Abstract
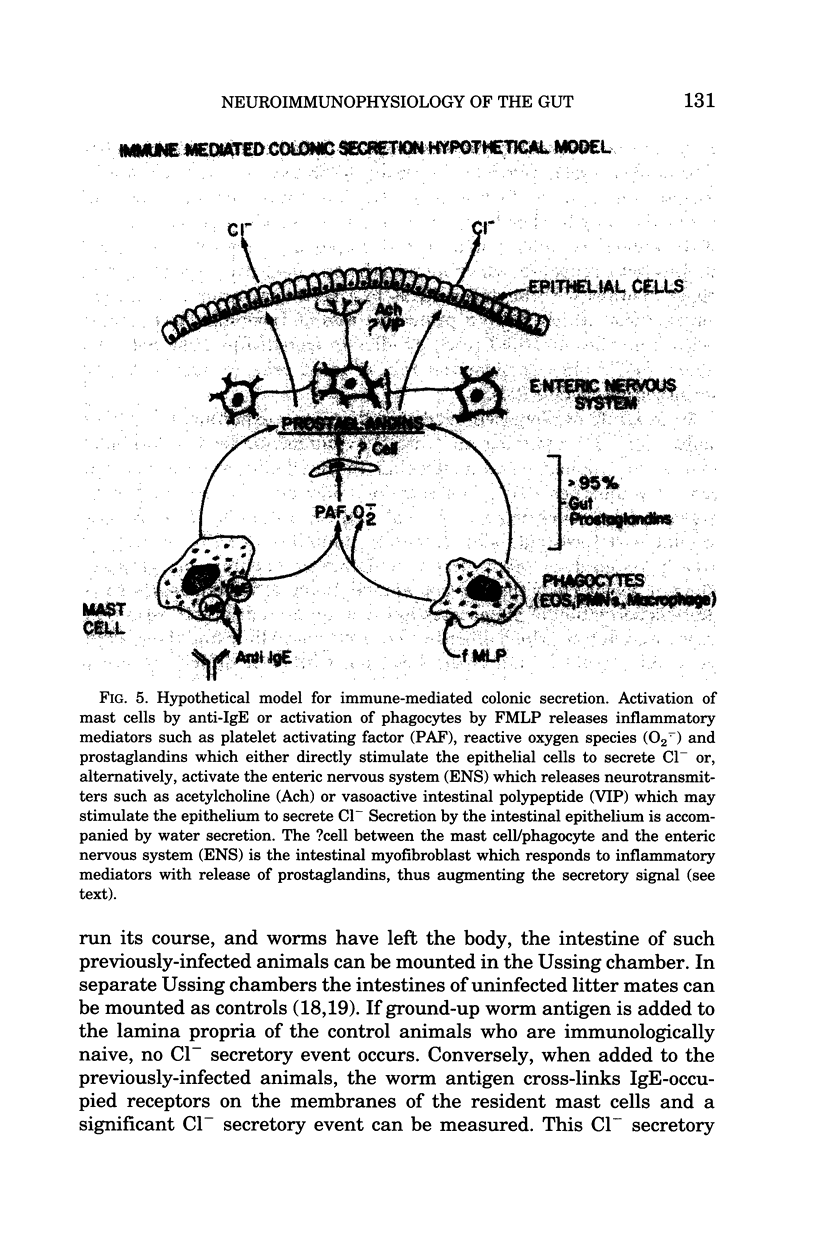
In conclusion, studies of the neuroimmunophysiology of the intestinal mucosa of the past 5-8 years have demonstrated an important role for the immune system in modulating water and electrolyte transport as well as intestinal motility in the gut. Activation of mast cells and phagocytes leads to heightened Cl- and water secretion, as well as changes in intestinal motility which leads to diarrheal states. These diarrheal responses are self-protective; they rid the intestine of offending microorganisms and antigens. Our investigation of this response has uncovered a new immune accessory cell Cz, the intestinal myofibroblast. This cell seems to play an important role in amplifying the immune signal. This cell is probably also important for the secretion of growth factors onto the epithelium and also the secretion of collagen which results in fibrosis under diseased states. These intestinal myofibroblasts are prolific prostaglandin producers, an important finding because prostaglandin synthesis inhibition has been shown to decrease the development of neoplasia in the gut. Thus, these intestinal myofibroblasts may have other important roles in addition to just modulating water and electrolyte secretion or gut motility. Our laboratory is now engaged in studying these intestinal myofibroblasts in some detail hoping to better understand the biology of these interesting cells.
Full text
PDF

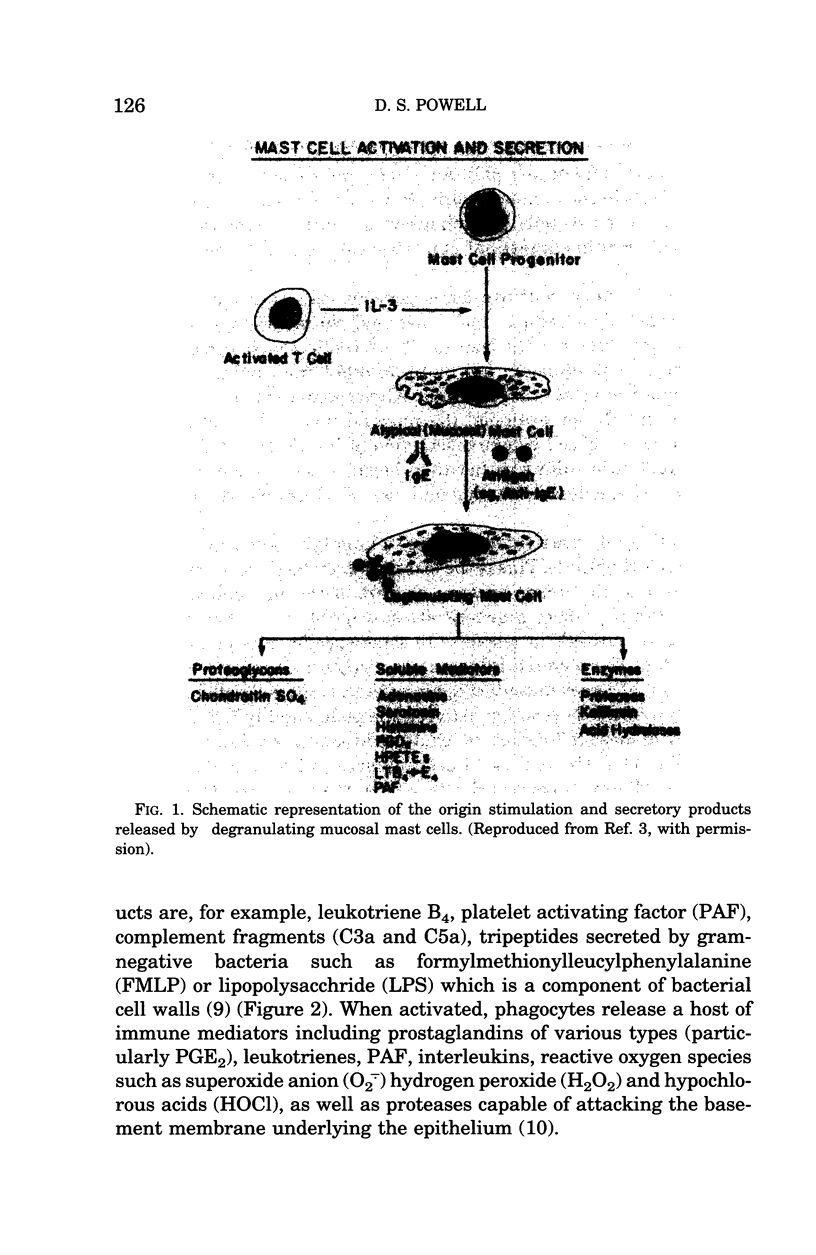
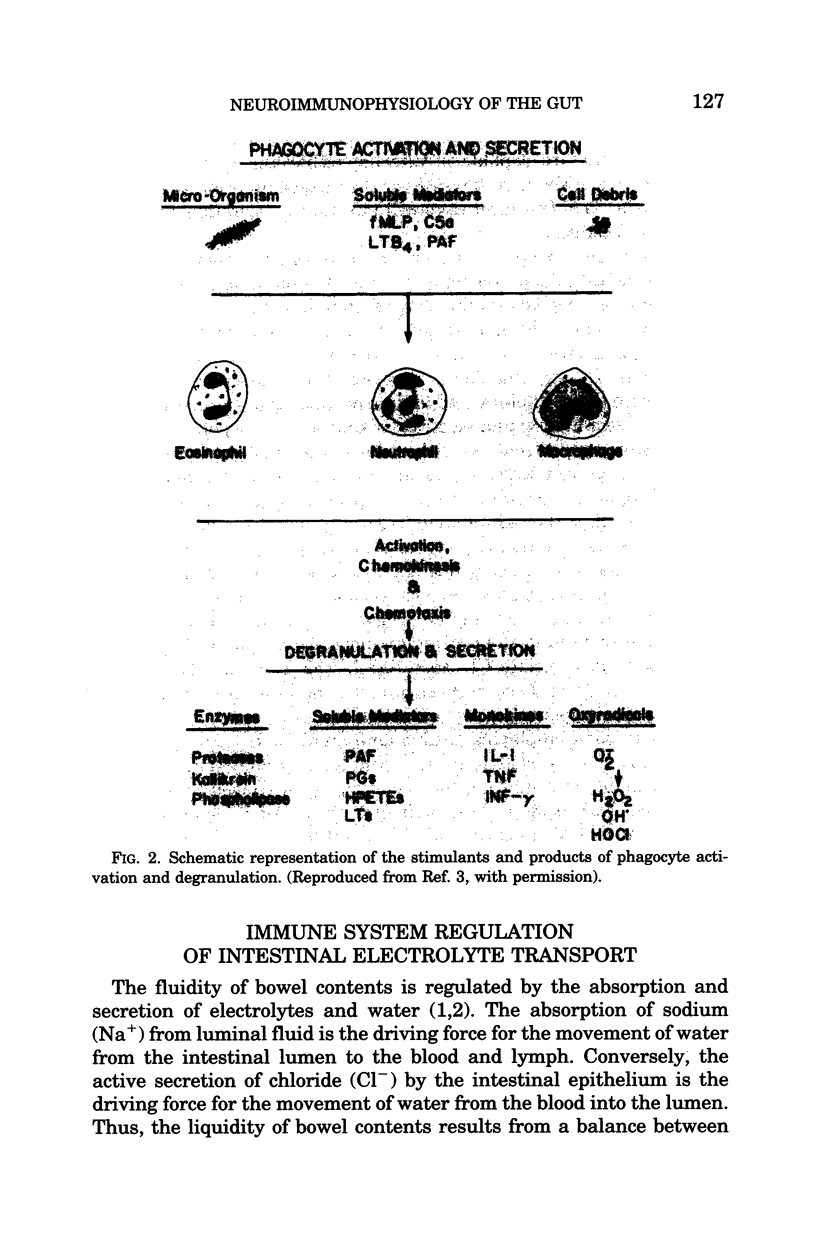

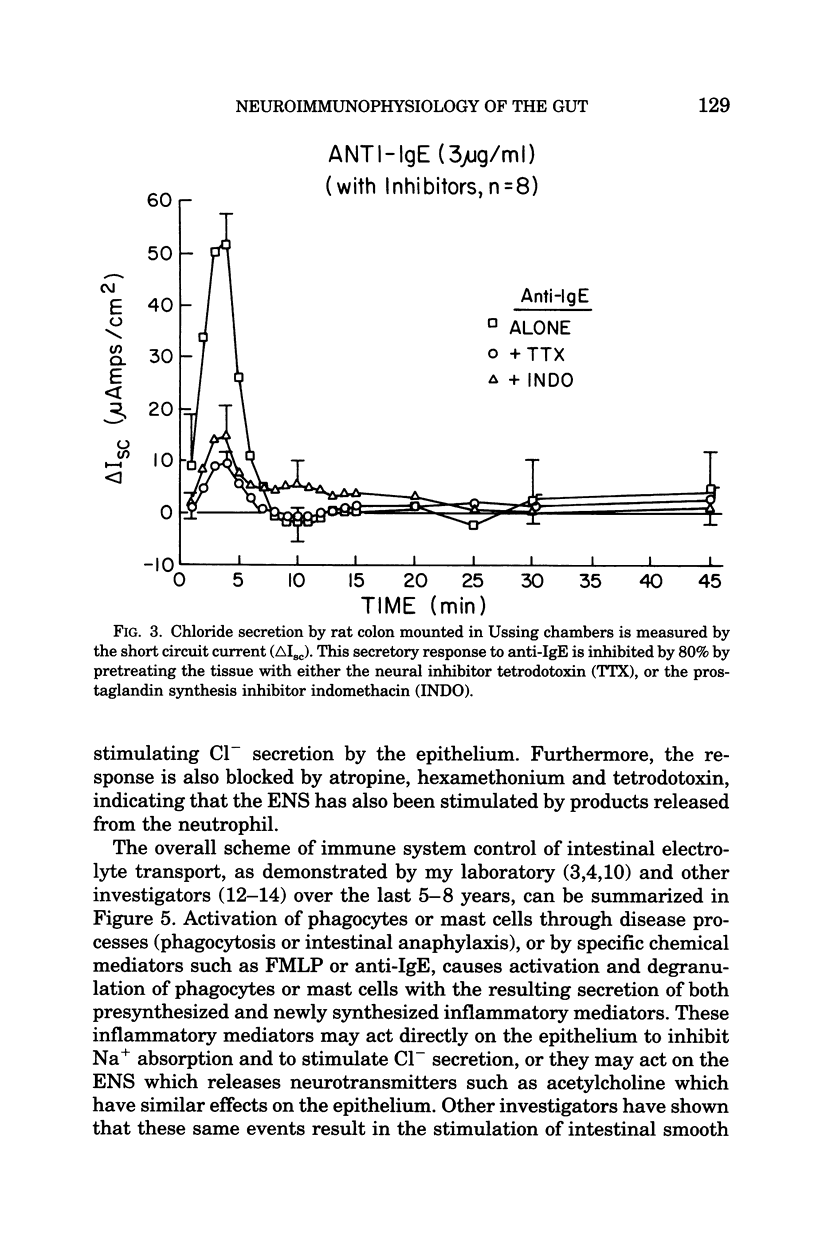
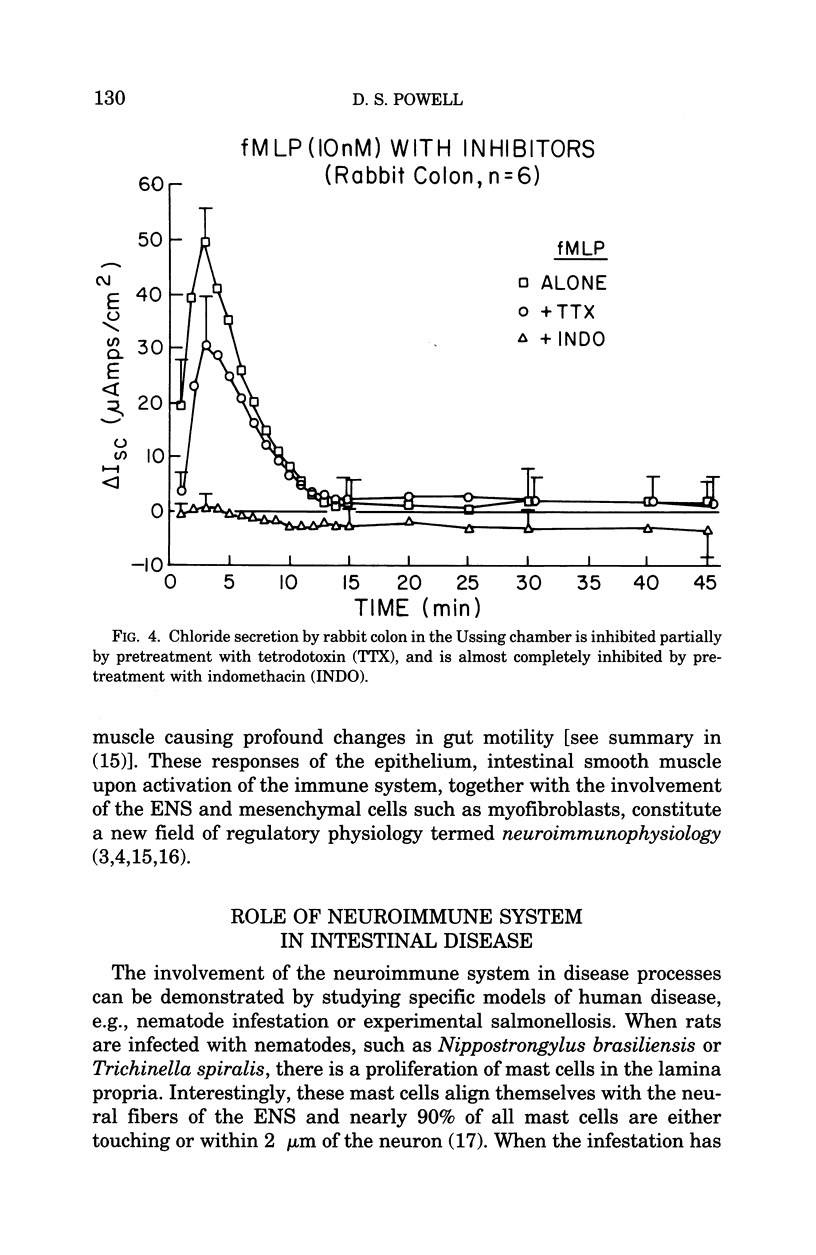








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baird A. W., Cuthbert A. W., Pearce F. L. Immediate hypersensitivity reactions in epithelia from rats infected with Nippostrongylus brasiliensis. Br J Pharmacol. 1985 Aug;85(4):787–795. doi: 10.1111/j.1476-5381.1985.tb11077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeken W., Mieremet-Ooms M., Ginsel L. A., Leijh P. C., Verspaget H. Enrichment of macrophages in cell suspensions of human intestinal mucosa by elutriation centrifugation. J Immunol Methods. 1984 Oct 12;73(1):189–201. doi: 10.1016/0022-1759(84)90044-9. [DOI] [PubMed] [Google Scholar]
- Bern M. J., Sturbaum C. W., Karayalcin S. S., Berschneider H. M., Wachsman J. T., Powell D. W. Immune system control of rat and rabbit colonic electrolyte transport. Role of prostaglandins and enteric nervous system. J Clin Invest. 1989 Jun;83(6):1810–1820. doi: 10.1172/JCI114086. [DOI] [PMC free article] [PubMed] [Google Scholar]
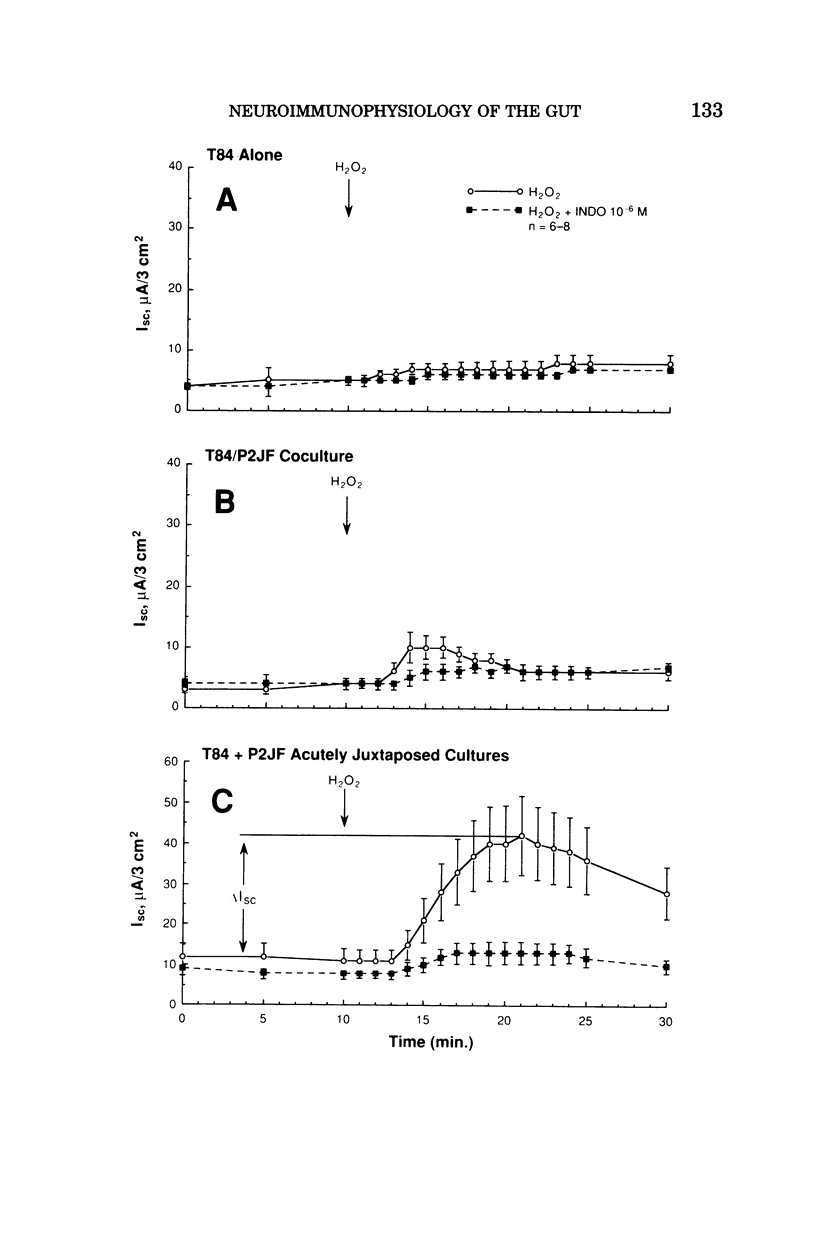
- Berschneider H. M., Powell D. W. Fibroblasts modulate intestinal secretory responses to inflammatory mediators. J Clin Invest. 1992 Feb;89(2):484–489. doi: 10.1172/JCI115610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro G. A., Harari Y., Russell D. Mediators of anaphylaxis-induced ion transport changes in small intestine. Am J Physiol. 1987 Oct;253(4 Pt 1):G540–G548. doi: 10.1152/ajpgi.1987.253.4.G540. [DOI] [PubMed] [Google Scholar]
- Cuthbert A. W., McLaughlan P., Coombs R. R. Immediate hypersensitivity reaction to beta-lactoglobulin in the epithelium lining the colon of guinea pigs fed cows' milk. Int Arch Allergy Appl Immunol. 1983;72(1):34–40. doi: 10.1159/000234837. [DOI] [PubMed] [Google Scholar]
- Eckmann L., Kagnoff M. F., Fierer J. Epithelial cells secrete the chemokine interleukin-8 in response to bacterial entry. Infect Immun. 1993 Nov;61(11):4569–4574. doi: 10.1128/iai.61.11.4569-4574.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannella R. A. Importance of the intestinal inflammatory reaction in salmonella-mediated intestinal secretion. Infect Immun. 1979 Jan;23(1):140–145. doi: 10.1128/iai.23.1.140-145.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannella R. A., Rout W. R., Formal S. B. Effect of indomethacin on intestinal water transport in salmonella-infected rhesus monkeys. Infect Immun. 1977 Jul;17(1):136–139. doi: 10.1128/iai.17.1.136-139.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinterleitner T. A., Powell D. W. Immune system control of intestinal ion transport. Proc Soc Exp Biol Med. 1991 Jul;197(3):249–260. doi: 10.3181/00379727-197-43252. [DOI] [PubMed] [Google Scholar]
- Joyce N. C., Haire M. F., Palade G. E. Morphologic and biochemical evidence for a contractile cell network within the rat intestinal mucosa. Gastroenterology. 1987 Jan;92(1):68–81. doi: 10.1016/0016-5085(87)90841-9. [DOI] [PubMed] [Google Scholar]
- Kaye G. I., Lane N., Pascal R. R. Colonic pericryptal fibroblast sheath: replication, migration, and cytodifferentiation of a mesenchymal cell system in adult tissue. II. Fine structural aspects of normal rabbit and human colon. Gastroenterology. 1968 May;54(5):852–865. [PubMed] [Google Scholar]
- Kennerly D. A., Sullivan T. J., Sylwester P., Parker C. W. Diacylglycerol metabolism in mast cells: a potential role in membrane fusion and arachidonic acid release. J Exp Med. 1979 Oct 1;150(4):1039–1044. doi: 10.1084/jem.150.4.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade E. A., Smith W. L., DeWitt D. L. Differential inhibition of prostaglandin endoperoxide synthase (cyclooxygenase) isozymes by aspirin and other non-steroidal anti-inflammatory drugs. J Biol Chem. 1993 Mar 25;268(9):6610–6614. [PubMed] [Google Scholar]
- Nathan C. F. Secretory products of macrophages. J Clin Invest. 1987 Feb;79(2):319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker F. G., Barnes E. N., Kaye G. I. The pericryptal fibroblast sheath. IV. Replication, migration, and differentiation of the subepithelial fibroblasts of the crypt and villus of the rabbit jejunum. Gastroenterology. 1974 Oct;67(4):607–621. [PubMed] [Google Scholar]
- Pascal R. R., Kaye G. I., Lane N. Colonic pericryptal fibroblast sheath: replication, migration, and cytodifferentiation of a mesenchymal cell system in adult tissue. I. Autoradiographic studies of normal rabbit colon. Gastroenterology. 1968 May;54(5):835–851. [PubMed] [Google Scholar]
- Perdue M. H., Gall D. G. Intestinal anaphylaxis in the rat: jejunal response to in vitro antigen exposure. Am J Physiol. 1986 Apr;250(4 Pt 1):G427–G431. doi: 10.1152/ajpgi.1986.250.4.G427. [DOI] [PubMed] [Google Scholar]
- Perdue M. H., Masson S., Wershil B. K., Galli S. J. Role of mast cells in ion transport abnormalities associated with intestinal anaphylaxis. Correction of the diminished secretory response in genetically mast cell-deficient W/Wv mice by bone marrow transplantation. J Clin Invest. 1991 Feb;87(2):687–693. doi: 10.1172/JCI115047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdue M. H., McKay D. M. Integrative immunophysiology in the intestinal mucosa. Am J Physiol. 1994 Aug;267(2 Pt 1):G151–G165. doi: 10.1152/ajpgi.1994.267.2.G151. [DOI] [PubMed] [Google Scholar]
- Powell D. W., Plotkin G. R., Maenza R. M., Solberg L. I., Catlin D. H., Formal S. B. Experimental diarrhea. I. Intestinal water and electrolyte transport in rat salmonella enterocolitis. Gastroenterology. 1971 Jun;60(6):1053–1064. [PubMed] [Google Scholar]
- Russell D. A., Castro G. A. Immunological regulation of colonic ion transport. Am J Physiol. 1989 Feb;256(2 Pt 1):G396–G403. doi: 10.1152/ajpgi.1989.256.2.G396. [DOI] [PubMed] [Google Scholar]
- Stead R. H., Tomioka M., Quinonez G., Simon G. T., Felten S. Y., Bienenstock J. Intestinal mucosal mast cells in normal and nematode-infected rat intestines are in intimate contact with peptidergic nerves. Proc Natl Acad Sci U S A. 1987 May;84(9):2975–2979. doi: 10.1073/pnas.84.9.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vane J. Towards a better aspirin. Nature. 1994 Jan 20;367(6460):215–216. doi: 10.1038/367215a0. [DOI] [PubMed] [Google Scholar]



