 bp, 123 bp and 117 bp, respectively.
NaV1.8-like and NaV1.9-like current expressed in capsaicin-sensitive versus capsaicin-insensitive nodose ganglion neurons
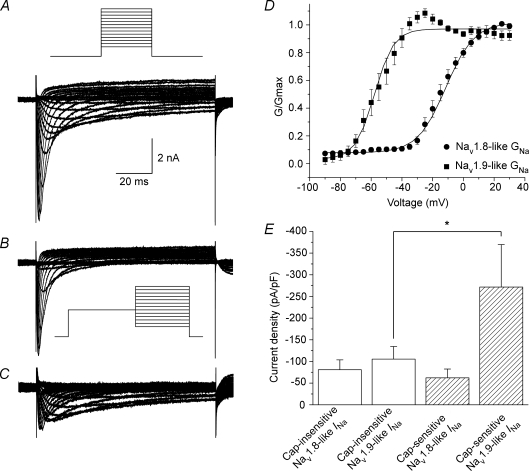
Of the nine subtypes of voltage gated sodium channels identified to date, three are categorized as TTX-R: NaV1.5, NaV1.8 and NaV1.9. Based on the results of our real-time RT-PCR data, the identity of the voltage gated sodium channel subtype that contributes to the TTX-R INa in nodose ganglion neurons is likely to be NaV1.8 and NaV1.9, either individually or in combination. We measured NaV1.8 and NaV1.9 currents to determine whether the currents were expressed either individually or in combination in guinea pig nodose ganglion neurons. Total TTX-R INa (Fig. 7A) was generated with 100 ms voltage steps (Fig. 7A, inset) from −90 mV to +30 mV from a resting potential of −120 mV in the presence of 0.3 μm TTX. To isolate the NaV1.8 current, the same voltage protocol was used but with a prepulse to −40 mV for 500 ms prior to the voltage steps (Fig. 7B). Subtraction of the NaV1.8-like current from the total TTX-R INa revealed the NaV1.9-like current (Fig. 7C). Of the 18 neurons studied, 17 displayed NaV1.8-like current of sufficient magnitude to be analysed. The NaV1.8-like current displayed half-activation potential and slope factor of −12.6 ± 2.0 mV and 7.4 ± 0.7 mV, respectively (Fig. 7D; Table 2). NaV1.8 activation kinetics recorded here is in agreement with that reported elsewhere (Rush & Waxman, 2004; Catterall et al. 2005). Of the same 18 neurons, 16 displayed measurable amounts of NaV1.9-like current. The NaV1.9-like current kinetics displayed half-activation potential and slope factor values of −54.8 ± 2.8 mV and 4.8 ± 0.7 mV (n = 13), respectively, which are consistent with reported values (Fig. 7D; Table 2) (Cummins et al. 1999; Coste et al. 2004; Maruyama et al. 2004; Rush & Waxman, 2004; Padilla et al. 2007).
Figure 7. Coexpression of NaV1.8-like and NaV1.9-like INa in a majority of lung-labelled nodose ganglion neurons.
A, TTX-R INa evoked with 100 ms voltage steps from −90 mV to 30 mV in 5 mV increments in the presence of 0.3 μm TTX. Holding potential was −120 mV. Fluoride was the primary anion in the pipette solution in this set of experiments. Inset: voltage protocol. B, a 500 ms prepulse to −40 mV prior to voltage steps protocol in A yielded the NaV1.8-like current. Inset: voltage protocol. C, digital subtraction of the NaV1.8-like current from the total TTX-R INa revealed the NaV1.9-like current. D, activation curves for NaV1.8 (•; n = 17) and NaV1.9 (▪; n = 13). Data were fitted with Boltzmann function (line). E, comparison of NaV1.8 and NaV1.9 current density among capsaicin-sensitive (hatched bars; n = 5) and -insensitive (open bars; n = 13) lung-specific nodose ganglion neurons. *Statistically significant comparison (P < 0.05). Data are means ±s.e.m.
Next, we examined whether the current density of each channel was different amongst capsaicin-sensitive and -insensitive neurons. A modest difference was detected in NaV1.9 current density between capsaicin-insensitive and -sensitive neurons, −105.4 ± 29.1 pA pF−1 and −271.6 ± 98.1 pA pF−1, respectively (P < 0.05) (Fig. 7E). There was no difference in NaV1.8 current density between capsaicin-insensitive and -sensitive neurons, −80.9 ± 22.8 pA pF−1 (n = 12) and −62.2 ± 20.3 pA pF−1 (n = 5), respectively.
Presence of NaV1.7, NaV1.8 and NaV1.9 immunoreactivity in the vagal ganglia
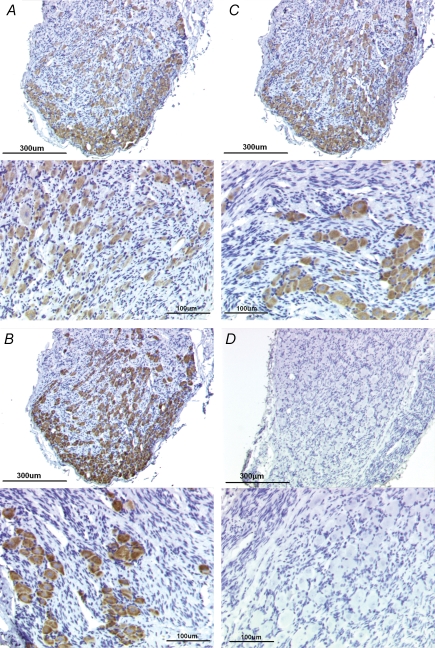
To validate the mRNA expression data obtained from single-cell RT-PCR, we used immunohistochemistry to detect NaV1.7, NaV1.8 and NaV1.9 VGSC protein in the nodose ganglia. Immunoreactivity for NaV1.7, NaV1.8 and NaV1.9 was analysed independently (single-labelling) from adjacent sections. From a random sampling of tissue sections, we observed that > 95% of neurons were positively stained for each of the three VGSC (Fig. 8A–C). The total number of neurons from representative fields of randomly selected tissue slices for NaV1.7, NaV1.8, and NaV1.9 were 162, 180 and 152, respectively. Although we did not attempt to analyse the colabelling of the VGSCs, the fact that an exceedingly high proportion of neurons expressed each of the VGSCs from adjacent sections is highly suggestive of the coexpression of the channel proteins in the vast majority of the nodose ganglion neurons. These data are consistent with the single-cell RT-PCR data, which showed that NaV1.7, NaV1.8 and NaV1.9 were colocalized in virtually every neuron tested. No immunoreactivity was detected in the isotype controls (Fig. 8D).
Figure 8. Immunoreactivity for NaV1.7, NaV1.8, and NaV1.9 found in virtually all cells in nodose ganglion.
A, representative low magnification (top) and high magnification (bottom) photomicrograph showing NaV1.7-like immunoreactivity using 1 : 1500 primary antibody concentration. Immunoreactivity was visualized using the chromagen DAG/horseradish peroxidase reaction. Mayer's haematoxylin was used to counterstain the tissues. B, low magnification (top) and high magnification (bottom) photomicrographs showing NaV1.8-like immunoreactivity (1 : 3000). C, low magnification (top) and high magnification (bottom) photomicrographs showing NaV1.9-like immunoreactivity (1 : 1500). D, rabbit IgG isotype control (2.5 μg ml−1). Top and bottom photomicrographs are at low and high magnification, respectively. Bars indicate 300 μm and 100 μm for low magnification and high magnification photomicrographs.
Discussion
The sodium channels in primary afferent neurons can be conveniently categorized into those that are sensitive to tetrodotoxin (TTX-S) and those that are resistant to TTX (TTX-R). In the dorsal root ganglia it has been shown that the TTX-R channels are preferentially expressed in small diameter capsaicin-sensitive nociceptive neurons. By contrast TTX-S channels carry most if not all the sodium current in larger diameter low-threshold mechano-sensitive neurons. A practical consequence of this information is that, in theory, sodium channel blockers could be developed that would selectively target nociceptive nerves. In the present study we addressed the question of whether selective expression of TTX-R channels also occurs in nodose vagal nociceptors, specifically in those that innervate the respiratory tract. Our data fail to support the hypothesis that TTX-R INa is selectively expressed in vagal nociceptors. The results from electrophysiological studies, gene expression studies, and immunohistochemical observations indicate that the TTX-R channels are similar amongst all respiratory nodose vagal afferent neurons including those that project capsaicin-sensitive C-fibre nociceptors, stretch-sensitive Aβ fibres, and touch-sensitive cough-evoking Aδ fibres.
The guinea pig lung is an ideal organ in which to assess the relative expression of TTX-R INa in nociceptive versus non-nociceptive nerve fibres. Both phenotypes of nerves innervate this organ in approximately equal numbers. The conclusion that the TTX-R INa is similar between nociceptive and non-nociceptive pulmonary afferent neurons, though, begs the question of the definition of pulmonary nociceptors. In the vagally innervated isolated lung preparation we have previously determined that the nodose ganglia project two types of fibres to the lung, one type being a low-threshold mechanosensors that can be stimulated by lung distension or smooth muscle contraction (Canning et al. 2004), in a manner equivalent to the RAR and SAR fibres described in this species in vivo (Bergren & Sampson, 1982). These relatively fast conducting A-fibres are not directly stimulated by capsaicin or various inflammatory mediators and are in no sense nociceptors. The other nodose nerve phenotype innervating the lungs is C-fibres. The guinea pig nodose C-fibres are not responsive to physiological stretch, but are sensitive to capsaicin, 5-HT (via 5-HT3 receptors), bradykinin (via B2 receptors), adenosine (via A1 and A2A receptors), ATP via (P2X receptors) as well as by noxious stretch (Undem et al. 2004; Chuaychoo et al. 2005, 2006). Accordingly these fibres fit Sherrington's definition of nociceptors as those nerves that are adapted to ‘attach to the (organ) a so-to-say specific sense of its own injuries’ (Sherrington, 1906). Based on this, the capsaicin sensitivity of a given nodose neuron labelled from the lungs is an ideal marker for its nociceptive quality. One must be cautious of a false-negative in our capsaicin response in the patch clamp studies. Nevertheless, it was reassuring that the percentage of capsaicin-sensitive neurons determined in the patch clamp studies approximated that observed in the extracellular recording studies of the nerves in the lungs. The finding that the capsaicin-sensitive and capsaicin-insensitive lung labelled neurons expressed similar TTX-R currents indicates that the TTX-R is expressed similarly in nociceptive and low-threshold mechanosensitive non-nociceptive nerves innervating the respiratory tract.
The vast majority (> 90%) of nodose fibres innervating guinea pig larynx, trachea and main bronchi are capsaicin-insensitive fibres (Riccio et al. 1996a). These nerve fibres, however, are easily differentiated from the capsaicin-insensitive stretch receptors in the lungs in that they do not respond to stretch and have conduction velocities in the Aδ range, about 3 times slower than the pulmonary stretch receptors. These fibres are also readily distinguishable from the nodose C-fibre nociceptors in that they do not respond directly to capsaicin or inflammatory mediators, but are exquisitely sensitive to punctate mechanical stimulation and to rapid decreases in pH (Kollarik & Undem, 2002). We have reported previously that these touch-sensitive Aδ fibres evoke cough in guinea pigs (Canning et al. 2004). That this third nerve phenotype shared a similar TTX-R response to the C-fibres and stretch-receptors indicates that the respiratory neurons in the guinea pig nodose ganglia may have a relatively uniform distribution with respect to expression of TTX-R currents. The respiratory tract also receives capsaicin-sensitive afferent fibres from the jugular and dorsal root ganglia. How the sodium currents in these C-fibre neurons compares to the sodium currents presented here in nodose neurons is not known.
That the non-nociceptive pulmonary neurons expressed substantial TTX-R is inconsistent with studies in the dorsal root ganglia, including spinal neurons innervating visceral tissue such as the colon where the capsaicin-insensitive population of neurons expressed little if any TTX-R INa. Most of the data presented here were carried out on neurons that were in culture for 24 h. It is possible that the culture conditions increased the TTX-R INa in the non-nociceptive neurons. We do not think this is likely. In a subset of studies we found that acutely dissociated capsaicin-insensitive nodose neurons revealed TTX-R that was not less than that observe following 24 h of culture. Moreover, the studies in which the neurons were studied in situ also revealed TTX-R INa in neurons that were not stimulated by capsaicin.
The sodium currents in vagal sensory neurons innervating the lungs has recently been evaluated in the rat (Kwong & Lee, 2005). In these studies the total sodium current density was significantly greater in capsaicin-insensitive neurons versus capsaicin-sensitive neurons. This difference from the present study may be due to differences in culture conditions or species differences. The density of TTX-R INa, however, as in the present study was similar between the two populations (accordingly the TTX-R as a percentage of the total INa was greater in capsaicin-sensitive rat neurons). These data support the hypothesis that TTX-R INa expressed in non-nociceptive pulmonary afferent nerves is not species specific.
Our data support the conclusion that both NaV1.8 and NaV1.9 are the major contributors of the TTX-R INa in nodose vagal respiratory capsaicin-sensitive and -insensitive neurons. With respect to the whole-cell TTX-R INa, NaV1.8 is the dominant channel, which is consistent with the fact that kinetics of the TTX-R INa is similar to NaV1.8 kinetics. Our data also indicate that NaV1.9 may play a greater role in nociceptive neurons than in non-nociceptive neurons, although how this channel contributes to neuronal function is currently unclear. NaV1.5 has recently been suggested to contribute to low-threshold TTX-R currents in small diameter dorsal root ganglion neurons (Renganathan et al. 2002). It is unlikely that NaV1.5 contributes much to TTX-R INa in the nodose sensory neurons. We did not detect NaV1.5-like currents, and the real-time RT-PCR data indicated that, as expected, NaV1.5 mRNA was expressed mainly in cardiac tissues.
Based on the data presented here, it would seem difficult to target sodium channels in respiratory vagal afferent nerves therapeutically in a manner that would selectively modulate the vagal C-fibre population. It should be kept in mind that this study focused on the cell body and the distribution of various sodium channel subtypes is not uniformly distributed throughout the neuron (Lai et al. 2004). It remains possible therefore, that the relative contribution of the TTX-R sodium channels to the sensitivity and pattern of action potential discharge at the nerve terminals may be different among the sensory nerve phenotypes innervating the respiratory tract depending on their anatomical distribution. There may be also be differences in the TTX-S component of respiratory nociceptive versus non-nociceptive vagal afferent nerves. Although this study did not focus on the identity of the TTX-S INa, it is likely that NaV1.7 plays a large role in the current, as assayed by single cell RT-PCR and immunohistochemistry. However, mRNA for other TTX-S sodium channels was also expressed in the ganglia, such as NaV1.1, NaV1.2 and NaV1.6, which may differentially contribute to total TTX-S INa in the different vagal afferent phenotypes innervating the respiratory tract.
Acknowledgments
The authors are indebted to Dr Christina Nassenstein for her guidance in the single-cell RT-PCR experiments. The authors thank Drs Marian Kollarik and Thomas Taylor-Clark for helpful suggestions. The authors thank Dr Taylor-Clark also for critical review of the manuscript. Funding was provided in part by NIH.
References
- Akopian AN, Sivilotti L, Wood JN. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature. 1996;379:257–262. doi: 10.1038/379257a0. [DOI] [PubMed] [Google Scholar]
- Amaya F, Wang H, Costigan M, Allchorne AJ, Hatcher JP, Egerton J, Stean T, Morisset V, Grose D, Gunthorpe MJ, Chessell IP, Tate S, Green PJ, Woolf CJ. The voltage-gated sodium channel Nav1.9 is an effector of peripheral inflammatory pain hypersensitivity. J Neurosci. 2006;26:12852–12860. doi: 10.1523/JNEUROSCI.4015-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbuckle JB, Docherty RJ. Expression of tetrodotoxin-resistant sodium channels in capsaicin-sensitive dorsal root ganglion neurons of adult rats. Neurosci Lett. 1995;185:70–73. doi: 10.1016/0304-3940(94)11227-a. [DOI] [PubMed] [Google Scholar]
- Benn SC, Costigan M, Tate S, Fitzgerald M, Woolf CJ. Developmental expression of the TTX-resistant voltage-gated sodium channels Nav1.8 (SNS) and Nav1.9 (SNS2) in primary sensory neurons. J Neurosci. 2001;21:6077–6085. doi: 10.1523/JNEUROSCI.21-16-06077.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergren DR, Peterson DF. Identification of vagal sensory receptors in the rat lung: are there subtypes of slowly adapting receptors? J Physiol. 1993;464:681–698. doi: 10.1113/jphysiol.1993.sp019657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergren DR, Sampson SR. Characterization of intrapulmonary, rapidly adapting receptors of guinea pigs. Respir Physiol. 1982;47:83–95. doi: 10.1016/0034-5687(82)90094-9. [DOI] [PubMed] [Google Scholar]
- Caffrey JM, Eng DL, Black JA, Waxman SG, Kocsis JD. Three types of sodium channels in adult rat dorsal root ganglion neurons. Brain Res. 1992;592:283–297. doi: 10.1016/0006-8993(92)91687-a. [DOI] [PubMed] [Google Scholar]
- Canning BJ, Mazzone SB, Meeker SN, Mori N, Reynolds SM, Undem BJ. Identification of the tracheal and laryngeal afferent neurones mediating cough in anaesthetized guinea-pigs. J Physiol. 2004;557:543–558. doi: 10.1113/jphysiol.2003.057885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA, Goldin AL, Waxman SG. International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol Rev. 2005;57:397–409. doi: 10.1124/pr.57.4.4. [DOI] [PubMed] [Google Scholar]
- Chen C-L, Broom DC, Liu Y, de Nooij JC, Li Z, Cen C, Samad OA, Jessell TM, Woolf CJ, Ma Q. Runx1 determines nociceptive sensory neuron phenotype and is required for thermal and neuropathic pain. Neuron. 2006;49:365–377. doi: 10.1016/j.neuron.2005.10.036. [DOI] [PubMed] [Google Scholar]
- Chuaychoo B, Lee M-G, Kollarik M, Pullmann R, Jr, Undem BJ. Evidence for both adenosine A1 and A2A receptors activating single vagal sensory C-fibres in guinea pig lungs. J Physiol. 2006;575:481–490. doi: 10.1113/jphysiol.2006.109371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuaychoo B, Lee M-G, Kollarik M, Undem BJ. Effect of 5-hydroxytryptamine on vagal C-fiber subtypes in guinea pig lungs. Pulm Pharmacol Ther. 2005;18:269–276. doi: 10.1016/j.pupt.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Coste B, Osorio N, Padilla F, Crest M, Delmas P. Gating and modulation of presumptive NaV1.9 channels in enteric and spinal sensory neurons. Mol Cell Neurosci. 2004;26:123–134. doi: 10.1016/j.mcn.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Cummins TR, Dib-Hajj SD, Black JA, Akopian AN, Wood JN, Waxman SG. A novel persistent tetrodotoxin-resistant sodium current in SNS-null and wild-type small primary sensory neurons. J Neurosci. 1999;19:RC43. doi: 10.1523/JNEUROSCI.19-24-j0001.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dib-Hajj SD, Tyrrell L, Black JA, Waxman SG. NaN, a novel voltage-gated Na channel, is expressed preferentially in peripheral sensory neurons and down-regulated after axotomy. Proc Natl Acad Sci U S A. 1998;95:8963–8968. doi: 10.1073/pnas.95.15.8963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flake NM, Gold MS. Inflammation alters sodium currents and excitability of temporomandibular joint afferents. Neurosci Lett. 2005;384:294–299. doi: 10.1016/j.neulet.2005.04.091. [DOI] [PubMed] [Google Scholar]
- Gold MS, Reichling DB, Shuster MJ, Levine JD. Hyperalgesic agents increase a tetrodotoxin-resistant Na+ current in nociceptors. Proc Natl Acad Sci U S A. 1996;93:1108–1112. doi: 10.1073/pnas.93.3.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold MS, Weinreich D, Kim C-S, Wang R, Treanor J, Porreca F, Lai J. Redistribution of NaV1.8 in uninjured axons enables neuropathic pain. J Neurosci. 2003;23:158–166. doi: 10.1523/JNEUROSCI.23-01-00158.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold MS, Zhang L, Wrigley DL, Traub RJ. Prostaglandin E2 modulates TTX-R INa in rat colonic sensory neurons. J Neurophysiol. 2002;88:1512–1522. doi: 10.1152/jn.2002.88.3.1512. [DOI] [PubMed] [Google Scholar]
- Hirsh AE, Fraser HB. Protein dispensability and rate of evolution. Nature. 2001;411:1046–1049. doi: 10.1038/35082561. [DOI] [PubMed] [Google Scholar]
- Kollarik M, Undem BJ. Mechanisms of acid-induced activation of airway afferent nerve fibres in guinea-pig. J Physiol. 2002;543:591–600. doi: 10.1113/jphysiol.2002.022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong K, Lee L-Y. Prostaglandin E2 potentiates a TTX-resistant sodium current in rat capsaicin-sensitive vagal pulmonary sensory neurones. J Physiol. 2005;564:437–450. doi: 10.1113/jphysiol.2004.078725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai J, Porreca F, Hunter JC, Gold MS. Voltage-gated sodium channels and hyperalgesia. Annu Rev Pharmacol Toxicol. 2004;44:371–397. doi: 10.1146/annurev.pharmtox.44.101802.121627. [DOI] [PubMed] [Google Scholar]
- Lancaster E, Weinreich D. Sodium currents in vagotomized primary afferent neurones of the rat. J Physiol. 2001;536:445–458. doi: 10.1111/j.1469-7793.2001.0445c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M-G, MacGlashan DW, Jr, Undem BJ. Role of chloride channels in bradykinin-induced guinea pig airway vagal C-fibre activation. J Physiol. 2005;566:205–212. doi: 10.1113/jphysiol.2005.087577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li BY, Schild JH. Patch clamp electrophysiology in nodose ganglia of adult rat. J Neurosci Methods. 2002;115:157–167. doi: 10.1016/s0165-0270(02)00010-9. [DOI] [PubMed] [Google Scholar]
- Maruyama H, Yamamoto M, Matsutomi T, Zheng T, Nakata Y, Wood JN, Ogata N. Electrophysiological characterization of the tetrodotoxin-resistant Na+ channel, Nav1.9, in mouse dorsal root ganglion neurons. Pflugers Arch. 2004;449:76–87. doi: 10.1007/s00424-004-1315-0. [DOI] [PubMed] [Google Scholar]
- Padilla F, Couble M-L, Coste B, Maingret F, Clerc N, Crest M, Ritter AM, Magloire H, Delmas P. Expression and localization of the Nav1.9 sodium channel in enteric neurons and in trigeminal sensory endings: Implication for intestinal reflex function and orofacial pain. Mol Cell Neurosci. 2007;35:138–152. doi: 10.1016/j.mcn.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Renganathan M, Dib-Hajj S, Waxman SG. Nav1.5 underlies the ‘third TTX-R sodium current’ in rat small DRG neurons. Brain Res Mol Brain Res. 2002;106:70–82. doi: 10.1016/s0169-328x(02)00411-4. [DOI] [PubMed] [Google Scholar]
- Riccio MM, Kummer W, Biglari B, Myers AC, Undem BJ. Interganglionic segregation of distinct vagal afferent fibre phenotypes in guinea-pig airways. J Physiol. 1996a;496:521–530. doi: 10.1113/jphysiol.1996.sp021703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio MM, Myers AC, Undem BJ. Immunomodulation of afferent neurons in guinea-pig isolated airway. J Physiol. 1996b;491:499–509. doi: 10.1113/jphysiol.1996.sp021234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Meth Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- Rush AM, Craner MJ, Kageyama T, Dib-Hajj SD, Waxman SG, Ranscht B. Contactin regulates the current density and axonal expression of tetrodotoxin-resistant but not tetrodotoxin-sensitive sodium channels in DRG neurons. Eur J Neurosci. 2005;22:39–49. doi: 10.1111/j.1460-9568.2005.04186.x. [DOI] [PubMed] [Google Scholar]
- Rush AM, Waxman SG. PGE2 increases the tetrodotoxin-resistant Nav1.9 sodium current in mouse DRG neurons via G-proteins. Brain Res. 2004;1023:264–271. doi: 10.1016/j.brainres.2004.07.042. [DOI] [PubMed] [Google Scholar]
- Sangameswaran L, Delgado SG, Fish LM, Koch BD, Jakeman LB, Stewart GR, Sze P, Hunter JC, Eglen RM, Herman RC. Structure and function of a novel voltage-gated, tetrodotoxin-resistant sodium channel specific to sensory neurons. J Biol Chem. 1996;271:5953–5956. doi: 10.1074/jbc.271.11.5953. [DOI] [PubMed] [Google Scholar]
- Sherrington CS. The Integrative Action of the Nervous System. New Haven, CT, USA: Yale University Press; 1906. [Google Scholar]
- Tate S, Benn S, Hick C, Trezise D, John V, Mannion RJ, Costigan M, Plumpton C, Grose D, Gladwell Z, Kendall G, Dale K, Bountra C, Woolf CJ. Two sodium channels contribute to the TTX-R sodium current in primary sensory neurons. Nat Neurosci. 1998;1:653–655. doi: 10.1038/3652. [DOI] [PubMed] [Google Scholar]
- Undem BJ, Chuaychoo B, Lee MG, Weinreich D, Myers AC, Kollarik M. Subtypes of vagal afferent C-fibres in guinea-pig lungs. J Physiol. 2004;556:905–917. doi: 10.1113/jphysiol.2003.060079. [DOI] [PMC free article] [PubMed] [Google Scholar]