Abstract
Release of hemoglobin from the erythrocyte during intravascular hemolysis contributes to the pathology of a variety of diseased states. This effect is partially due to the enhanced ability of cell-free plasma hemoglobin, which is primarily found in the ferrous, oxygenated state, to scavenge nitric oxide. Oxidation of the cell-free hemoglobin to methemoglobin, which does not effectively scavenge nitric oxide, using inhaled nitric oxide has been shown to be effective in limiting pulmonary and systemic vasoconstriction. However, the ferric heme species may be reduced back to ferrous hemoglobin in plasma and has the potential to drive injurious redox chemistry. We propose that compounds that selectively convert cell-free hemoglobin to ferric, and ideally iron-nitrosylated heme species that do not actively scavenge nitric oxide would effectively treat intravascular hemolysis. We show here that nitroxyl, generated by Angeli’s salt (Sodium α-oxyhyponitrite, Na2N2O3), preferentially reacts with cell-free hemoglobin compared to that encapsulated in the red blood cell under physiologically relevant conditions. Nitroxyl oxidizes oxygenated ferrous hemoglobin to methemoglobin and can convert the methemoglobin to a more stable, less toxic species, iron-nitrosyl hemoglobin. These results support the notion that Angeli’s salt or a similar compound could be used to effectively treat conditions associated with intravascular hemolysis.
Keywords: Hemoglobin, Nitric Oxide, Nitroxyl, Angeli’s salt, red blood cell, hemolysis
Introduction
Endothelial nitric oxide synthase produces nitric oxide (NO) which can then diffuse from the endothelial cells to the smooth muscle where it causes vasodilation via activation of soluble guanylate cyclase [1–5]. NO also modulates vascular permeability [6–8], angiogenesis [9,10], platelet adhesion and aggregation [11–13], and leukocyte adhesion [14–16]. Thus, NO bioavailabilty is important in maintaining several aspects of homeostasis and its dysfunction contributes to a large variety of diseased states [17–24].
NO primarily reacts with ferrous hemoglobin (Hb) in both the oxygenated (oxyHb) state, producing methemoglobin (metHb, where the heme is oxidized to FeIII) and nitrate as described in Equation 1. NO also reacts with ferrous Hb in the deoxygenated (deoxyHb) state, resulting in formation of nitrosylHb (HbNO) as described in Equation 2.
| (1) |
| (2) |
The kinetics of these reactions are limited by the rate of diffusion of the ligand to the heme pocket [25], with second order rate constants of 5–8 × 107 M−1s−1 [25–28] for the reaction described by Equation 1, and 3 × 107 M−1s−1 for the NO binding reaction to deoxyHb [29,30].
The production of nitrate from the reaction involving oxyHb is a dead end with respect to NO bioactivity. In addition, any NO that is slowly released from iron nitrosyl Hb (on the order of 10−3 to 10−5 s−1 [29,31,32]) is likely to be scavenged by oxyHb, thereby eliminating its activity. The fact that NO activity is effectively diminished in the presence of Hb was an important property that aided in its identification as the endothelial derived relaxing factor [33]. That NO is made in a compartment adjacent to the blood where there is about 10 mM Hb (heme concentration*), led to questioning how it can function without being scavenged by the Hb [34]. In normal physiology, the reason that endothelial-derived NO is not scavenged to the extent predicted, based purely on kinetic calculations, is that red blood cell (RBC) encapsulated Hb in the blood reacts with NO much more slowly than does cell-free Hb [35–45]. Three mechanisms contribute to reduced NO scavenging by RBCs [46].: (1) the rate of the reaction is largely limited by external diffusion of NO through the plasma to the surface of the RBC [44], especially due to the presence of a red cell free zone adjacent to the vessel walls where NO is made [37–39]; (2) NO diffusion is partially blocked by a physical barrier across the RBC membrane [40,43,47]; and (3) RBC-encapsulated Hb is efficiently compartmentalized in the lumen; it does not extravasate into the endothelium and interstitium [44,48–52].
All three mechanisms are disrupted during intravascular hemolysis [46]. The increased ability of cell-free Hb to scavenge NO has been widely attributed to the hypertension, increased systemic and pulmonary vascular resistance, and morbidity and mortality associated with administration of hemoglobin-based oxygen carriers (HBOCs or “blood substitutes”) [53–67]. More recently, the importance of intravascular hemolysis on NO bioavailability in diseased states including hemolytic anemias like sickle cell disease and paroxysmal nocturnal hemoglobinuria (PNH), thalassemia intermedia, malaria, thrombotic thrombocytopenic purpura, hemolytic uremic syndrome and cardiopulmonary bypass has been elucidated [17–19,68–73]. It had been thought that cell-free Hb in the blood was mainly present as metHb, but it is in fact mostly oxyHb [19,74]. Reiter and coworkers found that responsiveness to NO administration was blunted by 80% in patients with sickle cell anemia who had plasma Hb concentrations greater than or equal to 6 μM [19]. The hemolysis in sickle cell disease is generally lower than other conditions with an average of 4.2 ± 1.1 μM during steady state (but increasing several fold during sickle cell crisis [75,76]) compared to 0.2 ± 0.1 μM for control normal volunteers [19]. We have conducted calculations demonstrating that only 1 μM cell-free Hb significantly reduces NO bioavailability, even in the background of the 10 mM or so Hb found in whole blood [77]. Thus, low NO bioavailability is likely to contribute as much or more to pathology in other conditions involving hemolysis. It has been shown that hemolysis in cardiopulmonary bypass surgery leads to renal tube injury and other complications [78]. Minneci and coworkers demonstrated that intravascular hemolysis leads to vasoconstriction and impairs renal function in a canine model [18]. Thus, a host of animal and human data support the notion that NO scavenging by cell-free Hb due to intravascular hemolysis contributes to disease.
MetHb binds NO with a rate constant of only 4 × 104 M−1s−1 and releases it with a rate constant of 1 s−1 [79]. Thus, metHb is not expected to be a very efficient scavenger of NO and preferential oxidation of cell-free oxyHb to metHb by inhaled NO should reduce NO scavenging. In 2002, Reiter and coworkers. showed that NO-inhalation therapy results in conversion of plasma oxyHb to metHb in patients with sickle cell disease, thereby reducing the enhanced NO scavenging by the plasma Hb [19]. Similarly, in the canine model, Minneci and coworkers showed that NO inhalation following hemolysis resulted in restoration of NO responsiveness to NO donors and attenuation of the hemolysis associated vasoconstriction [18]. These results support the approach of oxidizing the cell-free Hb to diminish NO scavenging. Indeed, NO inhalation therapy in sickle cell disease and other hemolytic conditions has been gaining increased attention [17,68,72,80]. Although use of NO inhalation therapy holds promise for treatment of hemolytic conditions, its use is not practical in a variety of settings, particularly where chronic treatment is desired. NO inhalation therapy is expensive and compliance in its use with portable gas cylinders is not likely to be great. In addition, formation of metHb as an end-product may not be ideal due to potential oxidative damage [81–83]. Methemoglobin reacts with hydrogen peroxide or superoxide to form ferryl hemoglobin leading to the formation of other radicals causing protein damage and heme degradation which in turn can lead to cellular and tissue damage [81].
Angeli’s salt (Sodium α-oxyhyponitrite, Na2N2O3) decomposes into nitrite and nitroxyl (HNO), which readily reacts with oxyHb to form metHb (see reactions 1–3 in Figure 1) [84],
Figure 1.
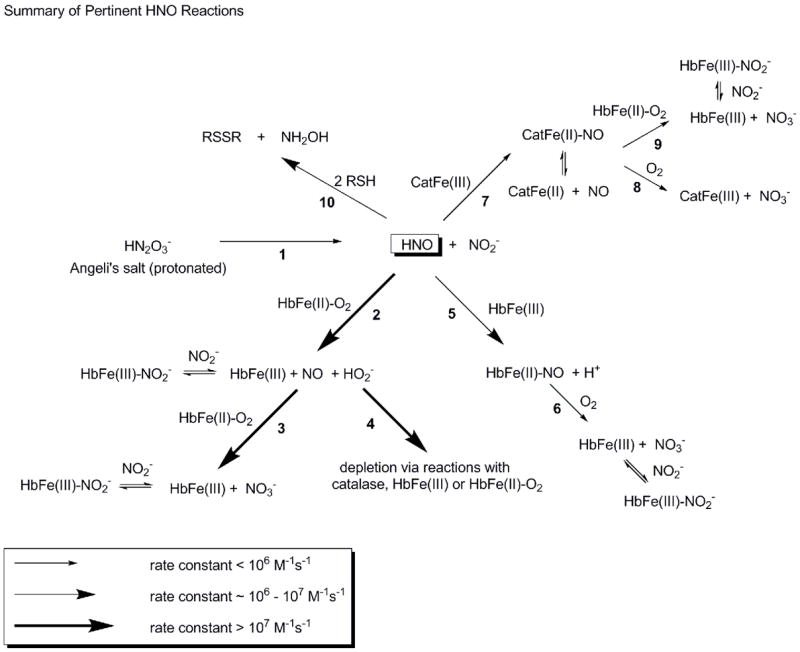
Schematic of reactions involving Angeli’s salt relevant to this study. Larger arrows indicate faster reactions as shown in the legend. Each reaction is numbered under the arrow. In reaction 1, AS decomposes to HNO and nitrite with a rate constant of about 6 × 10−4 s−1 in our studies, similar to that reported previously [84]. HNO reacts with oxyHb (reaction 2) with a rate constant of about 1 × 107 M−1s−1 (based on that reported for oxymyoglobin [85]), forming metHb, NO and peroxide. NO reacts with oxyHb to form metHb and nitrate with a rate constant of 5–8 × 107 M−1s−1 [25–28]. MetHb binds nitrite reversibly with a dissociation constant of 1 mM [98] or lower under certain conditions [102]. In our systems, peroxide formed by reaction 2 appears to be scavenged by catalase (reaction 4 where the initial step in this reaction occurs with a rate constant greater than 107 M−1s−1 [95]). HNO also reacts with metHb (reaction 5) to form HbNO, with a rate constant that is fast (8 × 105 M−1s−1(based on that reported for metmyoglobin [85])), but significantly slower than reaction 2 with oxyHb. HbNO reacts slowly with oxygen to form metHb (reaction 6) with a rate that may be governed by the dissociation rate constant of NO (about 10−3 s−1 for some dissociations [107]). HNO also reacts with catalase (reaction 7 occurring with a rate constant of 3 × 105 M−1s−1 [85]) to form a ferrous NO complex. The NO-catalase can release NO relatively rapidly [97] which can then make metHb from oxyHb via the reaction described in Equation 1 (reaction 9) or react with oxygen (reaction 8 occurring at about 5 × 10−4 M−1s−1 [97]). Finally, HNO can react with low molecular weight thiols (reaction 10) with a rate constant of 2 × 106 M−1s−1 [85]. HNO can also react with protein bound thiols but this reaction is likely to be slower.
| (3) |
| (4) |
The rate constants for the reaction of HNO with oxyHb (about 107 M−1s−1) [85] is comparable to NO, and thus it may preferentially react with cell-free Hb compared to RBC encapsulated Hb in a similar way as NO. In addition, HNO reacts with ferric hemes to form an iron nitrosyl with a rate constant of about 106 M−1s−1 [85,86], so we reasoned that HNO would also form iron nitrosyl Hb when reacted with oxyHb. A reaction scheme for Angeli’s salt (AS) and various Hb forms is presented in Figure 1.
In this paper we show that AS can be used to preferentially convert cell-free oxyHb to metHb in the presence of excess, physiologically relevant concentrations of RBC encapsulated Hb. In addition, we show that AS also forms potentially less redox active end-products including iron nitrosyl Hb and nitrite bound metHb. Thus, we propose that Angeli’s salt or a similar compound could be used to effectively treat hemolysis.
Materials and Methods
Reagents
Angelis salt was purchased from Cayman Chemical. All other chemicals were obtained from Sigma Chemical Company. Blood was obtained from volunteers or bought from the Interstate Blood Bank (Memphis, TN). RBCs were obtained by washing the blood three to five times in pH 7.4 phosphate buffered saline (PBS). Hb was purified as described previously [87,88]. The washed red blood cells were lysed by incubation with distilled water and the membranes spun out by centrifugation. After extensive dialysis against distilled water followed by dialysis against PBS, the Hb was pelleted in liquid nitrogen and stored at −80° C for storage. Note that this procedure does not eliminate catalase originally present in the red blood cell.
MetHb was prepared by incubation with excess ferricyanide and the excess ferricyanide was removed by column filtration (G-25) and dialysis. DeoxyHb was prepared by diluting Hb into deaerated PBS buffer obtained by flushing the buffer with nitrogen or argon in a septum capped flask with an exit needle also present. The Hb was further deoxygenated by purging the solution with argon or nitrogen in a septum capped flask (without inserting the purge needle into the solution). OxyHb was prepared by diluting the Hb into air-equilibrated buffer. The integrity of all Hb species was confirmed spectrophotmetrically. Ferryl hemoglobin was prepared by adding a two-fold molar excess hydrogen peroxide to metHb obtained from Sigma Chemical Company after sedimentation and filtration through G-25 columns.
Stock solutions of Angeli’s Salt were prepared in 0.01 M NaOH. The concentration was confirmed by UV absorbance at 237 nm with an extinction coefficient (ε) of 6.1 mM−1cm−1 (or, equivalently, ε = 8 mM−1cm−1 at 250 nm [89]). The release of HNO was initiated upon addition to reactions in 0.1 M phosphate buffer at pH 7.4. For anaerobic experiments the AS stock solutions were made with NaOH that had been purged with argon for at least thirty minutes.
Spectroscopy
Absorption spectroscopy on Hb was performed using a Cary 50 Bio Spectrometer in the visible wavelength range (Varian Inc.). Absorption spectroscopy of blood or RBCs was measured in the visible or near infra red range using a Perkin Elmer Life Sciences Lambda 9 spectrometer equipped with an integrating sphere to detect scattered light. Septum capped cells were used for experiments performed in other than ambient atmospheric conditions.
Electron paramagnetic resonance (EPR) spectroscopy was performed using a Bruker EMX 10/12 spectrometer cooled using liquid helium and operating at 9.4 GHz. Iron nitrosyl Hb was detected at 110 K using 5-G modulation, 10.1-milliwatt power, 655.36-ms time constant, and 167.77-s scan or 327.68-ms time constant and 83.89-s scans over 600 G. MetHb was measured by EPR (at low field using 15-G modulation, 10.1-milliwatt power, 81.92-ms time constant, and 41.94-s scan over 700 G) at 4 K using liquid helium. The concentration of each species was determined by performing a double integral of the EPR spectrum and comparing to standard samples.
Time resolved absorption spectroscopy was performed by mixing reactive species (Hb and AS) and taking absorption spectra at defined time intervals. Concentrations of known species were obtained by performing a least squares fit to known basis spectra of each species (Figure 2). In most cases spectral data were fit to all the species shown in Figure 2b except NO bound to metHb (FeIIINO-Hb) since this species is not expected to accumulate to a measurable amount. To ensure FeIIINO-Hb was not present, it was occasionally included in the fit parameters and always found not to be present at significant quantities or to improve the residuals significantly. The addition of ferrylHb to the fitting procedure was also found not to improve the fits (less than a factor of two improvement in residuals compared to when the fits were performed in the absence of ferrylHb) and was thus not included in the fits shown. Data presented on the percentage of each species includes those species that were present at greater than 1% according to the fits. Control experiments were performed where only nitrite was added at a concentration equivalent to that released by AS. No significant spectral changes where observed over the time course studied for these control experiments.
Figure 2.
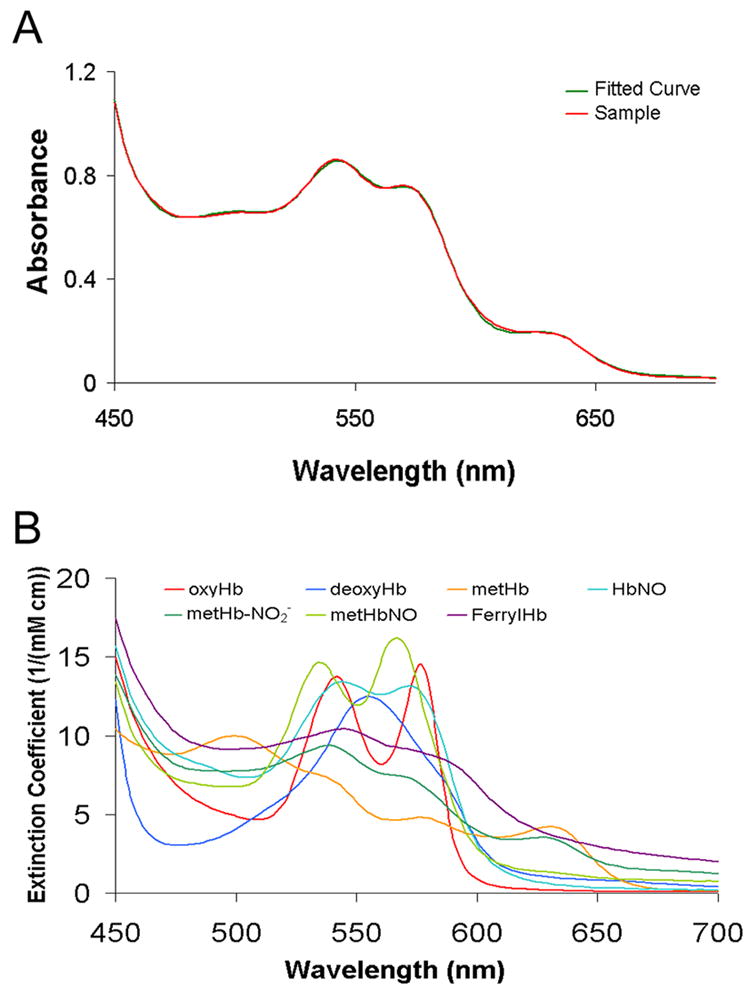
Spectral deconvolution of Hb species absortption. (A) Typical fit of an absorption spectrum showing both the raw data and the fit. HbNO found was 29%, the percentage of oxyHb was 5%, deoxyHb was 21%, the percentage of metHb was 41%, and metHb-NO2− was 4%. The data were taken from a partially oxygenated Hb sample (100 μM, 36% oxygen saturated) after incubating with 100 μM AS for 33 minutes. (B) The basis spectra used for fitting.
Competition Experiments
Preferential reactivity of AS with cell-free Hb compared to that encapsulated in red blood cells was performed based on the original conception of the Liao group [40] and modified as described in detail previously [45]. Briefly, AS (50 μM to 150 μM) was added to a mixture of Hb (final concentration of 30 μM to 100 μM) and RBCs at a hematocrit (Hct) comparable to that found in normal physiology (45%) or comparable to that of patients during sickle cell crisis (18% [75]). The RBCs were spun down and three samples were loaded into EPR tubes and frozen for analysis: (1) one containing a sample from the supernatant used to determine the amount of reacted cell-free Hb, (2) another from the pellet used to determine the amount of reacted RBC encapsulated Hb, and (3) one from the sample before centrifugation to determine the total amount of reacted Hb that should be equal to that in the other two EPR tubes. The preferential reactivity, , of AS is defined by the ratio of the bimolecular rate constant for the reaction of AS with cell-free Hb (kf) to that of the reaction of AS with RBC encapsulated Hb (kr). The preferential reactivity is calculated from the relation
| (5) |
where the subscripts r and f refer to the RBC encapsulated and cell-free Hb respectively. This equation states that the amount of metHb made in the red cell or cell-free fraction depends on the intrinsic, bimolecular (normalized by the concentration of Hb) rate constant and the amount of reacting material in each fraction. The concentrations (indicated by brackets) represent the moles of the species in the total volume. Thus,
| (6) |
where [metHb]s represents the concentration of metHb in the supernatant. A similar equation is used to determine [HbO2]f from the concentration of oxyHb in the supernatant ([HbO2]f = (1-Hct)* [HbO2]s where the subscript “s” refers to the supernatant). For partially deoxygenated samples, where the products included both HbNO and metHb, the sum of these products in each fraction was used to determine kf/kr. Since the concentration of cell-free Hb is not constant during the reaction, when necessary, a term accounting for this was included when calculating kf/kr as described previously [40]. In such a case [metHb]f/[oxyHb]f is replaced by ln(1 + [metHb]f/[oxyHb]f), where ln is the natural log. Note that ln(1 + x) ≅ x when x is small.
For each experiment, a control sample was prepared without AS and treated identically in other respects as the sample to which AS was added. Under aerobic or partially aerobic conditions, some MetHb could form due to autoxidation. Thus, we subtracted the amount of MetHb measured in the control sample from that in the samples to which AS was added. As described previously [45], a self-consistency check was imposed on the data whereby if the sum of the reacted Hb in the supernatant plus that in the red cells was significantly different from that in the whole mixture (leading to calculated values of kf/kr that differ by over 30%), the data were discarded.
Results
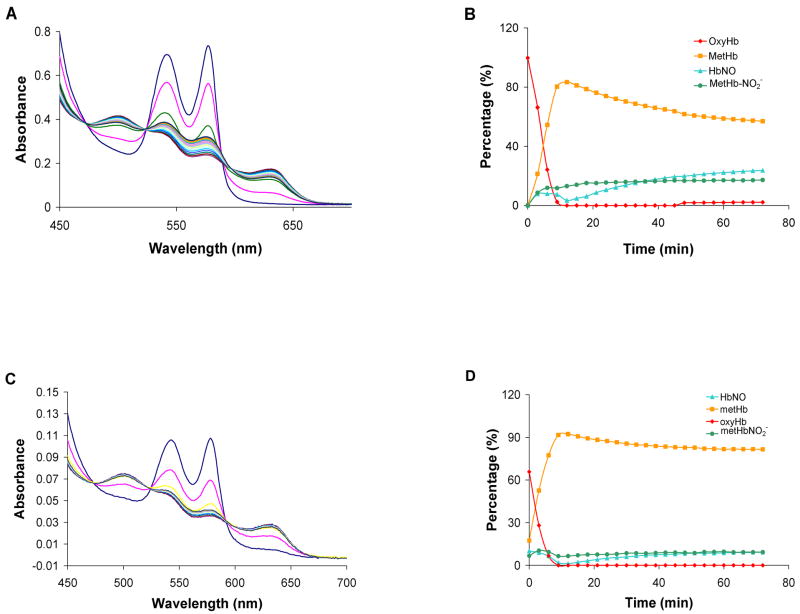
According to Equation 4, HNO converts oxyHb to metHb in a 1:2:2 stoichiometric ratio (Figure 1, reactions 2 and 3). In an earlier study, the rate of metHb formation was plotted against the concentration of AS and the slope of this line was compared to that for spontaneous AS decomposition (8.3 × 10−4 s−1) [84]. This comparison suggested the 2:1 metHb:AS stoichiometry [84]. We confirmed this stoichiometry using an alternative methodology (spectral deconvilution) when we mixed 50 μM of AS with 100 μM of oxyHb (Figure 3). Figure 3A shows representative time-resolved absorption spectra from a single experiment and Figure 3B shows the results from deconvoluting these spectra into their components. The apparent half life of AS (based on the time for the oxyHb to fall to half its initial value) is 16 minutes, consistent with previously measured values of the half-life under similar conditions [84]. The reaction (which is rate-limited by the decomposition of AS to HNO and nitrite) is essentially over after seventy minutes. At the final time point measured for the data shown in Figure 3B, 92% of the Hb is metHb and importantly, we observe that about 16% of this has nitrite bound. The average concentration of species at 72 minutes from three separate experiments was 93% MetHb with 10 ± 4 % having nitrite bound and 83 ± 5% being ligand free. Very little HbNO is made under these conditions (2 ± 1 % average over three experiments). It is possible that some of the oxyHb was converted to metHb via a direct reaction with nitrite. However, given the slow kinetics of the nitrite/oxyHb reaction [90], this reaction is not expected to be important. The rate constant for the slow (pre-autocatalytic phase) of the nitrite/oxyHb reaction is about 0.5 M−1s−1 at pH 7.4†. Under our conditions, without excess nitrite to Hb, one would not expect autocatalysis. We confirmed this by mixing 100 μM of nitrite with 100 μM of oxyHb for 72 minutes and we found that only 2.4 μM of metHb was formed (data not shown).
Figure 3.
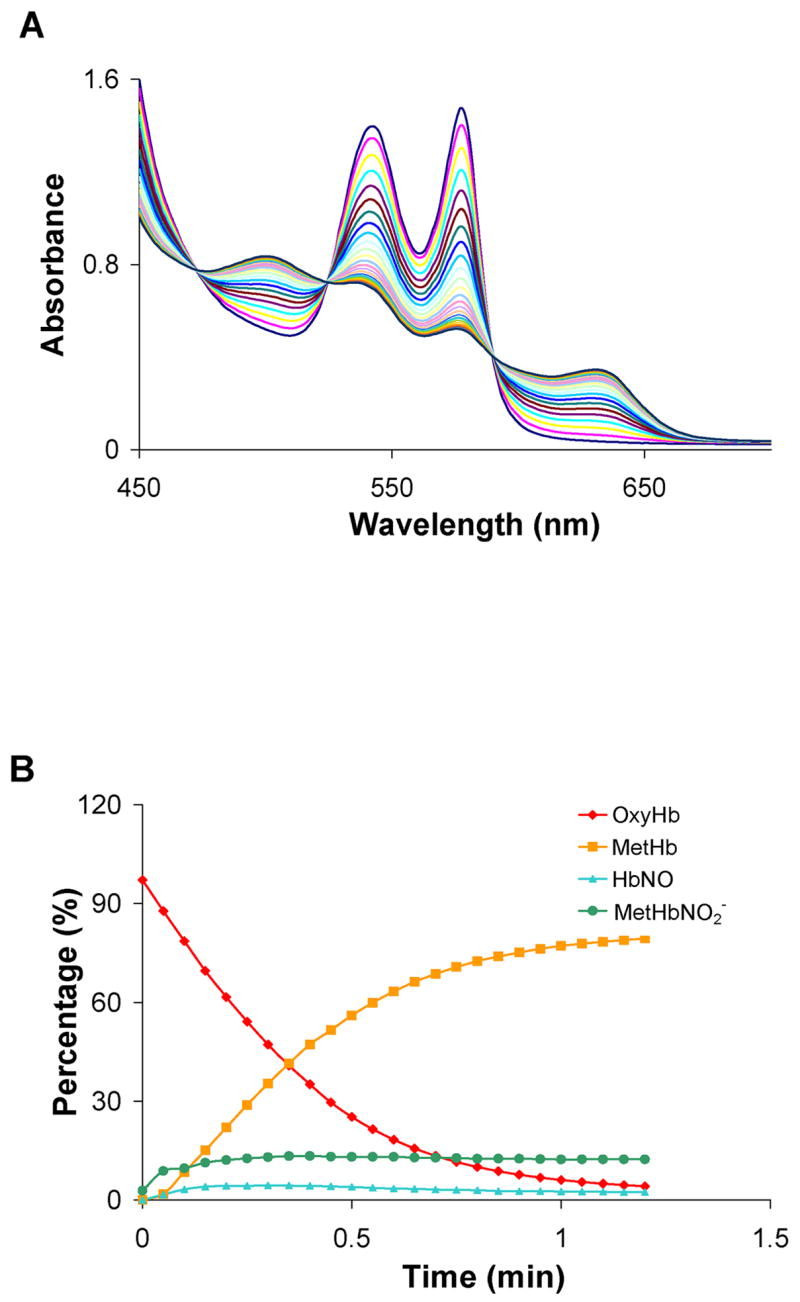
The reaction of Angeli’s salt with a molar excess of oxyHb. OxyHb (100 μM) was mixed with 50 μM Angeli’s salt in 0.1 M phosphate buffer under aerobic conditions. (A) UV-Vis spectra were recorded at 3.0 min intervals after the initial scan. (B) Each spectrum was fit to basis spectra to determine the percentage of each species at each time point. Data from a representative experiment are shown. The average amount of each species formed at 72 minutes from three different experiments was 3 ± 2 % oxyHb, 83 ± 5 % metHb, 2 ± 1 % HbNO, and 10 ± 4 % nitrite bound metHb. The remainder (about 2%) was fit as deoxyHb.
Figure 4 shows the results from mixing equimolar amounts of AS and oxyHb. Figure 4A shows representative spectra when 100 μM AS is reacted with 100 μM oxyHb. and Figure 4B shows the results from deconvolution into basis spectra. Here, the oxyHb is essentially gone within 10 minutes forming mostly metHb with some bound to nitrite and also forming some HbNO (at 12 minutes the concentrations are 0, 83, 13, and 3 μM for oxyHb, metHb, nitrite bound metHb, and HbNO). After 72 minutes 24% of the Hb is converted to HbNO form and 74% is metHb, 30% of which has nitrite bound. Similar results are obtained when 50 μM AS are reacted with 50 μM oxyHb (Figure 4c, d). Some differences in the final concentrations of MetHb and HbNO in the experiments with 50 μM vs 100 μM Hb may be due to the ratio of molecular oxygen to heme ratio in the two experiments.
Figure 4.
The reaction of Angeli’s salt with equi-molar oxyHb. (A) OxyHb (100 μM) was mixed with 100 μM Angeli’s salt in 0.1 M phosphate buffer under aerobic conditions. UV-Vis spectra were recorded at 3.0 min intervals after the initial scan. (B) Each spectrum from panel A was fit to basis spectra to determine the percentage of each species at each time point. (C) OxyHb (50 μM) was mixed with 50 μM Angeli’s salt in 0.1 M phosphate buffer under aerobic conditions. UV-Vis spectra were recorded at 3.0 min intervals after the initial scan. Note that the pathlength of the cell used here was smaller than that used to collect the data shown in panel A (0.2 cm compared to 0.5 cm). (D) Each spectrum from panel C was fit to basis spectra to determine the percentage of each species at each time point.
The HbNO detected when mixing AS with oxyHb is likely to be due to the reaction of metHb initially produced with HNO (i.e. reactions 2 and 3 followed by reaction 5 shown in Figure 1). To confirm this we reacted equimolar amounts of AS and metHb (Figure 5A and 5B) under aerobic conditions. HbNO is formed as expected. One AS will convert two oxyHb to two metHb (Figure 1, reactions 1–3). An additional AS can convert one metHb to one HbNO (Figure 1, reaction 5). If these were the only relevant reactions, with equimolar AS and oxyHb (as in Figure 4) one would expect that more of the Hb would be converted to HbNO after 72 minutes than is observed. Likewise, one would expect more HbNO to form from the mixture of equimolar amounts of metHb and HNO than is observed (Figure 5a,b). One reason for the lower yield is that some of the HbNO could be converted back to metHb via reactions involving oxygen [91]. These reactions are slow but could contribute to HbNO depletion. To test this we allowed 270 μM Hb that was 43% HbNO (formed by reaction of AS with metHb) to sit in room air for 82 minutes and found that 29% of the HbNO was converted to metHb (data not shown). To further explore the role of oxygen in affecting HbNO yield, we repeated the experiment shown in Figure 5A, mixing 100 μM AS with 100 μM metHb, only this time used anaerobic conditions. As shown in Figure 5C and 5D, significantly more HbNO is made (about 60% of total Hb).
Figure 5.
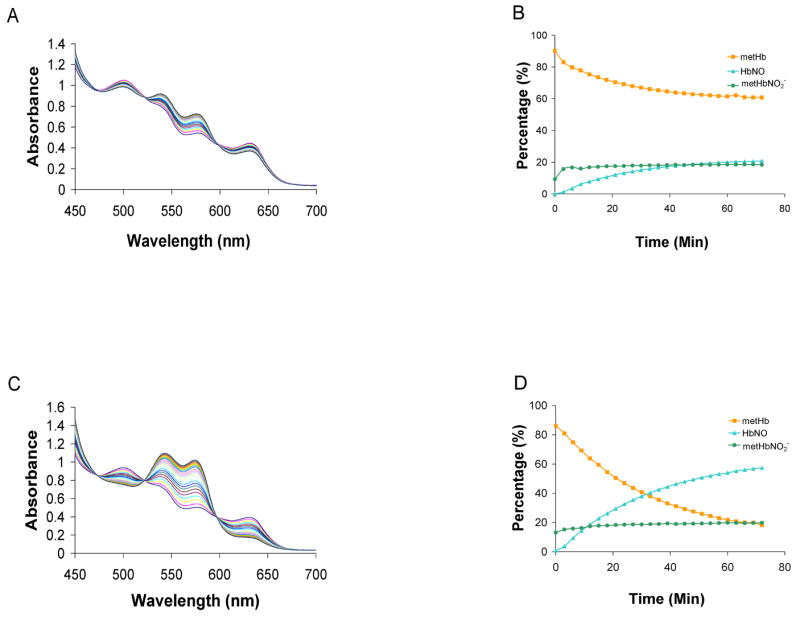
The reaction between metHb with Angeli’s salt. (A) MetHb (100 μM) was mixed with 100 μM Angeli’s salt in 0.1 M phosphate buffer equilibrated in aerobic conditions. Spectra are shown at 3 minute intervals over 72 minutes. (B) Each spectrum was fit to basis spectra to determine the percentage of each species at each time point. After 72 minutes, we found there to be 20% ± 2% HbNO, 64% ± 3% metHb, 15% ± 5% metHb-NO2− (n=3) (C) metHb (100 μM) was mixed with 100 μM Angeli’s salt in 0.1 M phosphate buffer equilibrated in anaerobic conditions. Spectra are shown at 3 minute intervals over 72 minutes. (D) Each spectrum was fit to basis spectra to determine the percentage of each species at each time point. After 72 minutes, we found there to be, 16% ± 9% metHb, 22% ± 3% metHb-NO2−, 54% ± 5% HbNO (n=3).
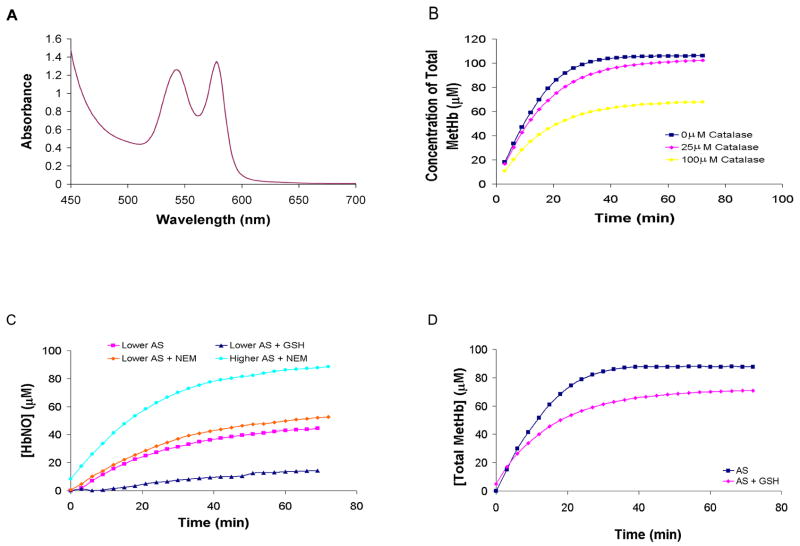
Although more HbNO is made when AS is added to metHb under anaerobic (Figure 5C and 5D) conditions than aerobic conditions (Figure 5A and 5B), the HbNO yield under anaerobic conditions is still less than what is expected (1 HbNO from 1 metHb, Figure 1 (reaction 5)). Thus, it is also unlikely that the lower than expected HbNO yield when equimolar AS is reacted with oxyHb (Figure 4) can be explained simply by invoking the HbNO + O2 reaction. Another possible explanation for lower HbNO yields could be due to the involvement of peroxide from the oxyHb + AS reaction (Equation 4 and Figure 1 reaction 2). Hydrogen peroxide could, among other things, react with metHb to form ferryl Hb and lead to heme degradation. However, a role for H2O2 in our reactions is unlikely due to the fact that our Hb preparations do not separate catalase from the Hb and thus contain substantial amounts of catalase so that the ratio of Hb to catalase would be about the same as that found in the red blood cell (about 1000:1 [92–94]). Even though the amount of catalase present is much less than the amount of Hb, since catalase reacts with H2O2 about 106 fold faster than oxyHb [95,96], one would expect catalase to convert essentially all the peroxide into water and oxygen. This is demonstrated to be the case in Figure 6A where we added 33 mM H2O2 with 100 μM oxyHb and recorded absorption spectra every 3 minutes for 90 minutes. No change in the absorption spectra was detected even with such high peroxide concentrations (all the spectra overlap). In addition, as noted in the methods section, we did not detect any significant ferryl Hb in our reaction systems when the spectrum was included in our regression analyses.
Figure 6.
Effects of GSH, H2O2, and catalase. (A) OxyHb (100 μM) was reacted with 33 mM hydrogen peroxide. Absorption spectra were collected every 3 minutes for 90 minutes. Every spectrum was identical so that only the last one collected is visible. (B) Catalase (25 μM or 100 μM) was included in the reaction of 50 μM AS and 100 μM oxyHb. The metHb yield, calculated by fitting to basis spectra, as a function of time are compared to that when catalase was not present. (C) The HbNO yield, calculated by fitting to basis spectra, as a function of time is plotted for the reactions of 100 μM metHb with 82 μM AS (lower AS) with or without 200 μM GSH. The HbNO yield is also plotted when the metHb is treated with NEM for 82 μM AS (lower AS) and 100 μM AS (higher AS). (D) MetHb yield is plotted as a function of time for the reaction of 50 μM AS with 100 μM oxyHb with or without 200 μM GSH present.
We investigated whether the presence of catalase in our systems would affect the reaction of AS and oxyHb. Figure 6B shows that when a lot (25 to 100 μM) of catalase is added to the oxyHb preparation (which already contains a little catalase), the metHb yield decreases. This phenomenon is likely due to the fact that HNO derived from AS reacts with catalase to form a ferrous iron nitrosyl form of catalase (reaction 7, Figure 1) [97]. NO readily dissociates from this catalase complex and reacts with oxyHb to make metHb (Figure 1, reactions 7 and 9) [97]. Note that in this pathway, one AS derived HNO molecule results in a single conversion of one oxyHb to metHb rather than when HNO derived from AS results in the conversion of two oxyHb to two metHb (via reactions 2 and 3 in Figure 1), thereby decreasing the metHb yield. Although the effect of catalase on metHb yield is observed at high (equimolar to Hb) catalase concentration, the effect is already diminished when the ratio of catalase to oxyHb is 1 to 4. Given that the ratio is about 1 to 1000 in red cells [92–94] (and our hemolysate), catalase is not likely to affect our experiments other than scavenging any H2O2 that is formed.
The most likely explanation for reduced HbNO yield in Figures 4 and 5 is due to the reaction of HNO and thiols (reaction 10 in Figure 1). The ability of thiols to compete with heme reactions with HNO has been noted previously [84]. When 200 μM reduced glutathione (GSH) was added to the reaction of 82 μM AS and 100 μM metHb under anaerobic conditions, the HbNO yield was greatly diminished (Figure 6C). Blocking the reactivity of the Hb β-93 cysteine increased the HbNO yield, but the effect is not as great as adding GSH, indicating that the reaction of HNO with protein bound thiols may be slower than with those of small molecules like GSH (Figure 6C). When 100 μM AS was added to 100 μM metHb that had been treated with NEM, HbNO yield was almost 100% (Figure 6C). The inclusion of 200 μM GSH with the reaction of 50 μM AS and 100 μM oxyHb reduced metHb yield (Figure 6D), but the effect of the GSH on the oxyHb reaction was not as great as it was on the metHb reaction. This result is consistent with published reaction rate constants for these reactions ([85] and see Figure 1). The fact that addition of 50 μM AS to 100 μM oxyHb forms almost 100 μM metHb (Figure 3) suggests that Hb β-93 cysteine does not effectively compete with the heme reaction of oxyHb and AS derived HNO. Reactions of protein thiols are predicted to be more competitive with the reaction of HNO and metHb and therefore may have contributed to lowered HbNO yields in Figure 5.
In vivo, the oxygen pressure and Hb oxygen saturation is less than that present when Hb is prepared in solution under aerobic conditions. We therefore examined the reaction of AS derived HNO with partially oxygenated Hb (Figure 7A). The initial sample is composed of 52% oxyHb and 48% deoxyHb. As shown in Figure 7B, the percentage of metHb in the sample rises, presumably due to the reaction with oxyHb, and then falls, most likely due the reaction of metHb with HNO to form HbNO. The disappearance of oxyHb is slower while the formation of HbNO is larger in Figure 7 compared to what is observed in Figure 4a,b where the Hb was completely oxygenated. This is most likely partially due to NO that is formed from the reaction of oxyHb and HNO reacting with deoxygenated Hb instead of another oxyHb. After 72 minutes, about 45% of the sample is converted to HbNO (Figure 7C).
Figure 7.
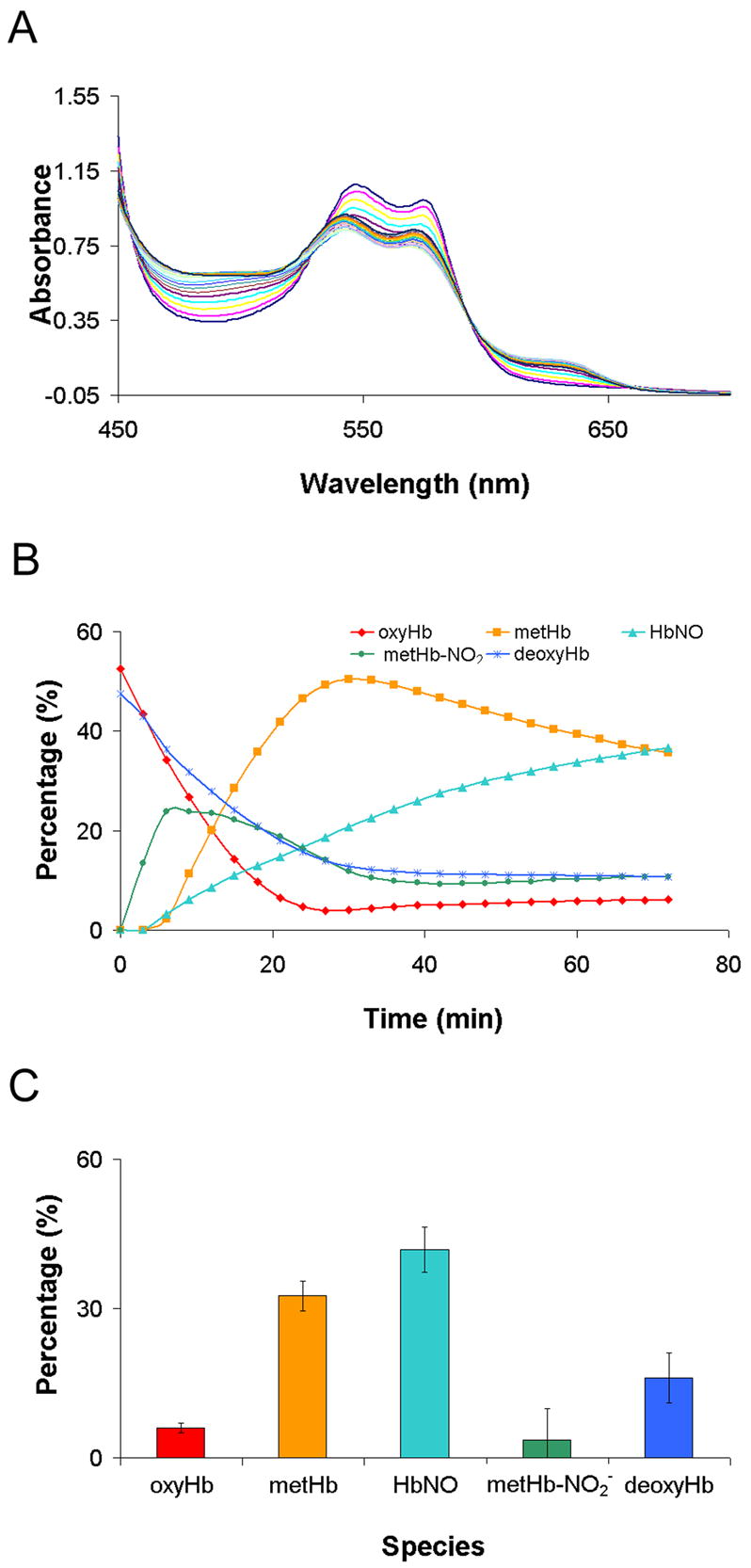
The reaction between partially oxygenated hemoglobin with Angeli’s salt. Partially oxygenated hemoglobin (100 μM ) was mixed with 100 μM Angeli’s salt in deoxygenated phosphate buffer. (A) Raw absorption spectra. (B) Each spectrum was fit to basis spectra to determine the percentage of each species at each time point. (C) The average amount of each species formed at 72 minutes from three different experiments. Standard deviations are also shown.
Figure 8 shows that AS preferentially reacts with cell-free Hb compared to RBC encapsulated Hb. As described in the methods section, competition experiments were performed where AS was added to a mixture of red cells and cell-free Hb and the relative rate of the reaction was monitored examining the products in each fraction after separation by sedimentation. Figure 8A shows EPR spectra taken from a mixture of 50 μM AS with 107 μM cell-free Hb and red cells at a hematocrit of 41% (corresponding to about 10 mM in Hb) after 30 minutes of incubation under aerobic conditions. Under these conditions, metHb is the only Hb product resulting from the addition of AS to oxyHb that is detectable by EPR (no HbNO). The presence of high spin metHb is evidenced by the low field EPR resonance at g = 6. Spectra are shown for the cell-free Hb (supernatant), red-cell encapsulated Hb (pellet), and the whole mixture (before sedimentation). The sum of the metHb in each fraction is equal to that in the whole mixture. This condition was used as a self-consistency check for inclusion of data sets for calculating the preferential reactivity, kf/kr. Since there was about 100 times more red-blood cell encapsulated Hb than cell-free Hb, if AS had no preferential reactivity (kf/kr = 1), one would expect only 1% of the metHb formed by reaction with AS to be in the cell-free Hb fraction. Instead, 45% of the reacted Hb is in the cell free fraction, giving a value of kf/kr = 75‡. Importantly when this experiment was repeated using whole blood, wherein 50 μM AS was reacted with whole blood with red cells at 41% Hct and 100 μM cell-free Hb, 26 μM metHb was formed in the supernatant (after correction to the total volume, data not shown). This is about 84% of the amount of metHb found when plasma was absent indicating that plasma thiols are likely to compete with oxyHb oxidation in plasma, but the oxidation of Hb will still be quite effective.
Figure 8.
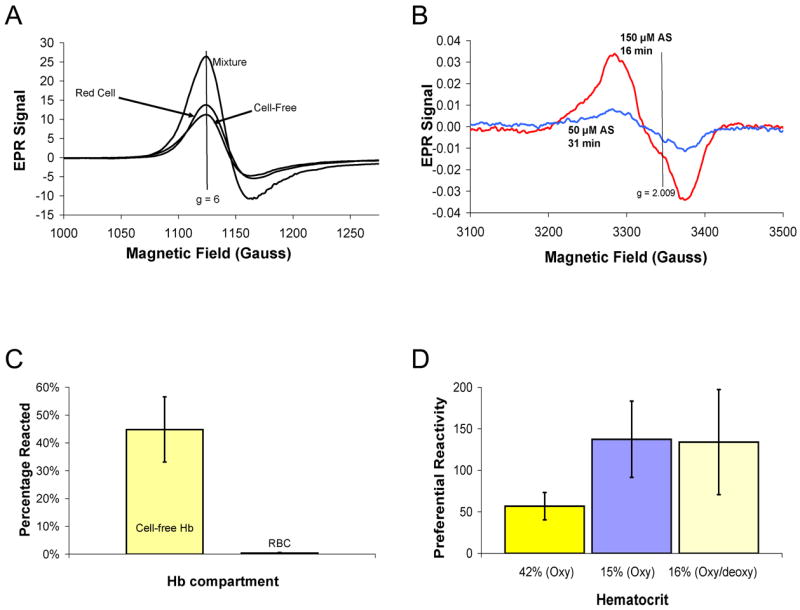
Preferential reactivity of AS. (A) Fifty micromolar AS was added to a mixture 107 μM cell-free Hb and RBCs at 41% hematocrit in aerobic conditions. After 31 minutes, the samples was analyzed for metHb formation by EPR. Spectra are shown for the cell-free Hb and red cell fractions as well as the mixture of the two. Double integration of the EPR peaks yielded 31 μM in the cell-free fraction, 36 μM in the red cell fraction, and 69 μM in the mixture. The magnetic field value corresponding to g = 6 is shown as a vertical line at 1120 G. For a control sample in which AS was not added, only 1.2 μM metHb formed due to autoxidation during 31 minutes. (B) The formation of iron-nitrosyl Hb in the cell-free fraction is demonstrated using EPR spectroscopy. One spectrum was taken from a sample where 50 μM AS was added to a mixture of RBCs at 17% hematocrit and 31 μM cell-free Hb for 31 minutes resulting in 0.5 μM HbNO in the cell-free fraction. The other spectrum was taken from a sample where 150 μM was added to a mixture of red cells at 16% hematocrit and 29 μM cell-free Hb resulting in the formation of 2 μM HbNO. The magnetic field value corresponding to g = 2.009 is shown as a vertical line at 3337 G. (C) A summary of percentage of cell-free and RBC Hb reacted after 50–150 μM AS was added to partially oxygenated (68 ± 12%) red cells at 16 ± 1% hematocrit and 30 ± 2 μM cell-free Hb after 6 minutes of the reaction. Each bar represent the percentage of reacted Hb (metHb and iron-nitrosyl Hb) compared to the total amount in that fraction. AS converted 45 ± 12 % of cell-free Hb (about 13.5 μM) to non-NO scavenging forms and only converted 0.4 ± 0.3 % of RBC encapsulated Hb (n=3). The data are shown as the average ± one standard deviation. (D) A summary of the preferential reactivity of AS is given for different conditions (data shown as average of three different preparations with error bars showing the standard deviation). The bar on the left is from data where 50 μM AS was added to red cells at 42 ± 1% hematocrit and 99 ± 7 μM cell-free Hb under completely aerobic conditions. The middle bar is from data where 50 μM AS was added to red cells at 15 ± 1% and 102 ± 2 μM cell-free Hb under completely aerobic conditions. The bar on the right is from when 50–150 μM AS was added to red cells at 16 ± 1% hematocrit and 30 ± 2 μM cell-free Hb under partially anaerobic conditions so that Hb oxygen saturation was 68 ± 12%.
When the ratio of AS to cell-free Hb increases and the system is partially deoxygenated, iron-nitrosyl Hb is detected both in the cell free fraction and red cell encapsulated fraction (Figure 8B). The formation of HbNO is evidenced by the appearance of an EPR resonance at g = 2.009. Figure 8C shows a summary of the percentage of reacted Hb in each fraction after 50–150 μM AS was added to red cells at 16 ± 1% hematocrit and 30 ± 2 μM cell-free Hb after 6 minutes of the reaction. AS converted 45 ± 12 % of cell-free Hb to non-NO scavenging forms (metHb and iron-nitrosyl Hb) and only converted 0.4 ± 0.3 % of RBC encapsulated Hb (n=3). Figure 8D shows a summary of the preferential reactivity (kf/kr) of AS from multiple trials under different conditions. The preferential reactivity is greater for lower hematocrits (such as in hemolytic anemias) as has been observed previously for NO [45].
Discussion
Consistent with previous studies we have shown (1) AS is efficient at converting oxyHb to metHb and (2) AS will further convert metHb to HbNO. Here we show that these reaction also produce nitrite bound metHb and, importantly, that AS preferentially reacts with cell-free Hb compared to RBC encapsulated Hb. These results suggest that AS may hold therapeutic promise in the context of reducing NO scavenging by cell-free Hb in pathological conditions associated with hemolysis.
Previous work has demonstrated the rapid reaction of HNO released from AS with oxyHb to form metHb [84–86]. The pathway likely involves co-oxidation of the iron atom and nitroxyl by the bound dioxygen to yield metHb, NO and an equivalent of hydrogen peroxide (reaction 2, Figure 1) [84],
| (7) |
The NO formed can then react with another oxyHb to form metHb and nitrate (reaction 3, Figure 1), giving the relation of Equation 4 where one AS derived HNO converts two oxyHb to two metHb [84]. Our results shown in Figure 3 are consistent with this stoichiometry. In addition, we find that that about 10% of the metHb has nitrite bound to it. Although the formation of metHb-NO2− has not (to our knowledge) been reported before, the result is to be expected given that AS forms nitrite and metHb binds nitrite [98]. MetHb-NO2− is less likely to cause oxidative damage than metHb alone. Peroxide or other reactants are less likely to be able to access the heme with nitrite bound.
Figures 4, 5 and 7 show the formation of HbNO. The kinetics of the reaction of HNO with oxyHb to form metHb is about ten times faster than the reaction of HNO with metHb to form HbNO [85,86]. Thus, one expects to convert most of the oxyHb to metHb before metHb is converted to HbNO. This is what is observed in Figures 4 and 7. We have shown that more HbNO is made when oxygen tension is lowered (Figures 5 and 7), most likely due to the reaction of oxygen with HbNO to form metHb. Formation of HbNO, which will be enhanced under conditions with lower oxygen tension, may benefit patients in that it is a relatively stable, non-toxic form of Hb. HbNO itself may not be infinitely stable in the plasma but its formation is likely to provide some protection against oxidative damage.
Other reactions besides those discussed so far may be considered to play a role when AS is added to oxyHb. Firstly, one may suggest that AS derived nitrite may react with the oxyHb, but that reaction is too slow to be a significant factor which we confirmed by adding nitrite to oxyHb and observing very little reaction. The reaction of nitrite with deoxyHb to form metHb and NO could play some role. This reaction is fastest (6 M−1s−1) when a sample is partially oxygenated so that some of the material is in the R quaternary state, but still much slower than the reaction of HNO with oxyHb [99,100]. Thus, the reaction of nitrite with deoxyHb could make some, but not a great, contribution to the yields we observe. Another reaction to consider would be the result of NO (formed from reaction of HNO with oxyHb) binding to metHb. Since the dissociation rate constant for NO from metHb is fast (about 1 s−1 [79]) and the association rate constant is slow (4 × 103 M−1s−1 [79] ) compared to the reaction of NO with oxyHb and NO binding to ferrous heme, very little if any NO bound metHb is likely to form. However, there is some possibility that a small amount that does form would undergo reductive nitrosyaltion forming nitrite and deoxyHb (which could subsequently be nitrosylated by another NO molecule) [101]. A third reaction that one might invoke is that of oxygen with HNO, but that reaction is several orders of magnitude slower than reactions of HNO with heme, so it is not expected to play a significant role [85]. Finally, one may consider the reactions of HNO with itself or with nitric oxide, but the steady-state levels of these predict that these reactions would not be significant.
We have shown (Figure 8) that AS reacts preferentially with cell-free Hb compared to that encapsulated in the red cell. Comparison of Figure 8A with Figure 3B indicates that the primary reaction of HNO in the RBC is with oxyHb. That this reaction successfully competes with reactions with thiols and other RBC constituents makes sense given that the oxyHb/HNO reaction is the fastest and oxyHb is the most abundant reacting molecule in the RBC. When AS was added to Hb alone (100 μM), a total of 69 μM metHb is made after 30 minutes, 13 μM of which had nitrite bound (Figure 3B). When AS was added to essentially the same concentration of Hb and almost 10 mM red cell encapsulated Hb (41% Hct), 67–69 μM metHb was made (Figure 8A). Since nitrite bound metHb is EPR silent [102], there is actually probably slightly more metHb made in the case with RBCs present. The reaction of nitrite with oxyHb in the RBC may also contribute slightly to metHb formation. This reaction is slow, but with 20 mM oxyHb in the red cell, it is expected to make some contribution. The reaction of nitrite with RBC Hb is not significantly impeded by RBC uptake [103]. When we reacted 50 μM nitrite (which is much more than the basal amount found in RBCs (< 1 μM) [103,104]) with RBCs for 30 minutes, 16 μM metHb formed (data not shown). Note that this is significantly more than would form from nitrite derived from 50 μM AS after 30 minutes since the AS does not decompose immediately.
That the preferential reactivity (kf/kr) is higher for lower hematocrit, demonstrates that a large factor in establishing the preferential reactivity is that the reaction with red cell encapsulated Hb is rate-limited by the time it take for the HNO to diffuse to the red cell (unstirred layer) rather than due to a physical red cell membrane diffusion barrier to HNO, as described for NO [45]. Rate limitation by a physical membrane barrier would not have a hematocrit dependence [45]. The values of kf/kr found here for AS are about 1/3 smaller than those measured for NO [45]. One reason for this is likely that the bimolecular rate for the reaction of NO with oxyHb is faster than that of HNO with oxyHb [28,85]. It has been shown both computationally [105] and experimentally [106] that when the intrinsic rate of reaction of Hb with a ligand is slower, kf/kr is smaller. On the other hand, the reaction of HNO with oxyHb is quite fast (about 107 M−1s−1 [85]) and so a kinetic barrier (where the reaction of HNO with red cell Hb will be rate limited by the time for the HNO to diffuse to the red cell) is expected. Our observations of a high preferential reactivity affirm this notion. Since AS releases nitrite, and nitrite reacts relatively slowly with Hb, its reactions may contribute to a smaller value of kf/kr for AS compared that of NO. The reaction with oxyHb is very slow and probably doesn’t contribute, but that with deoxyHb may be significant. In any case, the values of kf/kr that we measured were quite large (about 50 at 42% hematocrit and 130 at 16% hematocrit).
Under partially oxygenated conditions we measured HbNO in both the cell-free and red cell fractions when performing competition experiments. The HbNO formed under these conditions could be from the reaction of (1) metHb with HNO or (2) from the HNO with oxyHb to form metHb and NO with subsequent binding of NO to deoxyHb. The amount of HbNO made in the cell-free fraction was limited probably due to the fact the reaction of HNO with cell-free metHb does not have great preferential reactivity compared to the reaction with red cell oxyHb (or other Hb red cell reactions which are rate-limited by diffusion to the red cell). One may expect the cell-free HbNO yield to be greater in vivo due to the cell-free zone. Due to the fact that red cells travel fastest in the middle of blood vessels, a pressure gradient is formed pushing the red cells inward creating a red-cell free zone near the endothelium [37]. In this zone, AS will only react with cell-free Hb which is not pushed to the center of the vessel.
Much further work is required to establish the feasibility of using AS to treat hemolysis. AS has been infused in dogs at a rate of 10 μg/kg/min for ten minutes without adverse effects being reported [85], but the nature and extent of adverse effects for infusions at this and other doses remain to be determined. We have shown that AS reacts preferentially with cell-free Hb to form metHb and iron nitrosyl Hb. The preferential reactivity is greatest at low hematocrit as occurs in hemolytic anemias. As the products of the reaction do not effectively scavenge NO, AS may be useful to treat hemolysis to restore NO bioavailability in a similar way as NO inhalation therapy or use of NO donor molecules would. The decreased reaction rate of HNO with Hb encapsulated in the red cell compared to cell-free Hb may aid in allowing HNO to reach other therapeutic targets as less HNO will react with Hb than predicted based on rates determined using cell-free Hb.
Acknowledgments
This work was supported by NIH grant HL58091 (DK-S) and HL62198 (SBK). Further support is acknowledged through Career Award K02 HL078706 (DK-S).
List of abbreviations
- NO
Nitric oxide
- Hb
Hemoglobin
- oxyHb
Oxygenated hemoglobin
- metHb
Methemoglobin
- deoxyHb
deoxygenated hemoglobin
- HbNO
iron-nitrosyl hemoglobin (where NO is bound to the ferrous heme)
- RBC
Red blood cell
- HBOC
Hemoglobin based oxygen carrier
- PNH
Paroxysmal nocturnal hemoglobinuria
- HNO
nitroxyl
- AS
Angeli’s salt, Sodium α-oxyhyponitrite, Na2N2O3
- PBS
Phosphate buffered saline
- EPR
Electron paramagnetic resonace
- Hct
Hematocrit
- GSH
glutathione
Footnotes
Throughout this manuscript the concentration of Hb is given on a heme basis. So 2.5 mM Hb tetramers would herein be written as 10 mM Hb as there are 4 hemes per tetramer. 10 mM heme is equal to 16 g/dL, within the normal range of hemoglobin for an adult.
Neil Hogg, personal communication.
From Equation 5 we have kf/kr = ([oxyHb]r/[metHb]r)([metHb]f/[oxyHb]f), where the subscript refers to RBC and f refers to free as in cell-free Hb. For the data shown in Figure 8a: [oxy]r = 8,124 μM (40.6% Hct), [metHb]r = 36.3 μM, [metHb]f = 30.7 μM and the value [oxyHb]f at the time of the measurement (31 minutes after adding AS) was 78.3 μM. Using this value of [oxyHb]f is problematic as the amount of [oxyHb]f changes in time, so that Equation 5 is modified so that kf/kr = ([oxyHb]r/[metHb]r)(ln(1+ [metHb]f/[oxyHb]f)). Plugging in our values we have kf/kr = (224)(ln(1 + 0.392) = (224.)(0.331) = 74. Note that without the correction using natural log, one would have obtained a value of 88 which is an overestimation but not horribly different from the corrected value.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Furchgott RF, Zawadzki JV. The Obligatory Role of Endothelial-Cells in the Relaxation of Arterial Smooth-Muscle by Acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 2.Ignarro LJ, Byrns RE, Buga GM, Wood KS. Endothelium-Derived Relaxing Factor from Pulmonary-Artery and Vein Possesses Pharmacological and Chemical-Properties Identical to Those of Nitric-Oxide Radical. Circ Res. 1987;61:866–879. doi: 10.1161/01.res.61.6.866. [DOI] [PubMed] [Google Scholar]
- 3.Katsuki S, Arnold W, Mittal C, Murad F. Stimulation of Guanylate Cyclase by Sodium Nitroprusside, Nitroglycerin and Nitric-Oxide in Various Tissue Preparations and Comparison to Effects of Sodium Azide and Hydroxylamine. J Cyclic Nucleotide Res. 1977;3:23–35. [PubMed] [Google Scholar]
- 4.Palmer RMJ, Ferrige AG, Moncada S. Nitric-Oxide Release Accounts for the Biological-Activity of Endothelium-Derived Relaxing Factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 5.Ignarro LJ. Nitric Oxide Biology and Pathobiology. San Diego: Academic press; 2000. [Google Scholar]
- 6.Yuan SY. New insights into eNOS signaling in microvascular permeability. Am J Physiol Heart Circ Physiol. 2006;291:H1029–31. doi: 10.1152/ajpheart.00509.2006. [DOI] [PubMed] [Google Scholar]
- 7.Bucci M, Roviezzo F, Posadas I, Yu J, Parente L, Sessa WC, Ignarro LJ, Cirino G. Endothelial nitric oxide synthase activation is critical for vascular leakage during acute inflammation in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:904–8. doi: 10.1073/pnas.0408906102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Predescu D, Predescu S, Shimizu J, Miyawaki-Shimizu K, Malik AB. Constitutive eNOS-derived nitric oxide is a determinant of endothelial junctional integrity. American journal of physiology. 2005;289:L371–81. doi: 10.1152/ajplung.00175.2004. [DOI] [PubMed] [Google Scholar]
- 9.Murohara T, Asahara T, Silver M, Bauters C, Masuda H, Kalka C, Kearney M, Chen D, Symes JF, Fishman MC, Huang PL, Isner JM. Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. The Journal of clinical investigation. 1998;101:2567–78. doi: 10.1172/JCI1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooke JP. NO and angiogenesis. Atherosclerosis. 2003;4:53–60. doi: 10.1016/s1567-5688(03)00034-5. [DOI] [PubMed] [Google Scholar]
- 11.Battinelli EM, Loscalzo J. Nitric oxide and platelet-mediated hemostasis. In: Loscalzo J, Vita JA, editors. Nitric oxide and the cardiovascular system. Humana; Totowa, N.J: 2000. pp. 123–138. [Google Scholar]
- 12.Loscalzo J. Nitric oxide insufficiency, platelet activation, and arterial thrombosis. Circulation research. 2001;88:756–62. doi: 10.1161/hh0801.089861. [DOI] [PubMed] [Google Scholar]
- 13.Nakashima S, Tohmatsu T, Hattori H, Okano Y, Nozawa Y. Inhibitory action of cyclic GMP on secretion, polyphosphoinositide hydrolysis and calcium mobilization in thrombin-stimulated human platelets. Biochemical and biophysical research communications. 1986;135:1099–104. doi: 10.1016/0006-291x(86)91041-7. [DOI] [PubMed] [Google Scholar]
- 14.Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proceedings of the National Academy of Sciences of the United States of America. 1991;88:4651–5. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tailor A, Granger DN. Role of adhesion molecules in vascular regulation and damage. Current hypertension reports. 2000;2:78–83. doi: 10.1007/s11906-000-0063-6. [DOI] [PubMed] [Google Scholar]
- 16.Hickey MJ, Kubes P. Role of nitric oxide in regulation of leucocyte-endothelial cell interactions. Experimental physiology. 1997;82:339–48. doi: 10.1113/expphysiol.1997.sp004029. [DOI] [PubMed] [Google Scholar]
- 17.Rother RP, Bell L, Hillmen P, Gladwin MT. The clinical sequelae of intravascular hemolysis and extracellular plasma hemoglobin - A novel mechanism of human disease. Jama-J Am Med Assoc. 2005;293:1653–1662. doi: 10.1001/jama.293.13.1653. [DOI] [PubMed] [Google Scholar]
- 18.Minneci PC, Deans KJ, Zhi H, Yuen PST, Star RA, Banks SM, Schechter AN, Natanson C, Gladwin MT, Solomon SB. Hemolysis-associated endothelial dysfunction mediated by accelerated NO inactivation by decompartmentalized oxyhemoglobin. J Clin Invest. 2005;115:3409–3417. doi: 10.1172/JCI25040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reiter CD, Wang XD, Tanus-Santos JE, Hogg N, Cannon RO, Schechter AN, Gladwin MT. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med. 2002;8:1383–1389. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- 20.Coggins MP, Bloch KD. Nitric oxide in the pulmonary vasculature. Arterioscler Thromb Vasc Biol. 2007;27:1877–1885. doi: 10.1161/ATVBAHA.107.142943. [DOI] [PubMed] [Google Scholar]
- 21.Marin E, Sessa WC. Role of endothelial-derived nitric oxide in hypertension and renal disease. Current Opinion in Nephrology and Hypertension. 2007;16:105–110. doi: 10.1097/MNH.0b013e328017f893. [DOI] [PubMed] [Google Scholar]
- 22.Kato GJ, Gladwin MT, Steinberg MH. Deconstructing sickle cell disease: Reappraisal of the role of hemolysis in the development of clinical subphenotypes. Blood Rev. 2007;21:37–47. doi: 10.1016/j.blre.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ignarro LJ, Balestrieri ML, Napoli C. Nutrition, physical activity, and cardiovascular disease: An update. Cardiovasc Res. 2007;73:326–340. doi: 10.1016/j.cardiores.2006.06.030. [DOI] [PubMed] [Google Scholar]
- 24.Sobolewski P, Gramaglia I, Frangos J, Intaglietta M, van der Heyde HC. Nitric oxide bioavailability in malaria. Trends in Parasitology. 2005;21:415–422. doi: 10.1016/j.pt.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Eich RF, Li TS, Lemon DD, Doherty DH, Curry SR, Aitken JF, Mathews AJ, Johnson KA, Smith RD, Phillips GN, Olson JS. Mechanism of NO-induced oxidation of myoglobin and hemoglobin. Biochemistry-US. 1996;35:6976–6983. doi: 10.1021/bi960442g. [DOI] [PubMed] [Google Scholar]
- 26.Doyle MP, Hoekstra JW. Oxidation of Nitrogen-Oxides by Bound Dioxygen in Hemoproteins. J Inorg Biochem. 1981;14:351–358. doi: 10.1016/s0162-0134(00)80291-3. [DOI] [PubMed] [Google Scholar]
- 27.Herold S, Exner M, Nauser T. Kinetic and mechanistic studies of the NO center dot-mediated oxidation of oxymyoglobin and oxyhemoglobin. Biochemistry-US. 2001;40:3385–3395. doi: 10.1021/bi002407m. [DOI] [PubMed] [Google Scholar]
- 28.Huang KT, Huang Z, Kim-Shapiro DB. Nitric Oxide Red Blood Cell Membrane Permeability at high and low Oxygen Tension. Nitric Oxide. 2007;16:209–216. doi: 10.1016/j.niox.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cassoly R, Gibson QH. Conformation, Co-Operativity and Ligand-Binding in Human Hemoglobin. J Mol Biol. 1975;91:301–313. doi: 10.1016/0022-2836(75)90382-4. [DOI] [PubMed] [Google Scholar]
- 30.Huang KT, Huang Z, Kim-Shapiro DB. Nitric Oxide Red Blood Cell Membrane Permeability at high and low Oxygen Tension. Nitric Oxide. 2006;16:209–216. doi: 10.1016/j.niox.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sharma VS, Ranney HM. Dissociation of NO from Nitrosylhemoglobin. J Biol Chem. 1978;253:6467–6472. [PubMed] [Google Scholar]
- 32.Azizi F, Kielbasa JE, Adeyiga AM, Maree RD, Frazier M, Yakubu M, Shields H, King SB, Kim-Shapiro DB. Rates of nitric oxide dissociation from hemoglobin. Free Radic Biol Med. 2005;39:145–151. doi: 10.1016/j.freeradbiomed.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 33.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-Derived Relaxing Factor Produced and Released from Artery and Vein Is Nitric-Oxide. Proc Natl Acad Sci USA. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lancaster JR. Simulation of the Diffusion and Reaction of Endogenously Produced Nitric-Oxide. Proc Natl Acad Sci USA. 1994;91:8137–8141. doi: 10.1073/pnas.91.17.8137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carlsen E, Comroe JH. The rate of uptake of Carbon Monoxide and of Nitric Oxide by normal and human erythrocytes and experimentally produced spherocytes. J Gen Physiol. 1958;42:83–107. doi: 10.1085/jgp.42.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coin JT, Olson JS. Rate of Oxygen-Uptake by Human Red Blood-Cells. J Biol Chem. 1979;254:1178–1190. [PubMed] [Google Scholar]
- 37.Butler AR, Megson IL, Wright PG. Diffusion of nitric oxide and scavenging by blood in the vasculature. Biochim Biophys Acta. 1998;1425:168–176. doi: 10.1016/s0304-4165(98)00065-8. [DOI] [PubMed] [Google Scholar]
- 38.Vaughn MW, Kuo L, Liao JC. Estimation of nitric oxide production and reaction rates in tissue by use of a mathematical model. Am J Physiol-Heart Circul Physiol. 1998;43:H2163–H2176. doi: 10.1152/ajpheart.1998.274.6.H2163. [DOI] [PubMed] [Google Scholar]
- 39.Liao JC, Hein TW, Vaughn MW, Huang KT, Kuo L. Intravascular flow decreases erythrocyte consumption of nitric oxide. Proc Natl Acad Sci USA. 1999;96:8757–8761. doi: 10.1073/pnas.96.15.8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vaughn MW, Huang KT, Kuo L, Liao JC. Erythrocytes possess an intrinsic barrier to nitric oxide consumption. J Biol Chem. 2000;275:2342–2348. doi: 10.1074/jbc.275.4.2342. [DOI] [PubMed] [Google Scholar]
- 41.Vaughn MW, Huang KT, Kuo L, Liao JC. Erythrocyte consumption of nitric oxide: Competition experiment and model analysis. Nitric Oxide-Biol Ch. 2001;5:18–31. doi: 10.1006/niox.2000.0328. [DOI] [PubMed] [Google Scholar]
- 42.Liu XP, Samouilov A, Lancaster JR, Zweier JL. Nitric oxide uptake by erythrocytes is primarily limited by extracellular diffusion not membrane resistance. J Biol Chem. 2002;277:26194–26199. doi: 10.1074/jbc.M201939200. [DOI] [PubMed] [Google Scholar]
- 43.Huang KT, Han TH, Hyduke DR, Vaughn MW, Van Herle H, Hein TW, Zhang CH, Kuo L, Liao JC. Modulation of nitric oxide bioavailability by erythrocytes. Proc Natl Acad Sci USA. 2001;98:11771–11776. doi: 10.1073/pnas.201276698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu XP, Miller MJS, Joshi MS, Sadowska-Krowicka H, Clark DA, Lancaster JR. Diffusion-limited reaction of free nitric oxide with erythrocytes. J Biol Chem. 1998;273:18709–18713. doi: 10.1074/jbc.273.30.18709. [DOI] [PubMed] [Google Scholar]
- 45.Azarov I, Huang KT, Basu S, Gladwin MT, Hogg N, Kim-Shapiro DB. Nitric oxide scavenging by red blood cells as a function of hematocrit and oxygenation. J Biol Chem. 2005;280:39024–38032. doi: 10.1074/jbc.M509045200. [DOI] [PubMed] [Google Scholar]
- 46.Kim-Shapiro DB, Schechter AN, Gladwin MT. Unraveling the Reactions of Nitric Oxide, Nitrite, and Hemoglobin in Physiology and Therapeutics. Arterioscler Thromb Vasc Biol. 2006;26:697–705. doi: 10.1161/01.ATV.0000204350.44226.9a. [DOI] [PubMed] [Google Scholar]
- 47.Han TH, Liao JC. Erythrocyte nitric oxide transport reduced by a submembrane cytoskeletal barrier. Biochim Biophys Acta. 2005;1723:135–142. doi: 10.1016/j.bbagen.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 48.Loscalzo J. Nitric oxide binding and the adverse effects of cell-free hemoglobins: What makes us different from earthworms. J Lab Clin Med. 1997;129:580–583. doi: 10.1016/s0022-2143(97)90191-8. [DOI] [PubMed] [Google Scholar]
- 49.Sakai H, Hara H, Yuasa M, Tsai AG, Takeoka S, Tsuchida E, Intaglietta M. Molecular dimensions of Hb-based O-2 carriers determine constriction of resistance arteries and hypertension. Am J Physiol-Heart Circul Physiol. 2000;279:H908–H915. doi: 10.1152/ajpheart.2000.279.3.H908. [DOI] [PubMed] [Google Scholar]
- 50.Matheson B, Kwansa HE, Bucci E, Rebel A, Koehler RC. Vascular response to infusions of a nonextravasating hemoglobin polymer. J Appl Physiol. 2002;93:1479–1486. doi: 10.1152/japplphysiol.00191.2002. [DOI] [PubMed] [Google Scholar]
- 51.Dull RO, DeWitt BJ, Dinavahi R, Schwartz L, Hubert C, Pace N, Fronticelli C. Quantitative assessment of hemoglobin-induced endothelial barrier dysfunction. J Appl Physiol. 2004;97:1930–1937. doi: 10.1152/japplphysiol.00102.2004. [DOI] [PubMed] [Google Scholar]
- 52.Lamkin-Kennard KA, Jaron D, Buerk DG. Impact of the Fahraeus effect on NO and O-2 biotransport: A computer model. Microcirculation. 2004;11:337–349. doi: 10.1080/10739680490437496. [DOI] [PubMed] [Google Scholar]
- 53.Vogel WM, Dennis RC, Cassidy G, Apstein CS, Valeri CR. Coronary Constrictor Effect of Stroma-Free Hemoglobin-Solutions. Am J Physiol. 1986;251:H413–H420. doi: 10.1152/ajpheart.1986.251.2.H413. [DOI] [PubMed] [Google Scholar]
- 54.Hess JR, Macdonald VW, Brinkley WW. Systemic and Pulmonary-Hypertension after Resuscitation with Cell-Free Hemoglobin. J Appl Physiol. 1993;74:1769–1778. doi: 10.1152/jappl.1993.74.4.1769. [DOI] [PubMed] [Google Scholar]
- 55.Lee R, Neya K, Svizzero TA, Vlahakes GJ. Limitations of the Efficacy of Hemoglobin-Based Oxygen-Carrying Solutions. J Appl Physiol. 1995;79:236–242. doi: 10.1152/jappl.1995.79.1.236. [DOI] [PubMed] [Google Scholar]
- 56.Murray JA, Ledlow A, Launspach J, Evans D, Loveday M, Conklin JL. The Effects of Recombinant Human Hemoglobin on Esophageal Motor Function in Humans. Gastroenterology. 1995;109:1241–1248. doi: 10.1016/0016-5085(95)90584-7. [DOI] [PubMed] [Google Scholar]
- 57.Ulatowski JA, Nishikawa T, MathesonUrbaitis B, Bucci E, Traystman RJ, Koehler RC. Regional blood flow alterations after bovine fumaryl beta beta-crosslinked hemoglobin transfusion and nitric oxide synthase inhibition. Crit Care Med. 1996;24:558–565. doi: 10.1097/00003246-199604000-00003. [DOI] [PubMed] [Google Scholar]
- 58.Sloan EP, Koenigsberg M, Gens D, Cipolle M, Runge J, Mallory MN, Rodman G. Diaspirin cross-linked hemoglobin (DCLHb) in the treatment of severe traumatic hemorrhagic shock - A randomized controlled efficacy trial. Jama-J Am Med Assoc. 1999;282:1857–1864. doi: 10.1001/jama.282.19.1857. [DOI] [PubMed] [Google Scholar]
- 59.Dou Y, Maillett DH, Eich RF, Olson JS. Myoglobin as a model system for designing heme protein based blood substitutes. Biophys Chem. 2002;98:127–148. doi: 10.1016/s0301-4622(02)00090-x. [DOI] [PubMed] [Google Scholar]
- 60.Doherty DH, Doyle MP, Curry SR, Vali RJ, Fattor TJ, Olson JS, Lemon DD. Rate of reaction with nitric oxide determines the hypertensive effect of cell-free hemoglobin. Nature Biotechnology. 1998;16:672–676. doi: 10.1038/nbt0798-672. [DOI] [PubMed] [Google Scholar]
- 61.Olson JS, Foley EW, Rogge C, Tsai AL, Doyle MP, Lemon DD. No scavenging and the hypertensive effect of hemoglobin-based blood substitutes. Free Radic Biol Med. 2004;36:685–697. doi: 10.1016/j.freeradbiomed.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 62.Patel RP. Biochemical aspects of the reaction of hemoglobin and NO: Implications for Hb-based blood substitutes. Free Radic Biol Med. 2000;28:1518–1525. doi: 10.1016/s0891-5849(00)00259-8. [DOI] [PubMed] [Google Scholar]
- 63.Gulati A, Barve A, Sen AP. Pharmacology of hemoglobin therapeutics. J Lab Clin Med. 1999;133:112–119. doi: 10.1016/s0022-2143(99)90003-3. [DOI] [PubMed] [Google Scholar]
- 64.Abassi Z, Kotob S, Pieruzzi F, Abouassali M, Keiser KR, Fratantoni JC, Alayash AI. Effects of polymerization on the hypertensive action of diaspirin cross-linked hemoglobin in rats. J Lab Clin Med. 1997;129:603–610. doi: 10.1016/s0022-2143(97)90194-3. [DOI] [PubMed] [Google Scholar]
- 65.Sharma AC, Singh G, Gulati A. Role of No Mechanism in Cardiovascular Effects of Diaspirin Cross-Linked Hemoglobin in Anesthetized Rats. Am J Physiol-Heart Circul Physiol. 1995;38:H1379–H1388. doi: 10.1152/ajpheart.1995.269.4.H1379. [DOI] [PubMed] [Google Scholar]
- 66.Thompson A, McGarry AE, Valeri CR, Lieberthal W. Stroma-Free Hemoglobin Increases Blood-Pressure and Gfr in the Hypotensive Rat - Role of Nitric-Oxide. J Appl Physiol. 1994;77:2348–2354. doi: 10.1152/jappl.1994.77.5.2348. [DOI] [PubMed] [Google Scholar]
- 67.Sampei K, Ulatowski JA, Asano Y, Kwansa H, Bucci E, Koehler RC. Role of nitric oxide scavenging in vascular response to cell-free hemoglobin transfusion. Am J Physiol-Heart Circul Physiol. 2005;289:H1191–H1201. doi: 10.1152/ajpheart.00251.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reiter CD, Gladwin MT. An emerging role for nitric oxide in sickle cell disease vascular homeostasis and therapy. Curr Opin Hematol. 2003;10:99–107. doi: 10.1097/00062752-200303000-00001. [DOI] [PubMed] [Google Scholar]
- 69.Gladwin MT. Unraveling the hemolytic subphenotype of sickle cell disease. Blood. 2005;106:2925–2926. [Google Scholar]
- 70.Nolan VG, Wyszynski DF, Farrer LA, Steinberg MH. Hemolysis-associated priapism in sickle cell disease. Blood. 2005;106:3264–3267. doi: 10.1182/blood-2005-04-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Morris CR, Vichinsky EP, Kato GJ, Gladwin MT, Hazen S, Morris SM. Arginine metabolism, pulmonary hypertension, and sickle cell disease - In reply. Jama-J Am Med Assoc. 2005;294:2433–2434. doi: 10.1001/jama.294.19.2433-a. [DOI] [PubMed] [Google Scholar]
- 72.Gladwin MT, Sachdev V, Jison ML, Shizukuda Y, Plehn JF, Minter K, Brown B, Coles WA, Nichols JS, Ernst I, Hunter LA, Blackwelder WC, Schechter AN, Rodgers GP, Castro O, Ognibene FP. Pulmonary hypertension as a risk factor for death in patients with sickle cell disease. New Engl J Med. 2004;350:886–895. doi: 10.1056/NEJMoa035477. [DOI] [PubMed] [Google Scholar]
- 73.Kato GJ, McGowan VR, Machado RF, Little JA, Taylor J, VI, Morris CR, Nichols JS, Wang X, Poljakovic M, Morris J, Sidney M, Gladwin MT. Lactate dehydrogenase as a biomarker of hemolysis-associated nitric oxide resistance, priapism, leg ulceration, pulmonary hypertension and death in patients with sickle cell disease. Blood. 2006;107:2279–2285. doi: 10.1182/blood-2005-06-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang XD, Tanus-Santos JE, Reiter CD, Dejam A, Shiva S, Smith RD, Hogg N, Gladwin MT. Biological activity of nitric oxide in the plasmatic compartment. Proc Natl Acad Sci USA. 2004;101:11477–11482. doi: 10.1073/pnas.0402201101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ballas SK, Marcolina MJ. Hyperhemolysis during the evolution of uncomplicated acute painful episodes in patients with sickle cell anemia. Transfusion. 2006;46:105–110. doi: 10.1111/j.1537-2995.2006.00679.x. [DOI] [PubMed] [Google Scholar]
- 76.Naumann H, Diggs L, Barreras L, Williams B. Plasma hemoglobin and hemoglobin fractions in sickle cell crisis. Am J Clin Pathol. 1971;56:137–147. doi: 10.1093/ajcp/56.2.137. [DOI] [PubMed] [Google Scholar]
- 77.Jeffers A, Gladwin MT, Kim-Shapiro DB. Computation of plasma hemoglobin nitric oxide scavenging in hemolytic anemias. Free Radic Biol Med. 2006;41:1557–1565. doi: 10.1016/j.freeradbiomed.2006.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tanaka K, Kanamori Y, Sato T, Kondo C, Katayama Y, Yada I, Yuasa H, Kusagawa M. Administration of Haptoglobin during Cardiopulmonary bypass surgery. Trans Am Soc Artif Intern Organs. 1991;37:M482–M483. [PubMed] [Google Scholar]
- 79.Cooper CE. Nitric oxide and iron proteins. Biochim Biophys Acta-Bioenerg. 1999;1411:290–309. doi: 10.1016/s0005-2728(99)00021-3. [DOI] [PubMed] [Google Scholar]
- 80.Head CA, Brugnara C, MartinezRuiz R, Kacmarek RM, Bridges KR, Kuter D, Bloch KD, Zapol WM. Low concentrations of nitric oxide increase oxygen affinity of sickle erythrocytes in vitro and in vivo. J Clin Invest. 1997;100:1193–1198. doi: 10.1172/JCI119631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Alayash AI. Oxygen therapeutics: Can we tame haemoglobin? Nat Rev Drug Discov. 2004;3:152–159. doi: 10.1038/nrd1307. [DOI] [PubMed] [Google Scholar]
- 82.Motterlini R, Foresti R, Vandegriff K, Intaglietta M, Winslow RM. Oxidative-Stress Response in Vascular Endothelial-Cells Exposed to Acellular Hemoglobin-Solutions. Am J Physiol-Heart Circul Physiol. 1995;38:H648–H655. doi: 10.1152/ajpheart.1995.269.2.H648. [DOI] [PubMed] [Google Scholar]
- 83.Balla J, Jacob HS, Balla G, Nath K, Eaton JW, Vercellotti GM. Endothelial-Cell Heme Uptake from Heme-Proteins - Induction of Sensitization and Desensitization to Oxidant Damage. Proc Natl Acad Sci USA. 1993;90:9285–9289. doi: 10.1073/pnas.90.20.9285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Doyle MP, Mahapatro SN, Broene RD, Guy JK. Oxidation and Reduction of Hemoproteins by Trioxodinitrate(Ii) - the Role of Nitrosyl Hydride and Nitrite. J Am Chem Soc. 1988;110:593–599. [Google Scholar]
- 85.Miranda KM, Paolocci N, Katori T, Thomas DD, Ford E, Bartberger MD, Espey MG, Kass DA, Feelisch M, Fukuto JM, Wink DA. A biochemical rationale for the discrete behavior of nitroxyl and nitric oxide in the cardiovascular system. Proc Natl Acad Sci USA. 2003;100:9196–9201. doi: 10.1073/pnas.1430507100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Miranda KM, Nims RW, Thomasa DD, Espey MG, Citrin D, Bartberger MD, Paolocci N, Fukuto JM, Feelisch M, Wink DA. Comparison of the reactivity of nitric oxide and nitroxyl with heme proteins - A chemical discussion of the differential biological effects of these redox related products of NOS. J Inorg Biochem. 2003;93:52–60. doi: 10.1016/s0162-0134(02)00498-1. [DOI] [PubMed] [Google Scholar]
- 87.Huang Z, Louderback JG, Goyal M, Azizi F, King SB, Kim-Shapiro DB. Nitric oxide binding to oxygenated hemoglobin under physiological conditions. Biochim Biophys Acta. 2001;1568:252–260. doi: 10.1016/s0304-4165(01)00227-6. [DOI] [PubMed] [Google Scholar]
- 88.Geraci G, Parkhurst LJ, Gibson QH. Preparation and properties of α- and β-chains from human hemoglobin. J Biol Chem. 1969;17:4664–4667. [PubMed] [Google Scholar]
- 89.Liochev SI, Fridovich I. Copper, Zinc Superoxide Dismutase as a Univalent NO- Oxidoreductase and as a Dichlorofluorescin Peroxidase. J Biol Chem. 2001;276:35253–35257. doi: 10.1074/jbc.M104237200. %R 10.1074/jbc.M104237200. [DOI] [PubMed] [Google Scholar]
- 90.Doyle MP, Pickering RA, Dykstra RL, Nelson CL, Boyer RF. Involvement of Peroxide and Superoxide in the Oxidation of Hemoglobin by Nitrite. Biochem Biophys Res Commun. 1982;105:127–132. doi: 10.1016/s0006-291x(82)80020-x. [DOI] [PubMed] [Google Scholar]
- 91.Arnold EV, Bohle DS. Isolation and oxygenation reactions of nitrosylmyoglobins. Methods Enzymol. 1996;269:41–55. doi: 10.1016/s0076-6879(96)69008-9. [DOI] [PubMed] [Google Scholar]
- 92.Nakamura K, Watanabe M, Sawai-Tanimoto S, Ikeda T. A low catalase activity in dog erythrocytes is due to a very low content of catalase protein despite having a normal specific activity. International Journal of Biochemistry & Cell Biology. 1998;30:823–831. doi: 10.1016/s1357-2725(98)00044-2. [DOI] [PubMed] [Google Scholar]
- 93.Goth L. Human-Erythrocyte Catalase, Isolation with an Improved Method, Characterization and Comparison to Bovine Liver Catalase. Enzyme. 1989;41:191–199. doi: 10.1159/000469078. [DOI] [PubMed] [Google Scholar]
- 94.Miyataasano M, Ito K, Ikeda H, Sekiguchi S, Arai K, Taniguchi N. Purification of Copper-Zinc Superoxide-Dismutase and Catalase from Human-Erythrocytes by Copper-Chelate Affinity-Chromatography. Journal of Chromatography. 1986;370:501–507. doi: 10.1016/s0021-9673(00)94720-4. [DOI] [PubMed] [Google Scholar]
- 95.Nicholls P, Fita I, Loewen PC. Enzymology and structure of catalases. Advances in Inorganic Chemistry. 2001;51:51–106. [Google Scholar]
- 96.D’Agnillo F, Alayash AI. Interactions of hemoglobin with hydrogen peroxide alters thiol levels and course of endothelial cell death. Am J Physiol–Heart Circul Physiol. 2000;279:H1880–H1889. doi: 10.1152/ajpheart.2000.279.4.H1880. [DOI] [PubMed] [Google Scholar]
- 97.Huang JM, Kim-Shapiro DB, King SB. Catalase-mediated nitric oxide formation from hydroxyurea. J Med Chem. 2004;47:3495–3501. doi: 10.1021/jm030547z. [DOI] [PubMed] [Google Scholar]
- 98.Rodkey FL. Mechanism for Conversion of Oxyhemoglobin to Methemoglobin by Nitrite. Clin Chem. 1976;22:1986–1990. [PubMed] [Google Scholar]
- 99.Huang Z, Shiva S, Kim-Shapiro DB, Patel RP, Ringwood LA, Irby CE, Huang KT, Ho C, Hogg N, Schechter AN, Gladwin MT. Enzymatic function of hemoglobin as a nitrite reductase that produces Nitric oxide under allosteric control. J Clin Invest. 2005;115:2099–2107. doi: 10.1172/JCI24650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Huang KT, Keszler A, Patel N, Patel RP, Gladwin MT, Kim-Shapiro DB, Hogg N. The Reaction Between Nitrite and Deoxyhemoglobin: Reassment of Reaction Kinetics and Stoichiometry. J Biol Chem. 2005;280:31126–31131. doi: 10.1074/jbc.M501496200. [DOI] [PubMed] [Google Scholar]
- 101.Fernandez BO, Ford PC. Nitrite catalyzes ferriheme protein reductive nitrosylation. J Am Chem Soc. 2003;125:10510–10511. doi: 10.1021/ja036693b. [DOI] [PubMed] [Google Scholar]
- 102.Basu S, Grubina R, Huang J, Conradie J, Huang Z, Jeffers A, Jiang A, He X, Azarov I, Seibert R, Mehta A, Patel R, King SB, Hogg N, Ghosh A, Gladwin MT, Kim-Shapiro DB. Catalytic generation of N2O3 by a concerted nitrite reductase and anhydrase activity of hemoglobin. Nature Chemical Biology. 2007;3:785–794. doi: 10.1038/nchembio.2007.46. [DOI] [PubMed] [Google Scholar]
- 103.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu XL, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 104.Dejam A, Hunter CJ, Pelletier MM, Hsu LL, Machado RF, Shiva S, Power GG, Kelm M, Gladwin MT, Schechter AN. Erythrocytes are the major intravascular storage sites of nitrite in human blood. Blood. 2005;106:734–739. doi: 10.1182/blood-2005-02-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tsoukias NM, Popel AS. Erythrocyte consumption of nitric oxide in presence and absence of plasma-based hemoglobin. Am J Physiol-Heart Circul Physiol. 2002;282:H2265–H2277. doi: 10.1152/ajpheart.01080.2001. [DOI] [PubMed] [Google Scholar]
- 106.Olson JS. Stopped-Flow, Rapid Mixing Measurements of Ligand Binding to Hemoglobin and Red Cells. Methods Enzymol. 1981;76:631–651. doi: 10.1016/0076-6879(81)76148-2. [DOI] [PubMed] [Google Scholar]
- 107.Kim-Shapiro DB. Hemoglobin-nitric oxide cooperativity: Is no the third respiratory ligand? Free Radic Biol Med. 2004;36:402–412. doi: 10.1016/j.freeradbiomed.2003.10.030. [DOI] [PubMed] [Google Scholar]