Abstract
The role of parathyroid hormone-related protein (PTHrP) in fetal calcium homeostasis and placental calcium transport was examined in mice homozygous for the deletion of the PTHrP gene (PTHrP−/− null; NL) compared to PTHrP+/+ (wild-type; WT) and PTHrP+/− (heterozygous; HZ) littermates. Fetal blood ionized calcium was significantly reduced in NL fetuses compared to WT and HZ groups at 18 days of pregnancy (dp) with abolition of the fetomaternal calcium gradient. In situ placental perfusion of the umbilical circulation at 18 dp was used to measure unidirectional clearance of 45Ca across the placenta in maternofetal (CaKmf) and fetoplacental (CaKfp) directions; CaKfp was < 5% of CaKmf for all genotypes. At 18 dp, CaKmf across perfused placenta and intact placenta (CaKmf(intact)) were similar and concordant with net calcium accretion rates in vivo. CaKmf was significantly raised in NL fetuses compared to WT and HZ littermates. Calcium accretion was significantly elevated in NL fetuses by 19 dp. Placental calbindin-D9K expression in NL fetuses was marginally enhanced (P < 0.07) but expression of TRPV6/ECaC2 and plasma membrane Ca2+-ATPase (PMCA) isoforms 1 and 4 were unaltered. We conclude that PTHrP is an important regulator of fetal calcium homeostasis with its predominant effect being on unidirectional maternofetal transfer, probably mediated by modifying placental calbindin-D9K expression. In situ perfusion of mouse placenta is a robust methodology for allowing detailed dissection of placental transfer mechanisms in genetically modified mice.
Provision of calcium to the developing fetus is essential for bone mineralization, which, if compromised, may increase the risk of developing osteoporosis later in life (Tobias & Cooper, 2004). Maximal fetal accretion of calcium occurs over the last third of pregnancy, and in the rat, as in other species, the rise in fetal calcium accretion is exponential over this period (Comar, 1956). Net calcium flux to the fetus (CaJnet) is the difference between unidirectional maternofetal (CaJmf) and fetomaternal (CaJfm) calcium fluxes (Sibley & Boyd, 1988). The rise in CaJnet over gestation in the rat is a function of increased CaJmf (Glazier et al. 1992). Considerable data from a variety of species suggest that CaJmf is an active process, maintaining hypercalcaemia of the fetus relative to its mother, a feature of all species studied (Sibley & Boyd, 1988; Atkinson et al. 2006). CaJfm is thought to be a diffusional process (Husain et al. 2004).
In common with other calcium transporting epithelia, calcium transport across the placenta could plausibly involve three main steps (Belkacemi et al. 2005; Atkinson et al. 2006): firstly, diffusion into the trophoblast from maternal plasma down an electrochemical gradient through epithelial Ca2+ channels of the transient receptor potential (TRP) gene family; secondly, transfer across the trophoblast cytoplasm bound to the calcium binding protein calbindin-D9K (Glazier et al. 1992); and, thirdly, active extrusion into the fetal compartment via plasma membrane Ca2+-ATPase (PMCA) localized to the fetal facing basal plasma membrane (Fisher et al. 1987; Borke et al. 1989). Over the last third of gestation, gene and protein expression for calbindin-D9K increases markedly in both mouse and rat placenta (Mathieu et al. 1989; Glazier et al. 1992; Hamilton et al. 2000; An et al. 2004) whereas rat placental Ca2+-ATPase expression shows only a 2- to 3-fold increase (Glazier et al. 1992). This increase in rat placental calbindin-D9K expression is associated with a concomitant, stoichiometric increase in unidirectional maternofetal 45Ca clearance over this period leading to the speculation that calbindin-D9K may be rate limiting for placental calcium transport in this species (Glazier et al. 1992).
One of the fetal factors implicated in the regulation of placental calcium transport is parathyroid hormone-related protein (PTHrP), which is produced by the placenta as well as by other fetal tissues (Tobias & Cooper, 2004). Although PTHrP was initially discovered as the humoral factor responsible for hypercalcaemia of malignancy, it is now known to have broad expression in a variety of fetal and adult tissues (reviewed in Philbrick et al. 1996). The versatility of PTHrP in performing multiple, yet distinct, biological effects through endocrine, autocrine, paracrine and intracrine activities arises from alternative splicing of the PTHrP gene, giving rise to three initial translation products which then undergo post-translational processing to generate N-terminal, mid-region and C-terminal mature peptide fragments.
Consistent with this concept, calcium transfer into blood used to perfuse the fetal circulation of the in situ placenta in thyroparathyroidectomised fetal lambs was increased by the addition of partially purified hPTHrP or recombinant PTHrP(1–84), PTHrP(1–108) and PTHrP(1–141) but not by synthetic PTHrP(1–34) (Abbas et al. 1989). This stimulation was rapid (within 1 h) and was also conferred by hPTHrP(67–86 amide) and hPTHrP(75–86 amide) fragments (Care et al. 1990) suggesting that this activity resides in the PTHrP(75–86) peptide sequence and that the N-terminus is not involved. By contrast, infusion of hPTHrP(1–34) or hPTHrP(75–86 amide) into the fetoplacental circulation of intact rat fetuses had no effect on the placental transport of calcium (Shaw et al. 1991).
The availability of fetal mice homozygous for deletion of the PTHrP gene (PTHrP−/− null; NL) (Karaplis et al. 1994) has allowed closer examination of the effect of fetal PTHrP on placental calcium transport. NL fetuses exhibit abnormalities of endochondral bone development with accelerated endochondral ossification, yet, despite broad PTHrP tissue expression in PTHrP+/+ wild-type (WT) conceptuses, no gross morphological abnormalities were apparent in other tissues from NL fetuses (Karaplis et al. 1994). In NL fetuses, fetal blood ionized Ca2+ concentration is significantly reduced resulting in the abolition of the fetomaternal Ca2+ gradient (Kovacs et al. 1996; Tucci et al. 1996). The ratio of maternofetal transfer of 45Ca normalized to that of 51Cr-EDTA (used as a marker of diffusional permeability) was significantly reduced in NL fetuses when compared to WT and PTHrP+/− heterozygous (HZ) littermates (Kovacs et al. 1996), suggesting dependence of CaJmf on fetal PTHrP. Reversal of this effect by fetal injection 90 min before of hPTHrP(1–86) and hPTHrP(67–86), but not of hPTHrP(1–34), supports the concept that the mid-molecule region of PTHrP is functionally important in eliciting these acute effects. Curiously, in the light of these observations, others report a higher fetal calcium content in NL fetuses compared to WT littermates (Tucci et al. 1996), suggesting that CaJnet is increased in the absence of fetal PTHrP. The explanation for these apparently divergent observations could be methodological, but may also reflect differences in the relative contribution of CaJmf and/or CaJfm to CaJnet. A more detailed examination of the role of fetal PTHrP on transplacental calcium fluxes is required.
To do this, we have developed a method in which the fetoplacental circulation is artificially perfused in situ in the mouse (Bond et al. 2006a) allowing separate measurement of Jmf and of fetoplacental calcium flux (Jfp) as an estimate of Jfm. This technique provides a powerful tool to explore the molecular mechanisms involved in the regulation of unidirectional placental solute fluxes.
Methods
Chemicals
All chemicals were purchased from either Sigma-Aldrich Co. Ltd (Poole, UK) or VWR International (Lutterworth, UK) unless otherwise stated.
Animals
Experiments were performed in accordance with the UK Animals (Scientific Procedures) Act of 1986. PTHrP knockout mice generated by the targeted disruption in embryonic stem cells of exon IV, the major exon encoding mature murine PTHrP protein (Karaplis et al. 1994), were a kind gift from Professor H. Kronenberg (Harvard Medical School, USA).
Females (8–12 weeks old) and males (6 weeks to 8 months old) heterozygous for the deleted PTHrP gene were mated and the first day of gestation determined by the discovery of a copulation plug (term, 19–20 days). All animals were provided with nesting material and housed in cages maintained under a constant 12 h light–dark cycle at 21–23°C with free access to food (Beekay Rat and Mouse Diet, Bantin & Kingman, Hull, UK) and tap water. At the end of the experiment, all animals were killed by Schedule 1 procedure in accordance with the UK Animals (Scientific Procedures) Act of 1986.
Fetuses were genotyped using genomic DNA extracted from fetal tail tips (DNeasy, Qiagen, Crawley, UK) with primers specific to gene sequences for PTHrP (sense, 5′-GCTACTGCATGACAAGGGCAAGTCC-3′; antisense, 5′-GAGCCCTGCTGAACACAGTGAACAG-3′) and neomycin resistance (sense, 5′-GGAGAGGCTATTCGGCTATGAC-3′; antisense, 5′-CGCATTGCATCAGCCATGATGG-3′).
Placental calcium transfer studies
Measurement of unidirectional solute transfer across either the intact or perfused placenta is often expressed as solute clearance (Meschia et al. 1967), a formulation without any underlying mechanistic assumptions (Sibley, 1994). In the present investigation, unidirectional maternofetal clearance was measured both across perfused (CaKmf) and intact (CaKmf(intact)) placenta. Unidirectional fetoplacental clearance (CaKfp) was measured using perfused placenta. Net flux (CaJnet) was calculated from calcium accretion rate in vivo. Unidirectional flux can be calculated as the algebraic product of unidirectional clearance and respective arterial concentration (Sibley, 1994).
Unidirectional maternofetal clearance of 45Ca (CaKmf) across the perfused placenta
CaKmf across the perfused placenta of mice at 18 days of pregnancy (dp) was measured as previously described (Bond et al. 2006a). The dam was anaesthetized with an intraperitoneal injection of 300 μl fentanyl/fluanisone (Hypnorm: Janssen Pharmaceutica, Belgium) and midazolam (Phoenix Pharma Ltd, Gloucester, UK) mixture (1 part fentanyl (0.315 mg ml−1) and fluanisone (10 mg ml−1), 1 part midazolam (5 mg ml−1), and 2 parts water). Additional doses of anaesthetic were given intraperitoneally (2–5 μl (g dam weight)−1) during the course of the experiment as required.
CaKmf was calculated (Sibley & Boyd, 1988) as:
| (1) |
where [v] is fetal side placental venous effluent radioisotope concentration (dpm μl−1), Q is the perfusion flow rate (μl min−1), W is the placental wet weight (g) and [A] is the maternal plasma radioisotope concentration (dpm μl−1) taken at the mid-point of the perfusate collection period by curvilinear interpolation from a pooled maternal 45Ca disappearance curve (Bond et al. 2006a).
Unidirectional fetoplacental clearance of 45Ca (CaKfp) using perfused placenta
In these experiments, the dam was similarly prepared on 18 dp as detailed previously for the measurement of CaKmf (Bond et al. 2006a), but the maternal tail vein was not cannulated. The arteriovenous difference of 45Ca in Krebs Ringer solution (containing 10 nCi 45CaCl2 ml−1) used to perfuse the fetoplacental circulation allowed calculation of CaKfp, as given in eqn (2):
| (2) |
where [a] is the radioisotope concentration of arterial inflowing perfusate (dpm μl−1).
Inclusion criteria for perfusion experiments
Data were analysed from those experiments where (a) perfusate effluent recovery was 95% of perfusate inflow, (b) 45CaCl2 stock injectate radioactivity (dpm μl−1) fell within 95% of the mean value for all experiments, and (c) the radioisotope injection volume was within 10% of the 50 μl injectate volume. Inclusion criteria (b) and (c) were introduced to ensure that the radioactivity injected into each mouse was comparable thereby minimizing variability in the maternal radioisotope disappearance curve (Bond et al. 2006a).
Unidirectional maternofetal clearance of 45Ca across the intact placenta (CaKmf(intact))
CaKmf(intact) across the intact, unperfused placenta was measured on 18 dp using an adaptation of the method of Flexner & Pohl (1941) as previously described (Bond et al. 2006a).
CaKmf(intact) was calculated as:
| (3) |
where, Nx is total radiolabel accumulation (dpm) by the fetus (corrected for the fetal tail tip retained for genotyping) at x min after injection of radiolabel into the maternal vein and 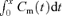 is the time integral of radioisotope concentration in maternal plasma (dpm · min μl−1) from 0 to x min (taken from the maternal plasma 45Ca disappearance curve; Bond et al. 2006a).
is the time integral of radioisotope concentration in maternal plasma (dpm · min μl−1) from 0 to x min (taken from the maternal plasma 45Ca disappearance curve; Bond et al. 2006a).
Maternal plasma 45Ca disappearance curve following injection into the maternal circulation
A maternal plasma 45Ca disappearance curve was constructed from 78 dams at 18 dp and fitted to a one-phase exponential decay model (r2 > 0.8) as previously described (Bond et al. 2006a).
Net transplacental flux of calcium (CaJnet)
Calcium content of fetal ash was measured at 17, 18 and 19 dp by atomic absorption spectrophotometry (Solaar S-Series, Thermo Elemental, Unicam Ltd, Cambridge, MA, USA) as previously described (Husain et al. 1994). Tail tips were taken for genotyping as described above.
CaJnet over 24 h (1440 min) between 17 to 18 or 18 to 19 dp was calculated as:
| (4) |
where W is mean placental wet weight (g) at either 18 or 19 dp, respectively.
Maternal whole blood ionized Ca2+ concentration
Terminal blood samples, taken at 18 dp from the carotid artery following decapitation, were analysed for ionized Ca2+ concentration (Bayer 865 Analyser, Siemens, Berkshire, UK).
Placental PTHrP expression
In order to assess the potential for endogenous placental production of PTHrP by maternal cells within decidua (Karperien et al. 1996), PTHrP gene and protein expression were examined in fetuses from within the same litter. Paired fetal genomic DNA and placental cDNA from each genotype was amplified using exon IV-specific primers (sense, 5′-GTTTCTTCCTCCACCATCTG-3′; antisense, 5′-ATCTGCCCTCATCGTCTG-3′) and placental PTHrP protein expression was examined by immunohistochemistry (see below).
Western blotting
Individual placentas harvested at 18 dp were homogenized in buffer containing 300 mm mannitol, 12 mm Hepes (pH 7.6) and 1% protease inhibitor cocktail (Sigma-Aldrich) for 30 s. The homogenate was retained or centrifuged at 2500 g for 5 min at 4°C (Sorvall Discovery 100SE, Kendro Laboratory Products, Bishop's Stortford, UK). Aliquots of this post-nuclear supernatant (PNS) were retained and the remaining PNS centrifuged at 100 000 g for 30 min at 4°C to obtain the membrane fraction. Both fractions were analysed for protein concentration (Bio-Rad Protein Assay) and stored at −80°C for further analysis.
Protein–SDS complexes were prepared with (Settle et al. 2004) or without (Bond et al. 2005) heat denaturation and SDS-PAGE performed followed by electrotransfer to nitrocellulose membranes (GE Healthcare, Little Chalfont, UK). The antisera used were rabbit polyclonal anti-human TRPV6 (ECaC2, 1: 200; Santa Cruz Biotechnology, Insight Biotechnology, Wembley, UK), rabbit polyclonal anti-rat calbindin-D9K (1: 1000; SWANT, Bellizona, Switzerland), rabbit polyclonal anti-human plasma membrane calcium ATPase isoforms 1 (PMCA1) and 4 (PMCA4, 1: 1000; SWANT), goat polyclonal anti-human PTHrP (1: 200; Santa Cruz Biotechnology) and rabbit polyclonal anti-human β-actin (1: 1000; Abcam, Cambridge, UK). Negative controls were prepared by omission of primary antibody or pre-absorption with excess blocking peptide. Immunoreactive species were detected with horseradish peroxidase-conjugated secondary antibodies (1: 2000, Dako, Ely, UK) using an enhanced chemiluminescence detection system (GE Healthcare). Immunoreactive signal density was measured by densitometry (Bio-Rad Molecular Analyst) and all signals fell within the linear range of detection.
PTHrP immunohistochemistry
PTHrP immunohistochemistry was performed on sections (5 μm) of paraffin-embedded placentas using standard techniques (Champion et al. 2004) after antigen retrieval by immersing slides in 0.01 m sodium citrate buffer pH 6 and microwaving on full power for 10 min. Non-specific binding was blocked with 10% rabbit serum. Goat polyclonal PTHrP antibody (2 μg ml−1; Santa Cruz) or non-immune goat IgG as negative control were applied overnight at 4°C followed by incubation with biotinylated rabbit anti-goat IgG (1: 100 Dako Ltd, UK) as secondary antibody. Sections were observed and photographed using a Leitz Dialux 22 microscope (Leica Microsystems, UK) and QICam 1394 digital camera (QImaging, UK).
Statistical analysis
All data are presented as mean ±s.e.m. where n is the number of fetuses/placentas. ANOVA with Bonferroni multiple comparison post hoc test was used to test the differences between experimental groups. Non-parametric comparisons were performed using Mann–Whitney and Kruskal–Wallis tests. P < 0.05 was taken as the significance level unless otherwise stated.
Abbreviations
PTHrP, parathyroid hormone-related protein; WT, wild-type; HZ, heterozygous; NL, null; TRPV, transient receptor potential vanilloid subfamily of calcium channels; ECaC, epithelial calcium channel; PMCA, plasma membrane Ca2+-ATPase; dp, day of pregnancy; PNS, postnuclear supernatant; IPYS, intraplacental yolk sac; CaKmf, unidirectional maternofetal clearance of calcium; CaKfp, unidirectional fetoplacental clearance of calcium; CaKmf(intact), unidirectional maternofetal clearance of calcium across intact (unperfused) placenta; CaJnet, net flux of calcium to the fetus; CaJmf, unidirectional maternofetal calcium flux; CaJfm, unidirectional fetomaternal calcium flux.
Results
Fetal and placental weight and fetal ionized Ca2+ concentration
Placental and fetal weights, fetal: placental weight ratio and fetal blood ionized Ca2+ concentration ([Ca2+]) for WT, HZ and NL fetuses at 18 dp are given in Table 1. Fetal weight was lower, and placental weight higher in NL fetuses compared to WT, with no difference with respect to other group comparisons. In the NL group, this was reflected in a significantly reduced fetal: placental weight ratio relative to HZ and WT groups.
Table 1.
Fetal and placental weights, fetal: placental weight ratio and fetal plasma ionized Ca2+ concentration in WT (PTHrP+/+), HZ (PTHrP+/−) and NL (PTHrP−/−) fetuses at 18 days of gestation
| WT | HZ | NL | |
|---|---|---|---|
| Fetal weight (g) | 0.90 ± 0.01 (74) | 0.89 ± 0.01 (131) | 0.84 ± 0.02b (45) |
| Placental weight (g) | 0.084 ± 0.001 (74) | 0.087 ± 0.001 (131) | 0.090 ± 0.002a (45) |
| Fetal: placental weight ratio | 10.9 ± 0.2 (74) | 10.3 ± 0.1 (131) | 9.5 ± 0.2c,d (45) |
| Fetal plasma ionized Ca2+ (mm) | 1.45 ± 0.02 (15) | 1.45 ± 0.02 (25) | 1.25 ± 0.07b,e (6) |
Data presented as mean ±s.e.m. (n).
P < 0.05,
P < 0.01,
P < 0.001 versus WT;
P < 0.05,
P < 0.001 versus HZ (ANOVA followed by Bonferroni's multiple comparison test).
[Ca2+] in NL fetuses was significantly lower than WT and HZ fetuses. There was a marked fetomaternal Ca2+ gradient apparent in both WT and HZ fetuses with [Ca2+] significantly higher (P < 0.001) than maternal [Ca2+] (1.16 ± 0.02 mm, n = 69). However, in NL fetuses the Ca2+ gradient was abolished as fetal [Ca2+] was not significantly different to maternal [Ca2+].
Unidirectional clearances of 45Ca across perfused and intact placenta
Table 2 gives unidirectional maternofetal clearance of calcium (CaKmf) across the perfused placenta (taken as the mean value of clearance over 25–45 min, the period over which CaKmf was shown to be in steady state for all three genotypes; ANOVA, data not shown). CaKmf was significantly higher across the perfused placentas of NL fetuses compared to their WT and HZ counterparts. Mirroring this, CaKmf across the intact placenta (CaKmf(intact)) of NL fetuses was 23–29% higher compared to WT and HZ groups (Table 2), the difference between groups being marginally significant (P < 0.07). For all genotypes CaKmf and CaKmf(intact) were not significantly different. There was no significant difference between groups in unidirectional fetoplacental clearance of calcium (CaKfp) (taken as the mean value of clearance over 10–45 min, over which period values were at steady state for all genotypes; ANOVA, data not shown). CaKfp was < 5% of CaKmf in all groups.
Table 2.
Unidirectional maternofetal (CaKmf) and fetoplacental (CaKmf) clearance of 45Ca across the perfused and intact placenta (CaKmf(intact)) of WT (PTHrP+/+), HZ (PTHrP+/−) and NL (PTHrP−/−) fetuses at 18 days of gestation
| WT | HZ | NL | |
|---|---|---|---|
| CaKmf (μl min−1 (g placenta)−1) | 86.4 ± 20.7* (3) | 94.9 ± 10.1** (13) | 172.0 ± 19.4 (7) |
| CaKfp (μl min−1 (g placenta)−1) | 1.2 ± 0.3a (5) | 3.4 ± 1.1b (4) | 1.6 ± 0.9b (5) |
| CaKmf(intact) (μl min−1 (g placenta)−1) | 128.5 ± 10.5 (42) | 123.0 ± 7.6 (67) | 158.1 ± 14.9† (28) |
Data presented as mean ±s.e.m. (n). *P < 0.05, **P < 0.01 versus NL (ANOVA followed by Bonferroni's multiple comparison test). aP < 0.05, bP < 0.005 versusCaKmf (Mann–Whitney test). †P < 0.07 for difference between groups (ANOVA).
Fetal calcium accretion and CaJnet
Table 3 shows fetal calcium content for all genotypes at 17, 18 and 19 dp normalized to ash weight. Ash weight, unlike wet weight (Table 1), was not significantly different between genotypes at each gestational age, in agreement with previous observations (Kovacs et al. 2001). Fetal calcium content was significantly elevated in NL fetuses at day 19 of pregnancy compared to WT and HZ fetuses, but no difference was observed between genotypes at the earlier gestational ages. The anticipated increase in fetal calcium accretion with advancing gestation was observed in all genotype groups between days 17–19 (P < 0.0005, ANOVA with Bonferroni's multiple comparison test). The calculated mean value for CaJnet at day 18 is within the 95% confidence limits for CaJmf measured at the same gestation (Table 3). The rise in CaJnet between day 18 and day 19 in NL fetuses was approximately double that for WT and HZ fetuses.
Table 3.
Fetal calcium content normalized to ash weight in WT (PTHrP+/+), HZ (PTHrP+/−) and NL (PTHrP−/−) fetuses at 17, 18 and 19 days of gestation and estimates of CaJmf and CaJnet
| WT | HZ | NL | |
|---|---|---|---|
| Day 17 (mmol (g ash)−1) | 2.23 ± 0.09 (6) | 2.53 ± 0.04 (28) | 2.46 ± 0.13 (9) |
| Day 18 (mmol (g ash)−1) | 3.15 ± 0.13 (30) | 3.17 ± 0.09 (64) | 3.56 ± 0.19 (17) |
| Day 19 (mmol (g ash)−1) | 3.45 ± 0.07** (29) | 3.63 ± 0.08* (57) | 4.12 ± 0.27 (18) |
| CaJmf† (nmol min−1 (g placenta)−1) | 149 ± 12 | 143 ± 9 | 183 ± 17 |
| CaJnet‡ (nmol min−1 (g placenta)−1) | |||
| Day 18 | 165 | 160 | 172 |
| Day 19 | 234 | 236 | 315 |
PTHrP gene expression
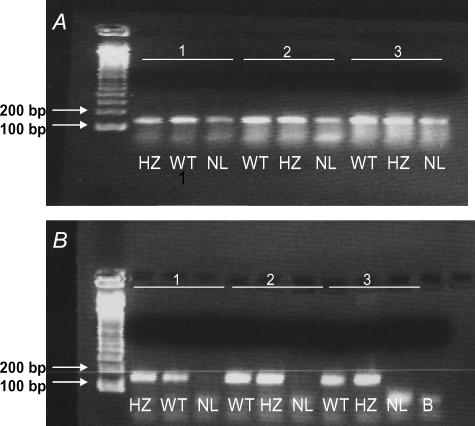
Placental cDNA from all three genotypes amplified PTHrP gene product (confirmed by sequencing) although product abundance appeared to be relatively lower in NL fetuses (Fig. 1A). However, no amplification product was visible in genomic DNA of NL fetuses (Fig. 1B).
Figure 1. PCR of paired placental cDNA (A) and fetal genomic DNA (B) from WT (PTHrP+/+), HZ (PTHrP+/−) and NL (PTHrP−/−) fetuses of 3 individual litters using exon IV-specific primers, the major exon encoding murine PTHrP.
Lane B, H2O replacement of DNA. 100 base pair (bp) ladder is shown on left. Amplification product of the predicted size (138 bp) was visible in placental cDNA from all three genotypes, whilst genomic DNA demonstrated the predicted pattern of amplification with no product observable in NL fetuses.
PTHrP immunohistochemistry
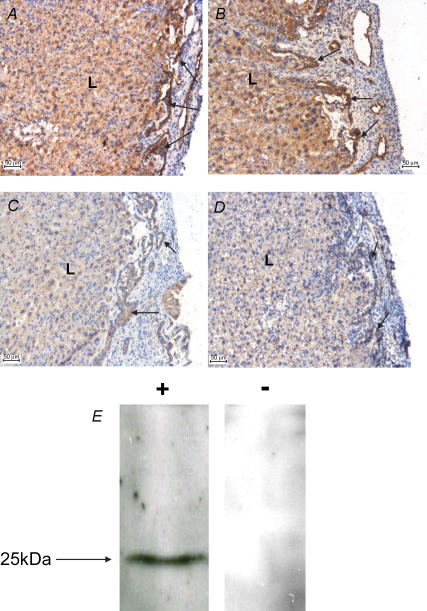
Figure 2 provides confirmation of the immunoreactive specificity of the anti-human PTHrP antibody for murine PTHrP and confirms negligible PTHrP protein expression in the placentas of NL fetuses. In placentas from WT and HZ fetuses, PTHrP immunoreactivity was intensely localized to the intraplacental yolk sac (IPYS) as well as being broadly distributed throughout the labyrinth trophoblast.
Figure 2. Immunohistochemistry of PTHrP in the placenta of mouse fetuses which were WT (PTHrP+/+) (A), HZ (PTHrP+/−) (B) or NL (PTHrP−/−) (C) for PTHrP allele.
Sections were incubated with goat anti-human PTHrP antibody (2 μg ml−1; A–C) or goat IgG (2 μg ml−1, D) as negative control. Intense staining was observed within the IPYS (arrows) and labyrinthine zone (L) of placentas of WT and HZ fetuses. In contrast in the NL fetus, placental staining was negligible and comparable to negative control, confirming a lack of placental PTHrP protein expression in the null mutant. All scale bars in A–D are 50 μm. E, Western blot of WT mouse placental lysate (50 μg protein lane−1) probed with either: +, affinity purified goat anti-human PTHrP antibody (1: 200; 1 μg ml−1) alone giving a single immunoreactive signal of predicted size; or −, in the presence of 5× excess (1: 40) blocking peptide which abolished signal.
Expression of calcium transport proteins
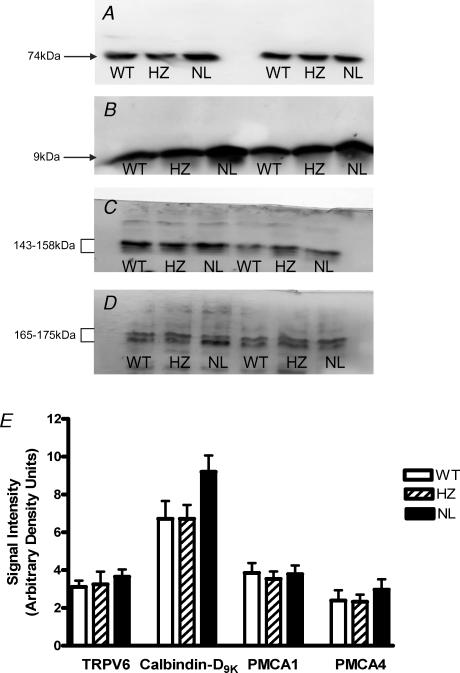
Probing placental protein for calcium transport proteins that may mediate calcium epithelial influx (TRPV6/ECaC2; Fig. 3A), cytosolic translocation (calbindin-D9K; Fig. 3B) and efflux (PMCA1 and PMCA4; Fig. 3C and D) revealed immunoreactive signals of appropriate size for all proteins. Signal intensity was not significantly different across genotype for TRPV6, PMCA1 and PMCA4 (Fig. 3E). However, in 7 of 9 litters examined calbindin-D9K expression was higher in the placentas of NL fetuses compared to their WT and HZ littermates, as visible in Fig. 3B, with a mean increase in calbindin-D9K expression of ∼37% (Fig. 3E; P < 0.07, Kruskal–Wallis test).
Figure 3. Western blots showing calcium transport protein expression in the placentas of WT (PTHrP+/+), HZ (PTHrP+/−) or NL (PTHrP−/−) fetuses, matched from two litters.
A, TRPV6 (ECaC2; 20 μg membrane protein lane−1, 30 s exposure); B, calbindin-D9K (20 μg PNS lane−1, 15 s exposure); C, PMCA1 (30 μg membrane protein lane−1, 5 min exposure); and D, PMCA4 (60 μg membrane protein lane−1, 5 min exposure). For all blots the immunoreactive species detected accord with the predicted molecular mass of the target protein, with signal abolished by omission of primary antibody (not shown). E, densitometric analysis (mean +s.e.m.) of immunoreactive signal intensity for TRPV6 (ECaC2; n = 6), calbindin-D9K (n = 9), PMCA1 (n = 6) and PMCA4 (n = 6) in placentas of WT, HZ and NL fetuses. Mean calbindin-D9K expression in NL fetuses was ∼37% higher than in WT and HZ littermates (P < 0.07, Kuskal–Wallis). All signals fell within the linear range of detection. For PMCA1 and PMCA4 signals comprising the triplet and doublet species, respectively, were analysed collectively. Equal protein loading was confirmed by densitometric analysis of replicate blots probed for β-actin (data not shown).
Discussion
In situ placental perfusion in the mouse (Bond et al. 2006a) has great potential as a methodology for measuring unidirectional clearances and fluxes in a species in which genetically modified strains are widely available. A striking finding of this study (Table 3) is the concordance between transport measurements for an actively transported solute, calcium, obtained in three ways: across fetal-side perfused placenta; across unperfused in situ placenta in anaesthetized animals; and in vivo. These observations therefore provide considerable confirmatory confidence in this methodology.
The values of CaKmf per unit placental weight obtained here and our demonstration that calcium flux across the mouse placenta is highly asymmetric, with CaJmf prevailing and approaching CaJnet, accord with previous observations in the rat (Štulc & Štulcová, 1986; Mughal et al. 1989; Robinson et al. 1989). Also, the very low ratio of CaKfm to CaKmf is consistent with that found in the rat (Štulc & Štulcová, 1986) and, to a lesser degree, in women (Štulc et al. 1994).
The phenotype of PTHrP-null mutants, a domed skull, short snout and mandible with protruding tongue and shortened limbs (Karaplis et al. 1994), were clearly discernible at 18 dp. In such mutants, PTHrP allele expression was confirmed to be absent using primers to exon IV, the major exon encoding murine PTHrP. The reduction of ∼6% in the weight of PTHrP-null fetuses is consistent with previous observations (Kovacs et al. 2001) although others report a similar body weight at birth (Karaplis et al. 1994). The efficiency of placental nutrient transfer may be attenuated in NL fetuses as evidenced by the reduced fetal: placental weight ratio.
In agreement with previous observations (Kovacs et al. 1996; Tucci et al. 1996), PTHrP-null mutants had a significantly lower fetal blood ionized Ca2+ concentration resulting in abolition of the fetomaternal Ca2+ concentration gradient. In contrast, fetal blood ionized Ca2+ concentration in WT and HZ fetuses was comparable and significantly higher than maternal Ca2+ concentration, also demonstrated previously (Kovacs et al. 1996; Tucci et al. 1996).
Previous studies have demonstrated a broad distribution of PTHrP mRNA and immunoreactivity in mouse placenta, being particularly abundant in the IPYS which lines the sinus of Duval connecting the yolk sac and the uterine lumen, but also detected in the labyrinth trophoblast (Kovacs et al. 2001, 2002), as observed here. As expected, PTHrP immunoreactivity was undetectable in the placental labyrinth of NL fetuses consistent with previous observations (Kovacs et al. 2001, 2002). We infer that PTHrP gene transcription in the placenta of NL fetuses (Fig. 1) arises from maternal cellular components of decidua (Karperien et al. 1996) although decidual PTHrP immunoreactivity in placenta from NL fetuses was undetectable (data not shown), suggesting paracrine effects of maternally derived PTHrP on placental function can be discounted.
Our starting hypothesis was that CaJmf would be down-regulated in placentas of NL fetuses based on previous evidence from PTHrP knockout mice (Kovacs et al. 1996) and studies examining the acute effects of PTHrP administration (outlined in the introduction). However, in contrast, our observations are all consistent in showing that calcium transport across the placenta of the NL fetus is, in fact, up-regulated. This assertion is based on the following evidence: (a) CaKmf was significantly elevated across the perfused placenta of the NL fetus compared to WT and HZ littermates, and at a significance level of P < 0.07 across the intact placenta (Table 2); (b) in the NL group there was a significant increase in fetal calcium accretion by day 19 of gestation consistent with an increased CaJnet (Table 3); (c) the very low and similar values of CaKfp in all genotypes (Table 2) implies different fetomaternal fluxes beween genotypes cannot account for (b); and (d) placental expression of calbindin-D9K, a molecular marker of placental calcium flux (Glazier et al. 1992) that predicts fetal calcium content (Verhaeghe et al. 1999), is probably raised (P < 0.07) in NL fetuses compared to WT and HZ littermates (Fig. 3).
Our previous flux measurements in the mouse implicate active calcium transport mechanisms (Bond et al. 2006a), compatible with our demonstration and that of others (Kovacs et al. 2002) that calcium transport proteins mediating influx (TRPV6; ECaC2), transcytosolic movement (calbindin-D9K) and efflux (PMCA) are co-expressed in mouse placenta. Of these, expression of calbindin-D9K appears, as noted previously (Kovacs et al. 2002), to be specifically altered by a lack of fetal PTHrP.
The up-regulation in calbindin-D9K expression parallels the increase in CaKmf, as in the rat where placental calbindin-D9K expression and CaKmf change in concert (Glazier et al. 1992; Husain et al. 1994). The cellular location of this altered calbindin-D9K expression remains uncertain, as a variety of cell types other than the labyrinth within the murine placenta express calbindin-D9K with a predominant localization to the IPYS (Mathieu et al. 1989; Ogura et al. 1998; Verhaeghe et al. 1999; Kovacs et al. 2002, 2005; Rummens et al. 2003). This distribution of calbindin-D9K to the IPYS, along with a host of other calcitropic hormones and receptors (Kovacs et al. 2002, 2005), has led to the proposal that the IPYS, a unique structure restricted to rodent placentas and formed by invagination of the primitive yolk sac into the chorioallantoic placenta (Ogura et al. 1998), provides a potential pathway for maternofetal calcium transfer in addition to exchange across the labyrinth trophoblast (Kovacs et al. 2002). However, the measurements of CaKmf made here do not allow relative contribution of these pathways to be addressed. Mutant mice lacking an IPYS (Ogura et al. 1998) may be useful in examining this issue further.
The collective evidence presented here supports the notion that maternofetal calcium transport by the placenta of NL fetuses is increased compared to WT and HZ littermates. This contrasts with the conclusions of Kovacs et al. (1996, 2001, 2002). They found diminished placental calcium transport across the intact placenta, normal fetal calcium content and reduced placental calbindin-D9K expression. However, there were several methodological differences between the two studies which might contribute to this disparity including: (a) the route, volume and amount of 45Ca tracer (intracardiac, 100 μl, 50 μCi 45Ca); (b) gestational age (17 dp); (c) anaesthetic (isoflurane inhalation); and (d) incorporation of 51Cr-EDTA (50 μCi) as a paracellular marker with data expressed as 45Ca/51Cr ratio. A change in the 45Ca/51Cr ratio as an index of calcium transport by Kovacs et al. (1996) allows for the possibility that either the numerator or denominator is altered. Related to this, we have previously shown that the maternofetal clearance of 14C-mannitol (a hydrophilic tracer with a similar diffusion coefficient in water to calcium) is significantly lower in the NL relative to its HZ and WT counterparts (Bond et al. 2006b), making interpretation following normalization to a paracellular marker under these circumstances a potential major source of error.
The interrelationships between fetal calcium homeostasis, placental calcium transport and fetal skeletal mineralization are complex, reliant upon the co-ordinated action of PTHrP and PTH (Tobias & Cooper, 2004; Miao et al. 2002). Fetal mice in which PTHrP or the PTHR1 gene is ablated or which lack parathyroid glands and so are unable to produce PTH (Hoxa3), all exhibit fetal hypocalcaemia, but show disparate patterns with regard to placental calcium transport, fetal PTH/PTHrP plasma concentration or fetal calcium accretion (Kovacs et al. 2001). This serves to emphasize that fetal calcaemia reflects the composite dynamics of calcium flux across both the placenta and fetal bone, calcium excretion by fetal kidney and its movement to and from amniotic fluid. The latter pathway is unlikely to confound the observations presented here as amniotic fluid calcium concentration is unaltered in NL fetuses (Kovacs et al. 2001).
The critical role of PTHrP in fetal skeletal morphogenesis is well established, acting in a paracrine manner to stimulate the proliferation of chondrocytes and delay their maturation (Karaplis et al. 1994; Miao et al. 2002; Tobias & Cooper, 2004). Derangement of this process by the targeted disruption of the PTHrP gene leads to lethal dyschondroplasia, premature differentiation of chondrocytes and advanced mineralization of the endochondral skeleton. This skeletal dysmorphogenesis is apparent as early as 14.5 days of gestation (Karaplis et al. 1994), a time at which PTHrP/PTH receptor expression is up-regulated in proliferating chondrocytes (MacLean & Kronenberg, 2005). Our demonstration that a lack of fetal PTHrP is associated with enhanced skeletal calcium deposition is compatible with the excessive skeletal mineralization and premature calcification/ossification found in NL fetuses (Karaplis et al. 1994) and agrees well with the observations of Tucci et al. (1996). In contrast, Kovacs et al. (2001) demonstrated comparable calcium content between genotypes, perhaps surprising in view of the excessive skeletal mineralization of NL fetuses. This excessive skeletal mineralization of the PTHrP-null mutant raises the possibility of an elevated fetal calcium demand, which we reason stimulates maternofetal calcium transport by a mechanism independent of fetal PTHrP, probably involving increased expression of calbindin-D9K.
The regulatory stimuli involved in evoking this calbindin-D9K response remain unclear. Fetal hypocalcaemia seems an unlikely candidate based on the lack of correspondence between placental calbindin-D9K expression which is reduced or unchanged in the face of fetal hypocalcaemia (Kovacs et al. 2001, 2002). Other studies showing that expression of calbindin-D9K in rodent placenta is not regulated by the biologically active form of vitamin D, 1,25(OH)2D3 (Glazier et al. 1995; Rummens et al. 2003; Kovacs et al. 2005) argue against its direct involvement. A significant elevation in fetal serum PTH in both PTHrP- and PTHR1-null fetuses (Kovacs et al. 2001) is not associated with a consistent trend in calbindin-D9K expression (Kovacs et al. 2002) suggesting a lack of direct regulation by PTH. Additionally PTH (1–84) had no effect on CaKmf across the placenta of intact rat fetuses (Robinson et al. 1989). Multiple regulatory factors may be involved in the trend to an altered placental calbindin-D9K expression observed here.
In summary, this study provides clear evidence of increased maternofetal calcium transport across the placenta of PTHrP-null fetuses with increased fetal calcium accretion despite pronounced fetal hypocalcaemia and abolition of the fetomaternal calcium gradient. The asymmetric nature of this response is indicated by the lack of effect on CaKfp. In situ placental perfusion of the mouse placenta provides a valuable tool to examine the regulation of unidirectional placental calcium flux, providing mechanistic insights regarding the role of fetal PTHrP.
Acknowledgments
This work was supported by a project grant from the Wellcome Trust (Grant number 076026/Z/04/Z). We are very grateful to Professor H. Kronenberg for providing the PTHrP knockout mice and to the staff at the University of Manchester BSU for all their kind help and co-operation with this project and also to Dr Rebecca Jones for her advice regarding the immunohistochemical studies.
References
- Abbas SK, Pickard DW, Rodda CP, Heath JH, Hammonds RG, Wood WI, Caple IW, Martin TJ, Care AD. Stimulation of ovine placental calcium transport by purified natural and recombinant parathyroid hormone-related protein (PTHrP) preparations. Q J Exp Physiol. 1989;74:549–552. doi: 10.1113/expphysiol.1989.sp003303. [DOI] [PubMed] [Google Scholar]
- An B-S, Choi K-C, Lee G-S, Leung PKC, Jeung E-B. Complex regulation of calbindin-D9k in the mouse placenta and extra-embryonic membrane during mid- and late pregnancy. Mol Cell Endocrinol. 2004;214:39–52. doi: 10.1016/j.mce.2003.11.029. [DOI] [PubMed] [Google Scholar]
- Atkinson DE, Boyd RDH, Sibley CP. Placental transfer. In: Neill JD, editor. Knobil and Neill's Physiology of Reproduction. 2. London: Elsevier; 2006. pp. 2787–2846. [Google Scholar]
- Belkacemi L, Bedard I, Simoneau L, Lafond J. Calcium channels, transporters and exchangers in placenta: a review. Cell Calcium. 2005;37:1–8. doi: 10.1016/j.ceca.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Bond H, Baker B, Boyd RDH, Cowley E, Glazier JD, Jones CJP, Sibley CP, Ward BS, Husain SM. Artificial perfusion of the fetal circulation of the in situ mouse placenta: methodology and validation. Placenta. 2006a;27(Suppl. A):S69–S75. doi: 10.1016/j.placenta.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Bond H, Baker B, Boyd R, Cowley E, Glazier J, Sibley C, Ward B, Husain S. Passive permeability of the mouse placenta to mannitol is reduced in parathyroid hormone related protein (PTHrP) null conceptuses. Proc Physiol Soc. 2006b;2:PC19. [Google Scholar]
- Bond H, Hamilton K, Balment R, Denton J, Freemont A, Garland H, Glazier J, Sibley C. Diabetes in pregnancy alters renal Ca2+ and Mg2+ reabsorption and bone formation in adult offspring. Diabetologia. 2005;48:1393–1400. doi: 10.1007/s00125-005-1804-5. [DOI] [PubMed] [Google Scholar]
- Borke JL, Caride A, Verma AK, Kelley LK, Smith CH, Penniston JT, Kumar R. Calcium pump epitopes in placental trophoblast plasma membranes. Am J Physiol Cell Physiol. 1989;257:C341–C346. doi: 10.1152/ajpcell.1989.257.2.C341. [DOI] [PubMed] [Google Scholar]
- Care AD, Abbas SK, Pickard DW, Barri M, Drinkhill M, Findlay JBC, White IR, Caple IW. Stimulation of ovine placental transport of calcium and magnesium by mid-molecule fragments of human parathyroid hormone related protein. Exp Physiol. 1990;75:605–608. doi: 10.1113/expphysiol.1990.sp003437. [DOI] [PubMed] [Google Scholar]
- Champion EE, Mann SJ, Glazier JD, Jones CJP, Rawlings JM, Sibley CP, Greenwood SL. System β and system A amino acid transporters in the feline endotheliochorial placenta. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1369–R1379. doi: 10.1152/ajpregu.00530.2003. [DOI] [PubMed] [Google Scholar]
- Comar CL. Radiocalcium studies in pregnancy. Ann N Y Acad Sci. 1956;64:281–298. [Google Scholar]
- Fisher GJ, Kelley LK, Smith CH. ATP-dependent calcium transport across basal plasma membranes of human placental trophoblast. Am J Physiol Cell Physiol. 1987;252:C38–C46. doi: 10.1152/ajpcell.1987.252.1.C38. [DOI] [PubMed] [Google Scholar]
- Flexner LB, Pohl HA. The transfer of radioactive sodium across the placenta of the rabbit. Am J Physiol. 1941;134:344–349. [Google Scholar]
- Glazier JD, Atkinson DE, Thornburg KL, Sharpe PT, Edwards D, Boyd RDH, Sibley CP. Gestational changes in Ca2+ transport across rat placenta and mRNA for calbindin9K and Ca2+-ATPase. Am J Physiol Regul Integr Comp Physiol. 1992;263:R930–R935. doi: 10.1152/ajpregu.1992.263.4.R930. [DOI] [PubMed] [Google Scholar]
- Glazier JD, Mawer EB, Sibley CP. Calbindin-D9K gene expression in rat chorioallantoic placenta is not regulated by 1,25-dihyroxyvitamin D3. Pediatr Res. 1995;37:720–725. doi: 10.1203/00006450-199506000-00008. [DOI] [PubMed] [Google Scholar]
- Hamilton K, Tein M, Glazier J, Mawer EB, Berry JL, Balment RJ, Boyd RDH, Garland HO, Sibley CP. Altered calbindin mRNA expression and calcium regulating hormones in rat diabetic pregnancy. J Endocrinol. 2000;164:67–76. doi: 10.1677/joe.0.1640067. [DOI] [PubMed] [Google Scholar]
- Husain SM, Birdsey TJ, Glazer JD, Mughal MZ, Garland HO, Sibley CP. Effect of diabetes mellitus on maternofetal flux of calcium and magnesium and calbindin9K mRNA expression in rat placenta. Pediatr Res. 1994;35:376–381. doi: 10.1203/00006450-199403000-00022. [DOI] [PubMed] [Google Scholar]
- Husain SM, Mughal MZ, Tsang RC. Calcium, phosphorus, and magnesium transport across the placenta. In: Polin RA, Fox WW, Abman SH, editors. Fetal and Neonatal Physiology. 3. Philadelphia: Saunders; 2004. pp. 314–322. [Google Scholar]
- Karaplis AC, Luz A, Glowacki J, Bronson RT, Tybulewicz VLJ, Kronenberg HM, Mulligan RC. Lethal skeletal dysplasia from targeted disruption of the parathyroid hormone-related peptide gene. Genes Dev. 1994;8:277–289. doi: 10.1101/gad.8.3.277. [DOI] [PubMed] [Google Scholar]
- Karperien M, Lanser P, De Laat SW, Boonstra J, Defize LHK. Parathyroid hormone related peptide mRNA expression during murine postimplantation development: evidence for involvement in multiple differentiation processes. Int J Dev Biol. 1996;40:599–608. [PubMed] [Google Scholar]
- Kovacs CS, Chafe LL, Woodland ML, McDonald KR, Fudge NJ, Wookey PJ. Calcitropic gene expression suggests a role for the intraplacental yolk sac in maternal-fetal calcium exchange. Am J Physiol Endocrinol Metab. 2002;282:E721–E732. doi: 10.1152/ajpendo.00369.2001. [DOI] [PubMed] [Google Scholar]
- Kovacs CS, Lanske B, Hunzelman JL, Guo J, Karaplis AC, Kronenberg HM. Parathyroid hormone-related peptide (PTHrP) regulates fetal-placental calcium transport through a receptor distinct from the PTH/PTHrP receptor. Proc Natl Acad Sci U S A. 1996;93:15233–15238. doi: 10.1073/pnas.93.26.15233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs CS, Manley NR, Moseley JM, Martin TJ, Kronenberg HM. Fetal parathyroids are not required to maintain placental calcium transport. J Clin Invest. 2001;107:1007–1015. doi: 10.1172/JCI11321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs CS, Woodland ML, Fudge NJ, Friel JK. The vitamin D receptor is not required for fetal mineral homeostasis or for the regulation of placental calcium transfer in mice. Am J Physiol Endocrinol Metab. 2005;289:E133–E144. doi: 10.1152/ajpendo.00354.2004. [DOI] [PubMed] [Google Scholar]
- MacLean HE, Kronenberg HM. Localization of Indian hedgehog and PTH/PTHrP receptor expression in relation to chondrocyte proliferation and mouse bone development. Dev Growth Differ. 2005;47:59–63. doi: 10.1111/j.1440-169x.2004.00781.x. [DOI] [PubMed] [Google Scholar]
- Mathieu CL, Burnett SH, Mills SE, Overpeck JG, Bruns DE, Bruns ME. Gestational changes in calbindin-D9k in rat uterus, yolk sac, and placenta: Implications for maternal-fetal calcium transport and uterine muscle function. Proc Natl Acad Sci U S A. 1989;86:3433–3437. doi: 10.1073/pnas.86.9.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meschia G, Battaglia FC, Bruns PD. Theoretical and experimental study of transplacental diffusion. J Appl Physiol. 1967;22:1171–1178. doi: 10.1152/jappl.1967.22.6.1171. [DOI] [PubMed] [Google Scholar]
- Miao D, He B, Karaplis AC, Goltzman D. Parathyroid hormone is essential for normal bone formation. J Clin Invest. 2002;109:1173–1182. doi: 10.1172/JCI14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mughal MZ, Ross R, Tsang RC. Clearance of calcium across in situ perfused placentas of intrauterine growth restricted rat fetuses. Pediatr Res. 1989;25:420–422. doi: 10.1203/00006450-198904000-00023. [DOI] [PubMed] [Google Scholar]
- Ogura Y, Takakura N, Yoshida H, Nishikawa S-I. Essential role of platelet-derived growth factor receptor α in the development of intraplacental yolk sac/sinus of Duval in mouse placenta. Biol Reprod. 1998;58:65–72. doi: 10.1095/biolreprod58.1.65. [DOI] [PubMed] [Google Scholar]
- Philbrick WM, Wysolmerski JJ, Holt GE, Orloff JJ, Yang KH, Vasavada RC, Weir EC, Broadus AE, Stewart AF. Defining the roles of parathyroid hormone-related protein in normal physiology. Physiol Rev. 1996;76:127–173. doi: 10.1152/physrev.1996.76.1.127. [DOI] [PubMed] [Google Scholar]
- Robinson NR, Sibley CP, Mughal MZ, Boyd RDH. Fetal control of calcium transport across the rat placenta. Pediatr Res. 1989;26:109–115. doi: 10.1203/00006450-198908000-00008. [DOI] [PubMed] [Google Scholar]
- Rummens K, van Cromphaut SJ, Carmeliet G, van Herck E, van Bree R, Stockmans I, Bouillon R, Verhaeghe J. Pregnancy in mice lacking the vitamin D receptor: Normal skeletal response but fetal hypomineralization rescued by maternal calcium supplementation. Pediatr Res. 2003;54:466–473. doi: 10.1203/01.PDR.0000081302.06915.D3. [DOI] [PubMed] [Google Scholar]
- Settle P, Mynett K, Speake P, Champion E, Doughty IM, Sibley CP, D'Souza SW, Glazier JD. Polarized lactate transporter activity and expression in the syncytiotrophoblast of the term human placenta. Placenta. 2004;25:496–504. doi: 10.1016/j.placenta.2003.11.009. [DOI] [PubMed] [Google Scholar]
- Shaw AJ, Mughal MZ, Maresh MJA, Sibley CP. Effects of two synthetic parathyroid hormone-related protein fragments on maternofetal transfer of calcium and magnesium and release of cyclic AMP by the in-situ perfused rat placenta. J Endocrinol. 1991;129:399–404. doi: 10.1677/joe.0.1290399. [DOI] [PubMed] [Google Scholar]
- Sibley CP. Mechanisms of ion transfer by rat placenta: a model for the human placenta? Placenta. 1994;15:675–691. doi: 10.1016/0143-4004(94)90030-2. [DOI] [PubMed] [Google Scholar]
- Sibley CP, Boyd RDH. Control of transfer across the mature placenta. In: Clarke J, editor. Oxford Reviews of Reproductive Biology. Vol. 10. Oxford: Oxford University Press; 1988. pp. 382–435. [PubMed] [Google Scholar]
- Štulc J, Štulcová B. Transport of calcium by the placenta of the rat. J Physiol. 1986;371:1–16. doi: 10.1113/jphysiol.1986.sp015958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Štulc J, Štulcová B, Ŝmíd M, Ŝach I. Parallel mechanisms of Ca++ transfer across the perfused human placental cotyledon. Am J Obstet Gynecol. 1994;170:162–167. doi: 10.1016/s0002-9378(94)70403-1. [DOI] [PubMed] [Google Scholar]
- Tobias JH, Cooper C. PTH/PTHrP activity and programming of skeletal development in utero. J Bone Miner Res. 2004;19:177–182. doi: 10.1359/JBMR.0301235. [DOI] [PubMed] [Google Scholar]
- Tucci J, Hammond V, Senior PV, Gibson A, Beck F. The role of fetal parathyroid hormone-related protein in transplacental calcium transport. J Mol Endocrinol. 1996;17:159–164. doi: 10.1677/jme.0.0170159. [DOI] [PubMed] [Google Scholar]
- Verhaeghe J, van Bree R, van Herck E, Rummens K, Vercruysse L, Bouillon R, Pijnenborg R. Pathogenesis of fetal hypomineralization in diabetic rats: Evidence for delayed bone maturation. Pediatr Res. 1999;45:209–217. doi: 10.1203/00006450-199902000-00009. [DOI] [PubMed] [Google Scholar]