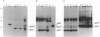Abstract
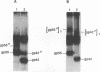
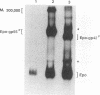
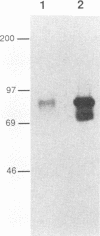
The env gene of Friend spleen focus-forming virus (SFFV) encodes a membrane glycoprotein (gp55) that is inefficiently (3 to 5%) processed from the rough endoplasmic reticulum to form a larger dimeric plasma membrane derivative (gp55p). Moreover, the SFFV env glycoprotein associates with erythropoietin receptors (EpoR) to cause proliferation of infected erythroblasts [J.-P. Li, A. D. D'Andrea, H. F. Lodish, and D. Baltimore, Nature (London) 343:762-764, 1990]. Interestingly, the mitogenic effect of SFFV is blocked in mice homozygous for the Fv-2r resistance gene, but mutant SFFVs can overcome this resistance. Recent evidence suggested that these mutants contain partial env deletions that truncate the membrane-proximal extracellular domain of the encoded glycoproteins (M. H. Majumdar, C.-L. Cho, M. T. Fox, K. L. Eckner, S. Kozak, D. Kabat, and R. W. Geib, J. Virol. 66:3652-3660, 1992). Mutant BB6, which encodes a gp42 glycoprotein that has a large deletion in this domain, causes erythroblastosis in DBA/2 (Fv-2s) as well as in congenic D2.R (Fv-2r) mice. Analogous to gp55, gp42 is processed inefficiently as a disulfide-bonded dimer to form cell surface gp42p. Retroviral vectors with SFFV and BB6 env genes have no effect on interleukin 3-dependent BaF3 hematopoietic cells, but they cause growth factor independency of BaF3/EpoR cells, a derivative that contains recombinant EpoR. After binding 125I-Epo to surface EpoR on these factor-independent cells and adding the covalent cross-linking reagent disuccinimidyl suberate, complexes that had immunological properties and sizes demonstrating that they consisted of 125I-Epo-gp55p and 125I-Epo-gp42p were isolated from cell lysates. Contrary to a previous report, SFFV or BB6 env glycoproteins did not promiscuously activate other members of the EpoR superfamily. Although the related env glycoproteins encoded by dualtropic murine leukemia viruses formed detectable complexes with EpoR, strong mitogenic signalling did not ensue. Our results indicate that the SFFV and BB6 env glycoproteins specifically activate EpoR; they help to define the glycoprotein properties important for its functions; and they strongly suggest that the Fv-2 leukemia control gene encodes an EpoR-associated regulatory factor.
Full text
PDF


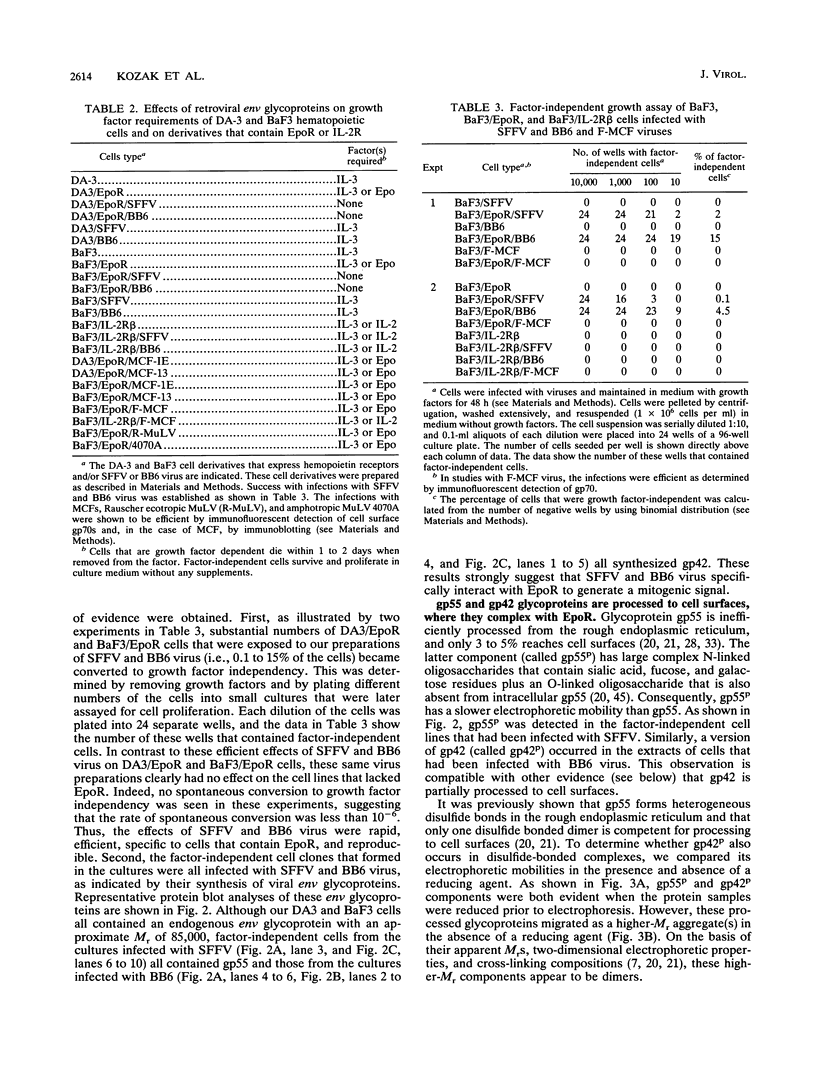




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi A., Sakai K., Kitamura N., Nakanishi S., Niwa O., Matsuyama M., Ishimoto A. Characterization of the env gene and long terminal repeat of molecularly cloned Friend mink cell focus-inducing virus DNA. J Virol. 1984 Jun;50(3):813–821. doi: 10.1128/jvi.50.3.813-821.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan J. F. Structural design and molecular evolution of a cytokine receptor superfamily. Proc Natl Acad Sci U S A. 1990 Sep;87(18):6934–6938. doi: 10.1073/pnas.87.18.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behringer R. R., Dewey M. J. Cellular site and mode of Fv-2 gene action. Cell. 1985 Feb;40(2):441–447. doi: 10.1016/0092-8674(85)90158-8. [DOI] [PubMed] [Google Scholar]
- Ben-David Y., Bernstein A. Friend virus-induced erythroleukemia and the multistage nature of cancer. Cell. 1991 Sep 6;66(5):831–834. doi: 10.1016/0092-8674(91)90428-2. [DOI] [PubMed] [Google Scholar]
- Bestwick R. K., Kozak S. L., Kabat D. Overcoming interference to retroviral superinfection results in amplified expression and transmission of cloned genes. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5404–5408. doi: 10.1073/pnas.85.15.5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budarf M., Huebner K., Emanuel B., Croce C. M., Copeland N. G., Jenkins N. A., D'Andrea A. D. Assignment of the erythropoietin receptor (EPOR) gene to mouse chromosome 9 and human chromosome 19. Genomics. 1990 Nov;8(3):575–578. doi: 10.1016/0888-7543(90)90047-x. [DOI] [PubMed] [Google Scholar]
- Casadevall N., Lacombe C., Muller O., Gisselbrecht S., Mayeux P. Multimeric structure of the membrane erythropoietin receptor of murine erythroleukemia cells (Friend cells). Cross-linking of erythropoietin with the spleen focus-forming virus envelope protein. J Biol Chem. 1991 Aug 25;266(24):16015–16020. [PubMed] [Google Scholar]
- Chesebro B., Miyazawa M., Britt W. J. Host genetic control of spontaneous and induced immunity to Friend murine retrovirus infection. Annu Rev Immunol. 1990;8:477–499. doi: 10.1146/annurev.iy.08.040190.002401. [DOI] [PubMed] [Google Scholar]
- Chesebro B., Wehrly K., Nishio J., Evans L. Leukemia induction by a new strain of Friend mink cell focus-inducing virus: synergistic effect of Friend ecotropic murine leukemia virus. J Virol. 1984 Jul;51(1):63–70. doi: 10.1128/jvi.51.1.63-70.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S. W., Wolff L., Ruscetti S. K. Transmembrane domain of the envelope gene of a polycythemia-inducing retrovirus determines erythropoietin-independent growth. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7957–7960. doi: 10.1073/pnas.86.20.7957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloyd M. W., Hartley J. W., Rowe W. P. Lymphomagenicity of recombinant mink cell focus-inducing murine leukemia viruses. J Exp Med. 1980 Mar 1;151(3):542–552. doi: 10.1084/jem.151.3.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rizzo D. F., Eskinazi D., Axelrad A. A. Negative regulation of DNA synthesis in early erythropoietic progenitor cells (BFU-E) by a protein purified from the medium of C57BL/6 mouse marrow cells. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4320–4324. doi: 10.1073/pnas.85.12.4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dizik M., Elliott R. W., Lilly F. Construction of a D2.B6-Fv-2r congenic mouse strain: nonlethality of the homozygous Fv-2' genotype. J Natl Cancer Inst. 1981 Apr;66(4):755–760. [PubMed] [Google Scholar]
- Eckner R. J., Hettrick K. L. Chronic infection of Fv-2-resistant hemopoietic cells by Friend spleen focus-forming virus. Leukemogenesis and control of stem cell differentiation by Fv-2. Virology. 1982 Oct 15;122(1):171–185. doi: 10.1016/0042-6822(82)90386-5. [DOI] [PubMed] [Google Scholar]
- Evans L. H., Cloyd M. W. Generation of mink cell focus-forming viruses by Friend murine leukemia virus: recombination with specific endogenous proviral sequences. J Virol. 1984 Mar;49(3):772–781. doi: 10.1128/jvi.49.3.772-781.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans L. H., Duesberg P. H., Scolnick E. M. Replication of spleen focus-forming Friend virus in fibroblasts from C57BL mice that are genetically resistant to spleen focus formation. Virology. 1980 Mar;101(2):534–539. doi: 10.1016/0042-6822(80)90469-9. [DOI] [PubMed] [Google Scholar]
- Famulari N. G. Murine leukemia viruses with recombinant env genes: a discussion of their role in leukemogenesis. Curr Top Microbiol Immunol. 1983;103:75–108. doi: 10.1007/978-3-642-68943-7_4. [DOI] [PubMed] [Google Scholar]
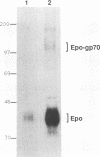
- Ferro F. E., Jr, Kozak S. L., Hoatlin M. E., Kabat D. Cell surface site for mitogenic interaction of erythropoietin receptors with the membrane glycoprotein encoded by Friend erythroleukemia virus. J Biol Chem. 1993 Mar 15;268(8):5741–5747. [PubMed] [Google Scholar]
- Geib R. W., Seaward M. B., Stevens M. L., Cho C. L., Majumdar M. RB virus: a strain of Friend virus that produces a 'Friend virus-like' disease in Fv-2rr mice. Virus Res. 1989 Oct;14(2):161–173. doi: 10.1016/0168-1702(89)90036-1. [DOI] [PubMed] [Google Scholar]
- Gliniak B. C., Kabat D. Leukemogenic membrane glycoprotein encoded by Friend spleen focus-forming virus: transport to cell surfaces and shedding are controlled by disulfide-bonded dimerization and by cleavage of a hydrophobic membrane anchor. J Virol. 1989 Sep;63(9):3561–3568. doi: 10.1128/jvi.63.9.3561-3568.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
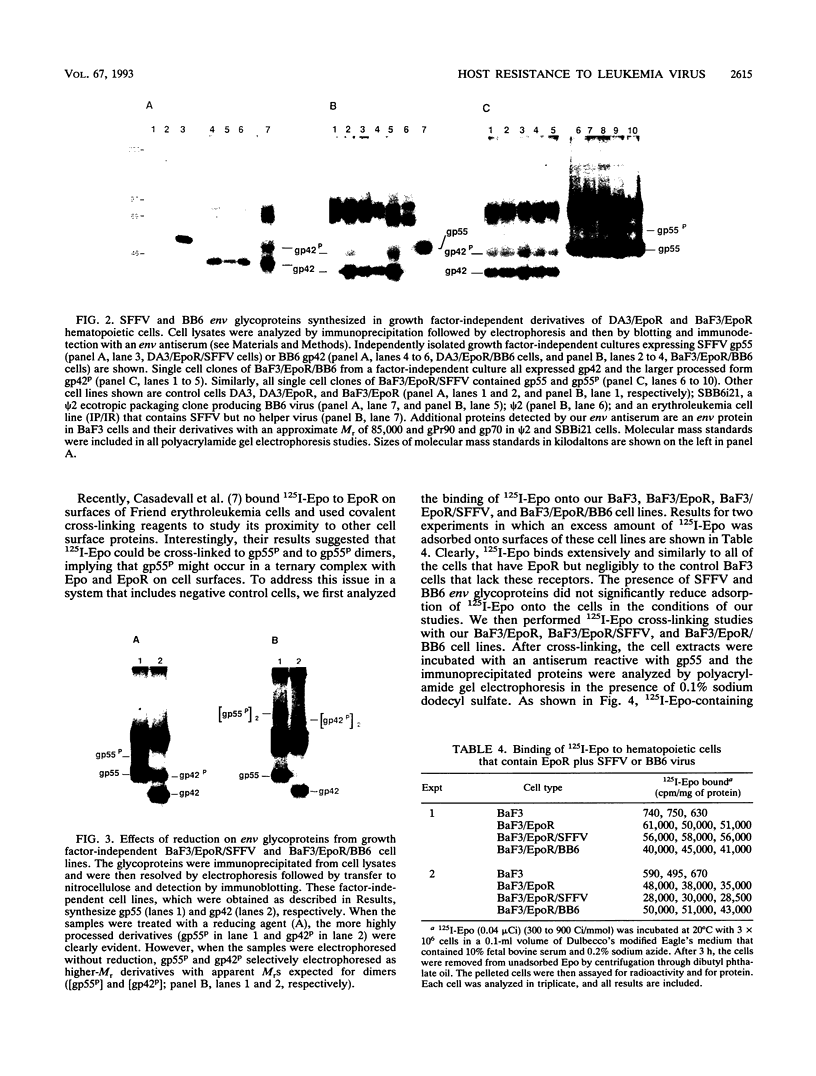
- Gliniak B. C., Kozak S. L., Jones R. T., Kabat D. Disulfide bonding controls the processing of retroviral envelope glycoproteins. J Biol Chem. 1991 Dec 5;266(34):22991–22997. [PubMed] [Google Scholar]
- Hankins W. D., Troxler D. Polycythemia- and anemia-inducing erythroleukemia viruses exhibit differential erythroid transforming effects in vitro. Cell. 1980 Dec;22(3):693–699. doi: 10.1016/0092-8674(80)90545-0. [DOI] [PubMed] [Google Scholar]
- Hatakeyama M., Tsudo M., Minamoto S., Kono T., Doi T., Miyata T., Miyasaka M., Taniguchi T. Interleukin-2 receptor beta chain gene: generation of three receptor forms by cloned human alpha and beta chain cDNA's. Science. 1989 May 5;244(4904):551–556. doi: 10.1126/science.2785715. [DOI] [PubMed] [Google Scholar]
- Hoatlin M. E., Kozak S. L., Lilly F., Chakraborti A., Kozak C. A., Kabat D. Activation of erythropoietin receptors by Friend viral gp55 and by erythropoietin and down-modulation by the murine Fv-2r resistance gene. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9985–9989. doi: 10.1073/pnas.87.24.9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H., Laigret F., Martin M. A., Repaske R. Characterization of a molecularly cloned retroviral sequence associated with Fv-4 resistance. J Virol. 1985 Sep;55(3):768–777. doi: 10.1128/jvi.55.3.768-777.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
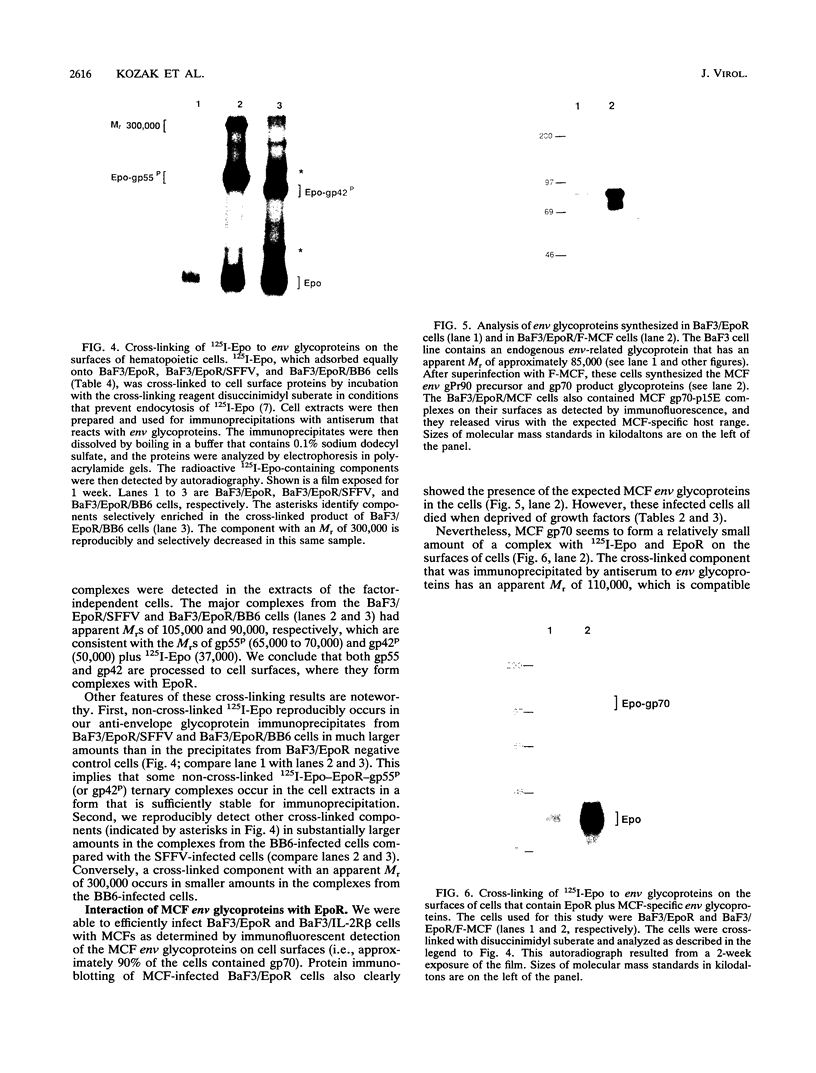
- Isfort R. J. Frequency and mechanisms of factor independence in IL-3-dependent cell lines. Somat Cell Mol Genet. 1990 Mar;16(2):109–121. doi: 10.1007/BF01233041. [DOI] [PubMed] [Google Scholar]
- Kabat D. Molecular biology of Friend viral erythroleukemia. Curr Top Microbiol Immunol. 1989;148:1–42. doi: 10.1007/978-3-642-74700-7_1. [DOI] [PubMed] [Google Scholar]
- Kay R., McPherson J. Hybrid pUC vectors for addition of new restriction enzyme sites to the ends of DNA fragments. Nucleic Acids Res. 1987 Mar 25;15(6):2778–2778. doi: 10.1093/nar/15.6.2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch W., Zimmermann W., Oliff A., Friedrich R. Molecular analysis of the envelope gene and long terminal repeat of Friend mink cell focus-inducing virus: implications for the functions of these sequences. J Virol. 1984 Mar;49(3):828–840. doi: 10.1128/jvi.49.3.828-840.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak C. A. Analysis of wild-derived mice for Fv-1 and Fv-2 murine leukemia virus restriction loci: a novel wild mouse Fv-1 allele responsible for lack of host range restriction. J Virol. 1985 Aug;55(2):281–285. doi: 10.1128/jvi.55.2.281-285.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak S. L., Kabat D. Ping-pong amplification of a retroviral vector achieves high-level gene expression: human growth hormone production. J Virol. 1990 Jul;64(7):3500–3508. doi: 10.1128/jvi.64.7.3500-3508.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. P., Baltimore D. Mechanism of leukemogenesis induced by mink cell focus-forming murine leukemia viruses. J Virol. 1991 May;65(5):2408–2414. doi: 10.1128/jvi.65.5.2408-2414.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. P., Bestwick R. K., Spiro C., Kabat D. The membrane glycoprotein of Friend spleen focus-forming virus: evidence that the cell surface component is required for pathogenesis and that it binds to a receptor. J Virol. 1987 Sep;61(9):2782–2792. doi: 10.1128/jvi.61.9.2782-2792.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. P., D'Andrea A. D., Lodish H. F., Baltimore D. Activation of cell growth by binding of Friend spleen focus-forming virus gp55 glycoprotein to the erythropoietin receptor. Nature. 1990 Feb 22;343(6260):762–764. doi: 10.1038/343762a0. [DOI] [PubMed] [Google Scholar]
- Machida C. A., Bestwick R. K., Boswell B. A., Kabat D. Role of a membrane glycoprotein in Friend virus-induced erythroleukemia: studies of mutant and revertant viruses. Virology. 1985 Jul 15;144(1):158–172. doi: 10.1016/0042-6822(85)90314-9. [DOI] [PubMed] [Google Scholar]
- Majumdar M. K., Cho C. L., Fox M. T., Eckner K. L., Kozak S., Kabat D., Geib R. W. Mutations in the env gene of friend spleen focus-forming virus overcome Fv-2r-mediated resistance to Friend virus-induced erythroleukemia. J Virol. 1992 Jun;66(6):3652–3660. doi: 10.1128/jvi.66.6.3652-3660.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- Mathey-Prevot B., Nabel G., Palacios R., Baltimore D. Abelson virus abrogation of interleukin-3 dependence in a lymphoid cell line. Mol Cell Biol. 1986 Nov;6(11):4133–4135. doi: 10.1128/mcb.6.11.4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayeux P., Casadevall N., Lacombe C., Muller O., Tambourin P. Solubilization and hydrodynamic characteristics of the erythropoietin receptor. Evidence for a multimeric complex. Eur J Biochem. 1990 Nov 26;194(1):271–278. doi: 10.1111/j.1432-1033.1990.tb19453.x. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Law M. F., Verma I. M. Generation of helper-free amphotropic retroviruses that transduce a dominant-acting, methotrexate-resistant dihydrofolate reductase gene. Mol Cell Biol. 1985 Mar;5(3):431–437. doi: 10.1128/mcb.5.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluthero F. G., Axelrad A. A. Superoxide dismutase as an inhibitor of erythroid progenitor cell cycling. Ann N Y Acad Sci. 1991;628:222–232. doi: 10.1111/j.1749-6632.1991.tb17249.x. [DOI] [PubMed] [Google Scholar]
- Ruscetti S. K. Employment of a [3H]thymidine-incorporation assay to distinguish the effects of different Friend erythroleukemia-inducing retroviruses on erythroid cell proliferation. J Natl Cancer Inst. 1986 Jul;77(1):241–245. [PubMed] [Google Scholar]
- Ruscetti S., Davis L., Feild J., Oliff A. Friend murine leukemia virus-induced leukemia is associated with the formation of mink cell focus-inducing viruses and is blocked in mice expressing endogenous mink cell focus-inducing xenotropic viral envelope genes. J Exp Med. 1981 Sep 1;154(3):907–920. doi: 10.1084/jem.154.3.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruta M., Clarke S., Boswell B., Kabat D. Heterogeneous metabolism and subcellular localization of a potentially leukemogenic membrane glycoprotein encoded by Friend erythroleukemia virus. Isolation of viral and cellular processing mutants. J Biol Chem. 1982 Jan 10;257(1):126–134. [PubMed] [Google Scholar]
- Ruta M., Kabat D. Plasma membrane glycoproteins encoded by cloned Rauscher and Friend spleen focus-forming viruses. J Virol. 1980 Sep;35(3):844–853. doi: 10.1128/jvi.35.3.844-853.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya T., Niho Y., Mak T. W. Erythroleukemia induction by Friend leukemia virus. A host gene locus controlling early anemia or polycythemia and the rate of proliferation of late erythroid cells. J Exp Med. 1982 Aug 1;156(2):398–414. doi: 10.1084/jem.156.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver J. E., Fredrickson T. N. Susceptibility to Friend helper virus leukemias in CXB recombinant inbred mice. J Exp Med. 1983 Nov 1;158(5):1693–1702. doi: 10.1084/jem.158.5.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver J., Teich N. Expression of resistance to Friend virus-stimulated erythropoiesis in bone marrow chimeras containing Fv-2rr and Fv-2ss bone marrow. J Exp Med. 1981 Jul 1;154(1):126–137. doi: 10.1084/jem.154.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiro C., Gliniak B. C., Kabat D. Splenic accumulation of interleukin-3-dependent hematopoietic cells in Friend erythroleukemia. J Virol. 1989 Oct;63(10):4434–4437. doi: 10.1128/jvi.63.10.4434-4437.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S., Axelrad A. A. Fv-2 locus controls the proportion of erythropoietic progenitor cells (BFU-E) synthesizing DNA in normal mice. Cell. 1980 Jan;19(1):225–236. doi: 10.1016/0092-8674(80)90404-3. [DOI] [PubMed] [Google Scholar]
- Tsichlis P. N., Bear S. E. Infection by mink cell focus-forming viruses confers interleukin 2 (IL-2) independence to an IL-2-dependent rat T-cell lymphoma line. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4611–4615. doi: 10.1073/pnas.88.11.4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Gaag H. C., Axelrad A. A. Friend virus replication in normal and immunosuppressed C57BL/6 mice. Virology. 1990 Aug;177(2):837–839. doi: 10.1016/0042-6822(90)90561-5. [DOI] [PubMed] [Google Scholar]
- Watanabe N., Nishi M., Ikawa Y., Amanuma H. A deletion in the Friend spleen focus-forming virus env gene is necessary for its product (gp55) to be leukemogenic. J Virol. 1990 Jun;64(6):2678–2686. doi: 10.1128/jvi.64.6.2678-2686.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff L., Ruscetti S. Tissue tropism of a leukemogenic murine retrovirus is determined by sequences outside of the long terminal repeats. Proc Natl Acad Sci U S A. 1986 May;83(10):3376–3380. doi: 10.1073/pnas.83.10.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura A., D'Andrea A. D., Lodish H. F. Friend spleen focus-forming virus glycoprotein gp55 interacts with the erythropoietin receptor in the endoplasmic reticulum and affects receptor metabolism. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4139–4143. doi: 10.1073/pnas.87.11.4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura A., Lodish H. F. In vitro phosphorylation of the erythropoietin receptor and an associated protein, pp130. Mol Cell Biol. 1992 Feb;12(2):706–715. doi: 10.1128/mcb.12.2.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zon L. I., Moreau J. F., Koo J. W., Mathey-Prevot B., D'Andrea A. D. The erythropoietin receptor transmembrane region is necessary for activation by the Friend spleen focus-forming virus gp55 glycoprotein. Mol Cell Biol. 1992 Jul;12(7):2949–2957. doi: 10.1128/mcb.12.7.2949. [DOI] [PMC free article] [PubMed] [Google Scholar]