Abstract
The brain is the most complex organ of the human body. It is composed of several highly specialized and heterogeneous populations of cells, represented by neurones (e.g. motoneurons, projection neurons or interneurons), and glia represented by astrocytes, oligodendrocytes and microglia. In recent years there have been numerous studies demonstrating close bidirectional communication of neurons and glia at structural and functional levels. In particular, the excitatory transmitter glutamate has been shown to evoke a variety of responses in astrocytes and oligodendrocytes in the healthy as well as the diseased brain. Here we overview the multitude of glutamate sensing molecules expressed in glia and describe some general experiments which have been performed to identify the glutamate-responsive molecules, i.e. the ionotropic and metabotropic glutamate receptors as well as the glutamate transporters. We also discuss a transgenic mouse model that permits detailed and specific investigations of the role of glial glutamate receptors.
Keywords: AMPA, astrocytes, glia, glutamate transporters, glutamate, kainate, neuronal–glial interactions, NMDA, oligodendrocytes
Introduction: neuronal–glial circuits
The neuronal doctrine, which from the 1890s shaped the development of neuroscience (von Waldeyer, 1891; Exner, 1894), is giving way to more inclusive theories, which regard the brain as a system of interconnected neuronal and glial networks (Haydon, 2001; Perea & Araque, 2005; Volterra & Meldolesi, 2005; Verkhratsky, 2006a). Numerous studies have demonstrated that all glial cell types, as diverse as the neuroectoderm-derived astrocytes, oligodendrocytes and NG2 glia or the mesoderm-derived microglia, express the same variety of neurotransmitter receptors as neurons in both culture systems and in in situ preparations (Verkhratsky & Kettenmann, 1996; Verkhratsky et al. 1998; Verkhratsky & Steinhauser, 2000). Many of these receptors, when activated by neural activity, initiate glial Ca2+ responses, which produce long-range interglial Ca2+ waves (Cornell-Bell et al. 1990; Cornell-Bell & Finkbeiner, 1991; Dani et al. 1992; Verkhratsky, 2006b). Glial Ca2+ signals, in turn, activate release of ‘glio'transmitters (which include glutamate, ATP, taurine, d-serine and probably many others) that can signal back to neurons, thus functionally integrating neuronal and glial circuitries (Bezzi et al. 1998, 2004; Zhang et al. 2004a,b; Volterra & Meldolesi, 2005). Glial cells perform a multitude of functions, which to a large extent control neuronal development, appearance, maintenance and plasticity of synaptic contacts, energy support, and integration into neuron–glia vascular units (Alvarez-Buylla et al. 2001; Malatesta et al. 2003; Nedergaard et al. 2003; Newman, 2003; Zonta et al. 2003; Hirrlinger et al. 2004; Mulligan & MacVicar, 2004; Gotz & Huttner, 2005; Magistretti, 2006; Verkhratsky, 2006b). Moreover, probably the most numerous glial cells, the astrocytes, can form an extended physically connected syncytium endowed with sophisticated mechanisms of intercellular ‘volume’ transmission (Dermietzel, 1998), which may allow them to play an important, albeit yet unknown, role in supporting high cognitive functions.
Glutamate as an excitatory neurotransmitter
Probably the first contemplation of glutamate as an excitatory transmitter in the central nervous system was made by Hayashi (1954), based on his findings that injection of glutamate in the brain provokes convulsions. By that time it was well known that glutamate is present in the nervous system at very high concentrations, yet it was firmly believed that glutamate is primarily involved with brain metabolism, rather then with synaptic transmission. The first direct experiment, which gave birth to the theory of glutamatergic transmission, was reported by Curtis et al. (1959), who discovered that iontophoretically delivered glutamate excited neurons in the spinal cord; they, however, found that other amino acids had the same ability and concluded that glutamate was most probably a non-specific agent not connected with interneuronal signalling. This view of non-specificity of glutamate prevailed for almost 20 years, before the appearance of specific agonists and antagonists helped to identify neuronal glutamate receptors (Evans et al. 1979; Watkins & Evans, 1981). In the ensuing 25 years the concept of glutamate as a main excitatory neurotransmitter in the brain was fully developed.
Incidentally, glutamate excites not only neurons but also glial cells, which became apparent in 1984 when microelectrode recordings revealed glutamate-mediated depolarization of astrocytes and oligodendrocytes (Bowman & Kimelberg, 1984; Kettenmann et al. 1984a,b). Further experiments have clearly demonstrated that glutamate acts as a mediator of both interneuronal and neuronal–glial signalling.
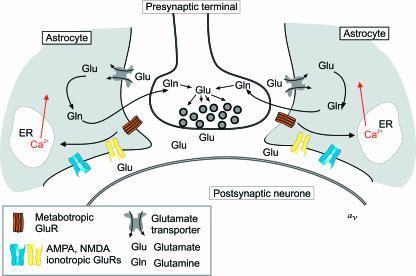
Overall, the glial cells interact with glutamate through three distinct molecular systems (Fig. 1), represented by ionotropic and metabotropic glutamate receptors as well as glutamate transporters. This review provides a concise overview of the diverse glutamate signalling pathways within the various glial cell types.
Fig. 1.
Glutamate-mediated neuronal–glial signalling. Synaptically released glutamate activates glial ionotropic (AMPA and NMDA) and metabotropic receptors. Activation of group I metabotropic receptors initiates phospholipase C-dependent synthesis of InsP3, which in turn triggers Ca2+ release from the endoplasmic reticulum (ER) Ca2+ store. The majority (~80%) of glutamate released during synaptic transmission is taken up by astroglial Na+/glutamate transporters; subsequently, glutamate is converted into glutamine, which is transported back to neurons, where it acts as a main source of newly synthesized glutamate (‘glutamate–glutamine shuttle’).
Ionotropic glutamate receptors
Three families of ionotropic glutamate receptors, defined by specific pharmacology and with distinct molecular structures, are present in the nervous system. These three families are AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid), kainate and NMDA (N-methyl-d-aspartate) receptors, which all belong to a broad family of cationic ligand-operated channels (for reviews see Wisden & Seeburg, 1993; Mayer & Armstrong, 2004; Mayer, 2005). All three families are expressed and functionally operational in the brain glia, although their distribution shows remarkable region- and cell-type specificity.
AMPA receptors are the most common in glia, being detected in various astroglial cells throughout the brain (Condorelli et al. 1999; Verkhratsky & Steinhauser, 2000; Seifert & Steinhauser, 2001), in white matter oligodendrocytes (Gottlieb & Matute, 1997; Bergles et al. 2000; Alberdi et al. 2005) and in NG2 glia (Lin & Bergles, 2002). Recently, they were also detected in microglia (Noda et al. 2000). The functional diversity of AMPA receptor-mediated membrane currents is determined by a variable assembly of four receptor subunits, GluR1 to GluR4, encoded by distinct genes (Wisden & Seeburg, 1993; Hollmann & Heinemann, 1994) and further post-transcriptional modification by alternative splicing and mRNA editing (Seeburg et al. 1998). AMPA receptors constructed from various subunits assemblies were identified in astroglial cells from virtually all brain areas, including hippocampus, cerebellum, neocortex and retina (Gallo & Ghiani, 2000; Verkhratsky & Steinhauser, 2000; Seifert & Steinhauser, 2001; Matthias et al. 2003) (Fig. 2). The comparative physiological analysis of glial cells from different brain regions was greatly facilitated by the use of transgenic mice with glia-specific expression of fluorescent proteins (Zhuo et al. 1997; Nolte et al. 2001; Mallon et al. 2002; Yuan et al. 2002; Hirrlinger et al. 2005). Importantly, in many types of glial cells, AMPA receptors are Ca2+ permeable, due to the relatively low abundance of the GluR2 subunit, which induces the Ca2+ impermeability of the receptor. The Ca2+ signals originating from Ca2+ influx through AMPA receptors were identified in several types of astrocytes, both in culture and in brain slices (Enkvist et al. 1989; Glaum et al. 1990; Burnashev et al. 1992; Muller et al. 1992; Jabs et al. 1994; Porter & McCarthy, 1995).
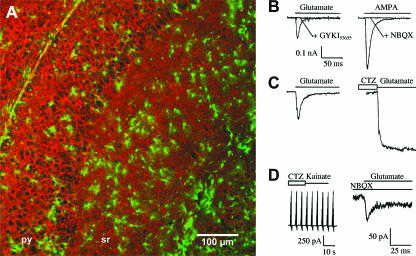
Fig. 2.
Glutamate responses in astroglial cells of the hippocampus. Within the hippocampus of TgN(GFAP-EGFP) transgenic mice two different astroglial cell populations can be distinguished by fluorescence intensities due to different levels of green fluorescent protein (EGFP) expression. These two cell populations respond to glutamate either by activation of glutamate transporters or AMPA-type glutamate receptors. (A) Confocal laser scanning microscopy of a hippocampal section obtained from TgN(GFAP-EGFP) mice and immunostained for the glutamate transporter GLT-1 (red). Astroglial cells (green) with different morphology can be readily distinguished, isolated and electrophysiologically characterized. (B) In two of the EGFP-positive astrocytes, rapid application of glutamate (left, postnatal day 9) or AMPA (right, postnatal day 15) evoked fast transient and completely desensitizing [τ (inactivation time constant) = 6 and 8.2 ms, respectively] inward currents that were completely inhibited by AMPA receptor antagonists GYKI53655 or NBQX. (C) In another EGFP-positive cell (postnatal day 13), the control response to glutamate (left) was enhanced two-fold in the presence of cyclothiazide (CTZ; right). (D) Cells with numerous processes and brightest fluorescence levels do not express functional AMPA receptors but possess functional glutamate transporters, since kainate cannot elicit currents while an inward current can still be observed in the presence of the selective AMPA receptor blocker NBQX. A: F. Kirchhoff, personal observations; B–D: modified from Matthias et al. (2003).
The second type of glutamate receptors, the kainate receptor, is assembled from five distinct subunits, the KA1 and KA2 and GluR5–7 (Huettner, 2003; Lerma, 2003). All these subunits were identified in certain types of astroglial cells (e.g. in bovine corpus callosum or in rodent perivascular astrocytes) at either the mRNA or the protein level (Garcia-Barcina & Matute, 1996; Brand-Schieber et al. 2004). Unfortunately, we still lack the evidence for functional kainate receptors determined as ion fluxes through the receptor pore. Incidentally, the GluR5 receptors are important for astroglial–neuronal signalling in hippocampus, where glutamate, released from the astroglia, signals to inhibitory neurons precisely through activation of neuronal GluR5 kainate receptors (Frerking, 2004; Liu et al. 2004). Kainate receptors were also identified in oligodendrocytes, where they may be important for mediating glutamate excitotoxicity (Alberdi et al. 2005, 2006).
The third type of glutamate receptor are the NMDA receptors encoded by seven genes, NR1, NR2A–D, and NR3A and B (Kew & Kemp, 2005). Similar to the AMPA or kainate receptors a heteromeric assembly of four subunits forms the ion-permeating pore of the NMDA receptor. The NR1 subunit is essential. NR2 or NR3 subunits alone are not sufficient to form a functional channel. NMDA receptors were for a long-time considered to be specifically confined to neurons. NMDA receptors display several important features, which conceptually made them relevant for neuronal integration and regulation of synaptic plasticity. These features include a very high Ca2+ permeability (PCa/PNa > 10; Mori & Mishina, 1995) and a specific Mg2+-dependent voltage-dependent block, which renders NMDA receptors barely active at hyperpolarized (–80 to –70 mV) membrane potentials (Mayer et al. 1984; Nowak et al. 1984). As a consequence, NMDA-mediated Ca2+ fluxes occur only when Mg2+ block is removed by sufficient depolarization of neurons, which provides for coincidence detection, relevant for various forms of synaptic plasticity (Collingridge & Bliss, 1995). As glial cells typically maintain a high degree of membrane polarization (resting potential ~ –80 mV) and are almost devoid of depolarizing ion permeabilities, it was firmly believed that NMDA receptors do not have any room for operation. Yet some time ago NMDA receptors subunits were detected in several types of astrocytes by immunohistochemistry, RT-PCR or in situ hybridization. In addition, NMDA-induced Ca2+ and membrane current responses were recorded from astroglia (e.g. Muller et al. 1993; Conti et al. 1996, 1997, 1999; Ziak et al. 1998; Schipke et al. 2001). Very recently, a series of papers substantiated the presence of functional NMDA receptors in cortical astrocytes (Fig. 3; Lalo et al. 2006) and demonstrated NMDA receptor expression and their fatal involvement in ischaemic insults in oligodendrocytes (Karadottir et al. 2005; Salter & Fern, 2005; Micu et al. 2006). Interestingly, in hippocampal astrocytes an up-regulation of the NMDA receptor subunit NR2B could be detected a few days after ischaemia and anoxia (Krebs et al. 2003). Incidentally, glial NMDA receptors are much less sensitive to Mg2+ block, which may result from a specific molecular composition (Lipton, 2006) being, as result, perfectly operational at the glial resting potentials.
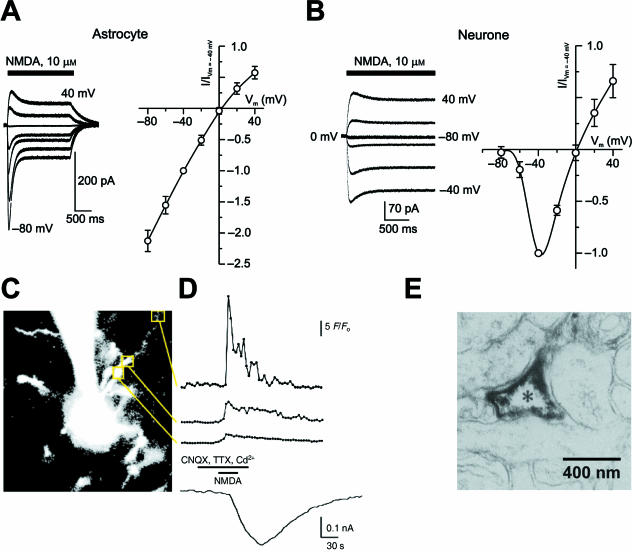
Fig. 3.
NMDA responses in astrocytes and in neurons. (A,B) Comparison of NMDA activated currents and corresponding I–V curves measured from an astrocyte (A) and a neuron (B) acutely dissociated from the same mouse cortical slice. The recordings were performed in normal physiological solution, containing 1 mm Mg2+. Note clear voltage-dependent relief from Mg2+ block in neurons vs. linear I–V curve in astrocytes. (C) A cell with the morphological features of an astrocyte was dialysed with Fluo-4 via the patch pipette in a cortical slice acutely isolated from a non-transgenic FVB/N mouse. Note the recording pipette approaching the cell from top. Changes in fluorescence (F/Fo) were analysed in the areas delineated by squares and are displayed in the top three traces in D; the lower trace shows simultaneously recorded membrane current response. NMDA (100 µm), CNQX, TTX and Cd2+ were applied as indicated. The largest fluorescent intensity changes, i.e. the highest Ca2+ influx, demonstrates high level of NMDA receptor expression in distal astroglial processes rather than in somatic regions. (E) Immunoelectron micrograph visualizing the expression of the NR1 subunit in astroglial processes of the rat cortex. A, B: reproduced from Lalo et al. (2006); C, D: modified from Schipke et al. (2001); E: modified from Aoki et al. (1994).
All in all, glial cells have a wide complement of ionotropic glutamate receptors, which have different sensitivity to glutamate, different kinetics and different ion permeability properties, thus allowing glial cells a high degree of sophistication in deciphering signals carried by external glutamate.
Metabotropic glutamate receptors
Metabotropic glutamate receptors are abundantly present in glial cells. These receptors, represented by eight different genes (mGluR1–8), belong to the family of seven-transmembrane-domain, G-protein-coupled receptors (Nakanishi, 1994; Kew & Kemp, 2005; Ferraguti & Shigemoto, 2006). Metabotropic glutamate receptors 1 and 5 (also known as group I mGluRs) are functionally linked to phospholipase C and synthesis of 1,4,5-inositol-trisphosphate (InsP3) and diacylglycerol (DAG); by contrast, mGluRs 2 and 3 (group II) and mGluRs 4 and 6–8 (Group III) are coupled to adenylate cyclase. Although it is assumed that glial cells can express all types of mGluRs, mGluR 3 and 5 remain the most abundant and best characterized glial mGluRs. These two types of mGluRs were also identified in situ, in astroglial processes at the ultrastructural level (Petralia et al. 1996; Aronica et al. 2000; Tamaru et al. 2001). Immunohistochemical identification of mGluR subtypes in oligodendroglia has not yet been shown. Both mGluR 3 and 5 could be demonstrated on cultured oligodendrocyte progenitor cells by Western blot analysis (Deng et al. 2004). Functionally, the group I mGluRs are important for triggering cytosolic Ca2+ signalling through stimulation of InsP3-induced Ca2+ release from the endoplasmic reticulum (ER) calcium store (Verkhratsky, 2005). Conceptually, the ER calcium store acts as a main source for glial calcium signalling; furthermore, it forms an intracellular excitable medium, which is instrumental for generation of long-range propagating glial Ca2+ waves (Verkhratsky & Kettenmann, 1996; Verkhratsky et al. 1998; Verkhratsky, 2006b). Stimulation of mGluR5 triggers elevation of cytosolic Ca2+ concentration in glia; these Ca2+ signals do not require extracellular Ca2+ and are sensitive to inhibitors of ER Ca2+ accumulation (thapsigargin or cyclopiazonic acid) and to heparin, which blocks InsP3 receptors residing in the ER membrane (Fig. 4; Kirischuk et al. 1999). Furthermore, at least in Bergmann glial cells, the mGluRs represent the main route for Ca2+ signal generation, as Ca2+ entry through AMPA receptors is limited by rapid desensitization of the receptors when activated by glutamate (cf. Fig. 2; Kirischuk et al. 1999).
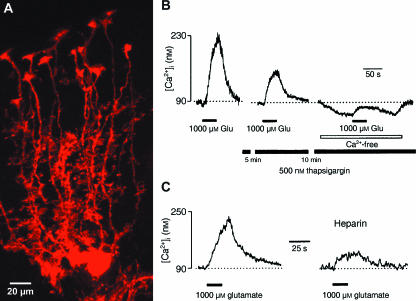
Fig. 4.
Metabotropic glutamate receptors in Bergmann glial cells. (A) Confocal laser scanning micrograph of single Bergmann glial cells in parasagittal brain sections of TgN(GFAP-mRFP1) mice, where Bergmann glial cells are selectively labelled by the expression of the red fluorescent protein mRFP1. (B) Incubation of slices with thapsigargin inhibits glutamate-induced [Ca2+]i transients. The [Ca2+]i transients were recorded from the Fura-2/AM ‘bulk’ loaded Bergmann glial cells; after recording the control response (first trace on the left) slices were incubated with 500 nm thapsigargin for 15 min before recordings re-commenced. (C) Heparin inhibits glutamate-triggered [Ca2+]i transients. The [Ca2+]i transient shown on the left panel was recorded from Fura-2/AM ‘bulk’ loaded Bergmann glial cell. Subsequently the cell was approached with patch pipette and the whole-cell patch clamp configuration was established. After 5 min of cell dialysis with the intrapipette solution containing 10 µm heparin, glutamate was applied for the second time. Note that heparin significantly reduced the amplitude of glutamate-triggered [Ca2+]i transient. A: from Scheller and F. Kirchhoff, own observations; B,C: modified from Kirischuk et al. (1999).
Glutamate transporters and glial Na+ homeostasis
Astroglial cells represent the main sink of glutamate in the brain. From the bulk of glutamate released during synaptic transmission, about 20% is accumulated into postsynaptic neurons and the remaining 80% is taken up by perisynaptic astrocytes (Swanson, 2005). This transport is achieved by specific glutamate transporters, represented, in the human brain, by five types, classified as EAAT1 to EAAT5 (where EAAT stands for excitatory amino acid transporter). EAAT1 and EAAT2 (analogues of which in rodent brain are known as glutamate/aspartate transporter, GLAST, and glutamate transporter-1, GLT-1) are expressed almost exclusively in astrocytes (Danbolt, 2001). Translocation of glutamate is powered by transmembrane ion gradients, and transport of a single glutamate molecule requires influx of three Na+ ions and one H+ ion together with efflux of one K+ ion. As a result Na+/glutamate transporter has an electrogenic effect, manifested in a net inward sodium current, and may substantially affect intracellular Na+ concentration.
Future experimental strategies: astroglia-specific and inducible gene deletion
Many studies have been performed to investigate the repertoire of glutamate signalling in glial cells. It has been shown that glial cells are sophisticated sensors of the excitatory transmitter glutamate, which is achieved through various response pathways. Remarkably, glial cells do not necessarily express significantly different glutamate receptors than their neighbouring neurons. Therefore, it was almost impossible to analyse the unique contribution of a given receptor subunit expressed in glia to the function of the whole brain or the behaviour of the freely moving animal. Any pharmacological intervention would have immediately affected both cell types, neurons and glia.
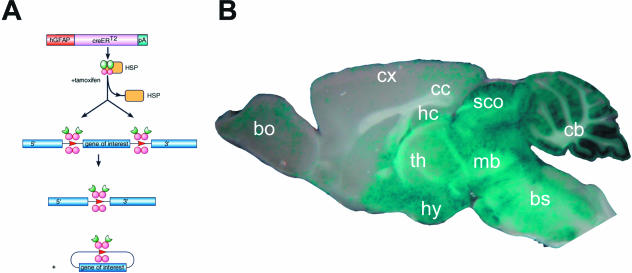
Very recently a series of transgenic mice have been developed which allow the selective ablation of defined genes either in astrocytes or in oligodendrocytes. Transgenic mice were generated by oocyte injection (non-homologous recombination) of transgenes composed of the human glial fibrillary acidic protein (GFAP) promoter (to achieve astroglia-specific expression), the coding sequence for a fusion protein of the DNA recombinase Cre and the mutated ligand binding domain of the human oestrogen receptor (Fig. 5; Hirrlinger et al. 2006). A different mouse line has been generated by direct targeting (homologous recombination) of the CreERT2 coding sequence into the astroglia-specific locus of the glutamate transporter GLAST (Mori et al. 2006). Inducible recombination in myelinating glia (oligodendrocytes and Schwann cells) is also possible by using the TgN(PLP-CreERT2) mice in which CreERT2 is targeted by the mouse proteolipid protein promoter into oligodendrocytes (Doerflinger et al. 2003; Leone et al. 2003).
Fig. 5.
Temporal control of astroglia-specific gene deletion in TgN(GFAP-CreERT2) mice by tamoxifen. (A) Principles of inducible gene recombination. Astrocytes of TgN(GFAP-CreERT2) mice express a fusion protein (CreERT2) of the DNA recombinase Cre and a mutated ligand binding domain of the oestrogen receptor. Under normal conditions CreERT2 is trapped in the cytoplasm by binding to heat shock proteins. The oestrogen receptor antagonist tamoxifen can liberate CreERT2 from this complex. After subsequent translocation into the nucleus, CreERT2 selectively excises DNA regions, which were flanked by loxP sites. (B) In cross-breeds of GFAP-CreERT2 mice with appropriate lacZ reporter mice widespread gene recombination in astrocytes can be achieved. The highest recombination rates are observed in the cerebellum (cb) and midbrain (mb). bulbus olfactorius (bo), brainstem (bs), corpus callosum (cc), cortex (cx), hippocampus (hc), hypothalamus (hy), and thalamus (th). Modified from Hirrlinger et al. (2006).
The transgenic CreERT2-mice can now be cross-bred to genetically modified mice in which relevant target genes, i.e. glutamate receptor genes, are flanked by loxP sites. The functional role of a given gene in a defined glial cell population can now be tested in the whole animal (e.g. by in vivo physiology or behavioural experiments) by temporal control of gene ablation via intraperitoneal injection of tamoxifen. Several mouse lines with appropriate modification of glutamate receptor genes are already available, for example to study different AMPA receptor genes GluR1–4 or the NR1 subunit of NMDA receptors (Tsien et al. 1996; Zamanillo et al. 1999). Unfortunately, similar models are not yet available for the metabotropic glutamate receptors.
Conclusions
Glutamate mediates neuronal–neuronal and neuronal–glial signalling. Synaptic release of glutamate triggers a complex response in glial cells, which involves activation of several distinct types of ionotropic and metabotropic receptors as well as glutamate transporters. These molecules, in turn, initiate a variety of intracellular signalling processes, most notably those associated with propagating cytoplasmic Ca2+ signals. Extended complement of glutamate sensors expressed in glial cell membranes allows the glia to decode neuronal activity and to synchronize and integrate neuronal and glial circuitries. Deployment of transgenic animal models with conditional deletion of single signalling components promises new discoveries of the pivotal functions of glial cells.
Acknowledgments
The research was supported by the National Institute of Health, the Royal Society, the Wellcome Trust, the Alzheimer Research Trust (UK), the Max Plank Society and Deutsche Forschungsgemeinschaft.
References
- Alberdi E, Sanchez-Gomez MV, Matute C. Calcium and glial cell death. Cell Calcium. 2005;38:417–425. doi: 10.1016/j.ceca.2005.06.020. [DOI] [PubMed] [Google Scholar]
- Alberdi E, Sanchez-Gomez MV, Torre I, et al. Activation of kainate receptors sensitizes oligodendrocytes to complement attack. J Neurosci. 2006;26:3220–3228. doi: 10.1523/JNEUROSCI.3780-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Garcia-Verdugo JM, Tramontin AD. A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci. 2001;2:287–293. doi: 10.1038/35067582. [DOI] [PubMed] [Google Scholar]
- Aoki C, Venkatesan C, Go CG, Mong JA, Dawson TM. Cellular and subcellular localization of NMDA-R1 subunit immunoreactivity in the visual cortex of adult and neonatal rats. J Neurosci. 1994;14:5202–5222. doi: 10.1523/JNEUROSCI.14-09-05202.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronica E, van Vliet EA, Mayboroda OA, Troost D, da Silva FH, Gorter JA. Upregulation of metabotropic glutamate receptor subtype mGluR3 and mGluR5 in reactive astrocytes in a rat model of mesial temporal lobe epilepsy. Eur J Neurosci. 2000;12:2333–2344. doi: 10.1046/j.1460-9568.2000.00131.x. [DOI] [PubMed] [Google Scholar]
- Bergles DE, Roberts JD, Somogyi P, Jahr CE. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature. 2000;405:187–191. doi: 10.1038/35012083. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Carmignoto G, Pasti L, et al. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature. 1998;391:281–285. doi: 10.1038/34651. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Gundersen V, Galbete JL, et al. Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nat Neurosci. 2004;7:613–620. doi: 10.1038/nn1246. [DOI] [PubMed] [Google Scholar]
- Bowman CL, Kimelberg HK. Excitatory amino acids directly depolarize rat brain astrocytes in primary culture. Nature. 1984;311:656–659. doi: 10.1038/311656a0. [DOI] [PubMed] [Google Scholar]
- Brand-Schieber E, Lowery SL, Werner P. Select ionotropic glutamate AMPA/kainate receptors are expressed at the astrocyte–vessel interface. Brain Res. 2004;1007:178–182. doi: 10.1016/j.brainres.2003.12.051. [DOI] [PubMed] [Google Scholar]
- Burnashev N, Khodorova A, Jonas P, et al. Calcium-permeable AMPA-kainate receptors in fusiform cerebellar glial cells. Science. 1992;256:1566–1570. doi: 10.1126/science.1317970. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Bliss TV. Memories of NMDA receptors and LTP. Trends Neurosci. 1995;18:54–56. [PubMed] [Google Scholar]
- Condorelli DF, Conti F, Gallo V, et al. Expression and functional analysis of glutamate receptors in glial cells. Adv Exp Med Biol. 1999;468:49–67. doi: 10.1007/978-1-4615-4685-6_5. [DOI] [PubMed] [Google Scholar]
- Conti F, DeBiasi S, Minelli A, Melone M. Expression of NR1 and NR2A/B subunits of the NMDA receptor in cortical astrocytes. Glia. 1996;17:254–258. doi: 10.1002/(SICI)1098-1136(199607)17:3<254::AID-GLIA7>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Conti F, Minelli A, DeBiasi S, Melone M. Neuronal and glial localization of NMDA receptors in the cerebral cortex. Mol Neurobiol. 1997;14:1–18. doi: 10.1007/BF02740618. [DOI] [PubMed] [Google Scholar]
- Conti F, Barbaresi P, Melone M, Ducati A. Neuronal and glial localization of NR1 and NR2A/B subunits of the NMDA receptor in the human cerebral cortex. Cereb Cortex. 1999;9:110–120. doi: 10.1093/cercor/9.2.110. [DOI] [PubMed] [Google Scholar]
- Cornell-Bell AH, Finkbeiner SM, Cooper MS, Smith SJ. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science. 1990;247:470–473. doi: 10.1126/science.1967852. [DOI] [PubMed] [Google Scholar]
- Cornell-Bell AH, Finkbeiner SM. Ca2+ waves in astrocytes. Cell Calcium. 1991;12:185–204. doi: 10.1016/0143-4160(91)90020-f. [DOI] [PubMed] [Google Scholar]
- Curtis DR, Phillis JW, Watkins JC. Chemical excitation of spinal neurones. Nature. 1959;183:611–612. doi: 10.1038/183611a0. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Progr Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Dani JW, Chernjavsky A, Smith SJ. Neuronal activity triggers calcium waves in hippocampal astrocyte networks. Neuron. 1992;8:429–440. doi: 10.1016/0896-6273(92)90271-e. [DOI] [PubMed] [Google Scholar]
- Deng W, Wang H, Rosenberg PA, Volpe JJ, Jensen FE. Role of metabotropic glutamate receptors in oligodendrocyte excitotoxicity and oxidative stress. Proc Natl Acad Sci USA. 2004;101:7751–7756. doi: 10.1073/pnas.0307850101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dermietzel R. Gap junction wiring: a ‘new’ principle in cell-to-cell communication in the nervous system? Brain Res Brain Res Rev. 1998;26:176–183. doi: 10.1016/s0165-0173(97)00031-3. [DOI] [PubMed] [Google Scholar]
- Doerflinger NH, Macklin WB, Popko B. Inducible site-specific recombination in myelinating cells. Genesis. 2003;35:63–72. doi: 10.1002/gene.10154. [DOI] [PubMed] [Google Scholar]
- Enkvist MO, Holopainen I, Akerman KE. Glutamate receptor-linked changes in membrane potential and intracellular Ca2+ in primary rat astrocytes. Glia. 1989;2:397–402. doi: 10.1002/glia.440020602. [DOI] [PubMed] [Google Scholar]
- Evans RH, Francis AA, Hunt K, Oakes DJ, Watkins JC. Antagonism of excitatory amino acid-induced responses and of synaptic excitation in the isolated spinal cord of the frog. Br J Pharmacol. 1979;67:591–603. doi: 10.1111/j.1476-5381.1979.tb08706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exner S. Entwurf zur physiologischen Erklärung der Psychischen Erscheinungen. Leipzig/Vienna: Deiticke; 1894. [Google Scholar]
- Ferraguti F, Shigemoto R. Metabotropic glutamate receptors. Cell Tissue Res. 2006;326:483–504. doi: 10.1007/s00441-006-0266-5. [DOI] [PubMed] [Google Scholar]
- Frerking M. When astrocytes signal, kainate receptors respond. Proc Natl Acad Sci USA. 2004;101:2649–2650. doi: 10.1073/pnas.0400474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo V, Ghiani CA. Glutamate receptors in glia: new cells, new inputs and new functions. Trends Pharmacol Sci. 2000;21:252–258. doi: 10.1016/s0165-6147(00)01494-2. [DOI] [PubMed] [Google Scholar]
- Garcia-Barcina JM, Matute C. Expression of kainate-selective glutamate receptor subunits in glial cells of the adult bovine white matter. Eur J Neurosci. 1996;8:2379–2387. doi: 10.1111/j.1460-9568.1996.tb01201.x. [DOI] [PubMed] [Google Scholar]
- Glaum SR, Holzwarth JA, Miller RJ. Glutamate receptors activate Ca2+ mobilization and Ca2+ influx into astrocytes. Proc Natl Acad Sci USA. 1990;87:3454–3458. doi: 10.1073/pnas.87.9.3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb M, Matute C. Expression of ionotropic glutamate receptor subunits in glial cells of the hippocampal CA1 area following transient forebrain ischemia. J Cereb Blood Flow Metab. 1997;17:290–300. doi: 10.1097/00004647-199703000-00006. [DOI] [PubMed] [Google Scholar]
- Gotz M, Huttner WB. The cell biology of neurogenesis. Nat Rev Mol Cell Biol. 2005;6:777–788. doi: 10.1038/nrm1739. [DOI] [PubMed] [Google Scholar]
- Hayashi T. Effects of sodium glutamate on the nervous system. Keio J Med. 1954;3:192–193. [Google Scholar]
- Haydon PG. GLIA: listening and talking to the synapse. Nat Rev Neurosci. 2001;2:185–193. doi: 10.1038/35058528. [DOI] [PubMed] [Google Scholar]
- Hirrlinger J, Hulsmann S, Kirchhoff F. Astroglial processes show spontaneous motility at active synaptic terminals in situ. Eur J Neurosci. 2004;20:2235–2239. doi: 10.1111/j.1460-9568.2004.03689.x. [DOI] [PubMed] [Google Scholar]
- Hirrlinger PG, Scheller A, Braun C, et al. Expression of reef coral fluorescent proteins in the central nervous system of transgenic mice. Mol Cell Neurosci. 2005;30:291–303. doi: 10.1016/j.mcn.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Hirrlinger PG, Scheller A, Braun C, Hirrlinger J, Kirchhoff F. Temporal control of gene recombination in astrocytes by transgenic expression of the tamoxifen-inducible DNA recombinase variant CreERT2. Glia. 2006;54:11–20. doi: 10.1002/glia.20342. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Huettner JE. Kainate receptors and synaptic transmission. Prog Neurobiol. 2003;70:387–407. doi: 10.1016/s0301-0082(03)00122-9. [DOI] [PubMed] [Google Scholar]
- Jabs R, Kirchhoff F, Kettenmann H, Steinhauser C. Kainate activates Ca2+-permeable glutamate receptors and blocks voltage-gated K+ currents in glial cells of mouse hippocampal slices. Pflugers Arch. 1994;426:310–319. doi: 10.1007/BF00374787. [DOI] [PubMed] [Google Scholar]
- Karadottir R, Cavelier P, Bergersen LH, Attwell D. NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature. 2005;438:1162–1166. doi: 10.1038/nature04302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettenmann H, Backus KH, Schachner M. Aspartate, glutamate and gamma-aminobutyric acid depolarize cultured astrocytes. Neurosci Lett. 1984a;52:25–29. doi: 10.1016/0304-3940(84)90345-8. [DOI] [PubMed] [Google Scholar]
- Kettenmann H, Gilbert P, Schachner M. Depolarization of cultured oligodendrocytes by glutamate and GABA. Neurosci Lett. 1984b;47:271–276. doi: 10.1016/0304-3940(84)90525-1. [DOI] [PubMed] [Google Scholar]
- Kew JN, Kemp JA. Ionotropic and metabotropic glutamate receptor structure and pharmacology. Psychopharmacology (Berl) 2005;179:4–29. doi: 10.1007/s00213-005-2200-z. [DOI] [PubMed] [Google Scholar]
- Kirischuk S, Kirchhoff F, Matyash V, Kettenmann H, Verkhratsky A. Glutamate-triggered calcium signalling in mouse Bergmann glial cells in situ: role of inositol-1,4,5-trisphosphate-mediated intracellular calcium release. Neuroscience. 1999;92:1051–1059. doi: 10.1016/s0306-4522(99)00067-6. [DOI] [PubMed] [Google Scholar]
- Krebs C, Fernandes HB, Sheldon C, Raymond LA, Baimbridge KG. Functional NMDA receptor subtype 2B is expressed in astrocytes after ischemia in vivo and anoxia in vitro. J Neurosci. 2003;23:3364–3372. doi: 10.1523/JNEUROSCI.23-08-03364.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalo U, Pankratov Y, Kirchhoff F, North RA, Verkhratsky A. NMDA receptors mediate neuron-to-glia signaling in mouse cortical astrocytes. J Neurosci. 2006;26:2673–2683. doi: 10.1523/JNEUROSCI.4689-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone DP, Genoud S, Atanasoski S, et al. Tamoxifen-inducible glia-specific Cre mice for somatic mutagenesis in oligodendrocytes and Schwann cells. Mol Cell Neurosci. 2003;22:430–440. doi: 10.1016/s1044-7431(03)00029-0. [DOI] [PubMed] [Google Scholar]
- Lerma J. Roles and rules of kainate receptors in synaptic transmission. Nat Rev Neurosci. 2003;4:481–495. doi: 10.1038/nrn1118. [DOI] [PubMed] [Google Scholar]
- Lin SC, Bergles DE. Physiological characteristics of NG2-expressing glial cells. J Neurocytol. 2002;31:537–549. doi: 10.1023/a:1025799816285. [DOI] [PubMed] [Google Scholar]
- Lipton SA. NMDA receptors, glial cells, and clinical medicine. Neuron. 2006;50:9–11. doi: 10.1016/j.neuron.2006.03.026. [DOI] [PubMed] [Google Scholar]
- Liu QS, Xu Q, Arcuino G, Kang J, Nedergaard M. Astrocyte-mediated activation of neuronal kainate receptors. Proc Natl Acad Sci USA. 2004;101:3172–3177. doi: 10.1073/pnas.0306731101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magistretti PJ. Neuron-glia metabolic coupling and plasticity. J Exp Biol. 2006;209:2304–2311. doi: 10.1242/jeb.02208. [DOI] [PubMed] [Google Scholar]
- Malatesta P, Hack MA, Hartfuss E, et al. Neuronal or glial progeny: regional differences in radial glia fate. Neuron. 2003;37:751–764. doi: 10.1016/s0896-6273(03)00116-8. [DOI] [PubMed] [Google Scholar]
- Mallon BS, Shick HE, Kidd GJ, Macklin WB. Proteolipid promoter activity distinguishes two populations of NG2-positive cells throughout neonatal cortical development. J Neurosci. 2002;22:876–885. doi: 10.1523/JNEUROSCI.22-03-00876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthias K, Kirchhoff F, Seifert G, et al. Segregated expression of AMPA-type glutamate receptors and glutamate transporters defines distinct astrocyte populations in the mouse hippocampus. J Neurosci. 2003;23:1750–1758. doi: 10.1523/JNEUROSCI.23-05-01750.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer ML, Westbrook GL, Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984;309:261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- Mayer ML, Armstrong N. Structure and function of glutamate receptor ion channels. Annu Rev Physiol. 2004;66:161–181. doi: 10.1146/annurev.physiol.66.050802.084104. [DOI] [PubMed] [Google Scholar]
- Mayer ML. Glutamate receptor ion channels. Curr Opin Neurobiol. 2005;15:282–288. doi: 10.1016/j.conb.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Micu I, Jiang Q, Coderre E, et al. NMDA receptors mediate calcium accumulation in myelin during chemical ischaemia. Nature. 2006;439:988–992. doi: 10.1038/nature04474. [DOI] [PubMed] [Google Scholar]
- Mori H, Mishina M. Structure and function of the NMDA receptor channel. Neuropharmacology. 1995;34:1219–1237. doi: 10.1016/0028-3908(95)00109-j. [DOI] [PubMed] [Google Scholar]
- Mori T, Tanaka K, Buffo A, Wurst W, Kuhn R, Gotz M. Inducible gene deletion in astroglia and radial glia – a valuable tool for functional and lineage analysis. Glia. 2006;54:21–34. doi: 10.1002/glia.20350. [DOI] [PubMed] [Google Scholar]
- Muller T, Moller T, Berger T, Schnitzer J, Kettenmann H. Calcium entry through kainate receptors and resulting potassium-channel blockade in Bergmann glial cells. Science. 1992;256:1563–1566. doi: 10.1126/science.1317969. [DOI] [PubMed] [Google Scholar]
- Muller T, Grosche J, Ohlemeyer C, Kettenmann H. NMDA-activated currents in Bergmann glial cells. Neuroreport. 1993;4:671–674. doi: 10.1097/00001756-199306000-00017. [DOI] [PubMed] [Google Scholar]
- Mulligan SJ, MacVicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature. 2004;431:195–199. doi: 10.1038/nature02827. [DOI] [PubMed] [Google Scholar]
- Nakanishi S. Metabotropic glutamate receptors: synaptic transmission, modulation, and plasticity. Neuron. 1994;13:1031–1037. doi: 10.1016/0896-6273(94)90043-4. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Ransom B, Goldman SA. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci. 2003;26:523–530. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Newman EA. New roles for astrocytes: regulation of synaptic transmission. Trends Neurosci. 2003;26:536–542. doi: 10.1016/S0166-2236(03)00237-6. [DOI] [PubMed] [Google Scholar]
- Noda M, Nakanishi H, Nabekura J, Akaike N. AMPA-kainate subtypes of glutamate receptor in rat cerebral microglia. J Neurosci. 2000;20:251–258. doi: 10.1523/JNEUROSCI.20-01-00251.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolte C, Matyash M, Pivneva T, et al. GFAP promoter-controlled EGFP-expressing transgenic mice: a tool to visualize astrocytes and astrogliosis in living brain tissue. Glia. 2001;33:72–86. [PubMed] [Google Scholar]
- Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Perea G, Araque A. Glial calcium signaling and neuron–glia communication. Cell Calcium. 2005;38:375–382. doi: 10.1016/j.ceca.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Niedzielski AS, Wenthold RJ. The metabotropic glutamate receptors, mGluR2 and mGluR3, show unique postsynaptic, presynaptic and glial localizations. Neuroscience. 1996;71:949–976. doi: 10.1016/0306-4522(95)00533-1. [DOI] [PubMed] [Google Scholar]
- Porter JT, McCarthy KD. GFAP-positive hippocampal astrocytes in situ respond to glutamatergic neuroligands with increases in [Ca2+]i. Glia. 1995;13:101–112. doi: 10.1002/glia.440130204. [DOI] [PubMed] [Google Scholar]
- Salter MG, Fern R. NMDA receptors are expressed in developing oligodendrocyte processes and mediate injury. Nature. 2005;438:1167–1171. doi: 10.1038/nature04301. [DOI] [PubMed] [Google Scholar]
- Schipke CG, Ohlemeyer C, Matyash M, Nolte C, Kettenmann H, Kirchhoff F. Astrocytes of the mouse neocortex express functional N-methyl-D-aspartate receptors. FASEB J. 2001;15:1270–1272. doi: 10.1096/fj.00-0439fje. [DOI] [PubMed] [Google Scholar]
- Seeburg PH, Higuchi M, Sprengel R. RNA editing of brain glutamate receptor channels: mechanism and physiology. Brain Res Brain Res Rev. 1998;26:217–229. doi: 10.1016/s0165-0173(97)00062-3. [DOI] [PubMed] [Google Scholar]
- Seifert G, Steinhauser C. Ionotropic glutamate receptors in astrocytes. Prog Brain Res. 2001;132:287–299. doi: 10.1016/S0079-6123(01)32083-6. [DOI] [PubMed] [Google Scholar]
- Swanson RA. Astrocyte neurotransmitter uptake. In: Kettenmann H, Ransom BR, editors. Neuroglia. Oxford: Oxford University Press; 2005. pp. 346–354. [Google Scholar]
- Tamaru Y, Nomura S, Mizuno N, Shigemoto R. Distribution of metabotropic glutamate receptor mGluR3 in the mouse CNS: differential location relative to pre- and postsynaptic sites. Neuroscience. 2001;106:481–503. doi: 10.1016/s0306-4522(01)00305-0. [DOI] [PubMed] [Google Scholar]
- Tsien JZ, Chen DF, Gerber D, et al. Subregion- and cell type-restricted gene knockout in mouse brain. Cell. 1996;87:1317–1326. doi: 10.1016/s0092-8674(00)81826-7. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Kettenmann H. Calcium signalling in glial cells. Trends Neurosci. 1996;19:346–352. doi: 10.1016/0166-2236(96)10048-5. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Orkand RK, Kettenmann H. Glial calcium: homeostasis and signaling function. Physiol Rev. 1998;78:99–141. doi: 10.1152/physrev.1998.78.1.99. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Steinhauser C. Ion channels in glial cells. Brain Res Brain Res Rev. 2000;32:380–412. doi: 10.1016/s0165-0173(99)00093-4. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A. Physiology and pathophysiology of the calcium store in the endoplasmic reticulum of neurons. Physiol Rev. 2005;85:201–279. doi: 10.1152/physrev.00004.2004. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A. Patching the glia reveals functional organisation of the brain. Pflugers Arch. 2006a;453:411–420. doi: 10.1007/s00424-006-0099-9. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A. Calcium ions and integration in neural circuits. Acta Physiol (Oxford) 2006b;187:357–369. doi: 10.1111/j.1748-1716.2006.01566.x. [DOI] [PubMed] [Google Scholar]
- Volterra A, Meldolesi J. Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci. 2005;6:626–640. doi: 10.1038/nrn1722. [DOI] [PubMed] [Google Scholar]
- von Waldeyer HWG. Über einige neuere Forschungen im Gebiete der Anatomie des Centralnervensystems. Deutsche Med Wochenschrift. 1891;44:1–44. [Google Scholar]
- Watkins JC, Evans RH. Excitatory amino acid transmitters. Annu Rev Pharmacol Toxicol. 1981;21:165–204. doi: 10.1146/annurev.pa.21.040181.001121. [DOI] [PubMed] [Google Scholar]
- Wisden W, Seeburg PH. Mammalian ionotropic glutamate receptors. Curr Opin Neurobiol. 1993;3:291–298. doi: 10.1016/0959-4388(93)90120-n. [DOI] [PubMed] [Google Scholar]
- Yuan X, Chittajallu R, Belachew S, Anderson S, McBain CJ, Gallo V. Expression of the green fluorescent protein in the oligodendrocyte lineage: a transgenic mouse for developmental and physiological studies. J Neurosci Res. 2002;70:529–545. doi: 10.1002/jnr.10368. [DOI] [PubMed] [Google Scholar]
- Zamanillo D, Sprengel R, Hvalby O, et al. Importance of AMPA receptors for hippocampal synaptic plasticity but not for spatial learning. Science. 1999;284:1805–1811. doi: 10.1126/science.284.5421.1805. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Fukuda M, Van Bockstaele E, Pascual O, Haydon PG. Synaptotagmin IV regulates glial glutamate release. Proc Natl Acad Sci USA. 2004a;101:9441–9446. doi: 10.1073/pnas.0401960101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Pangrsic T, Kreft M, et al. Fusion-related release of glutamate from astrocytes. J Biol Chem. 2004b;279:12724–12733. doi: 10.1074/jbc.M312845200. [DOI] [PubMed] [Google Scholar]
- Zhuo L, Sun B, Zhang CL, Fine A, Chiu SY, Messing A. Live astrocytes visualized by green fluorescent protein in transgenic mice. Dev Biol. 1997;187:36–42. doi: 10.1006/dbio.1997.8601. [DOI] [PubMed] [Google Scholar]
- Ziak D, Chvatal A, Sykova E. Glutamate-, kainate- and NMDA-evoked membrane currents in identified glial cells in rat spinal cord slice. Physiol Res. 1998;47:365–375. [PubMed] [Google Scholar]
- Zonta M, Angulo MC, Gobbo S, et al. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]