Abstract
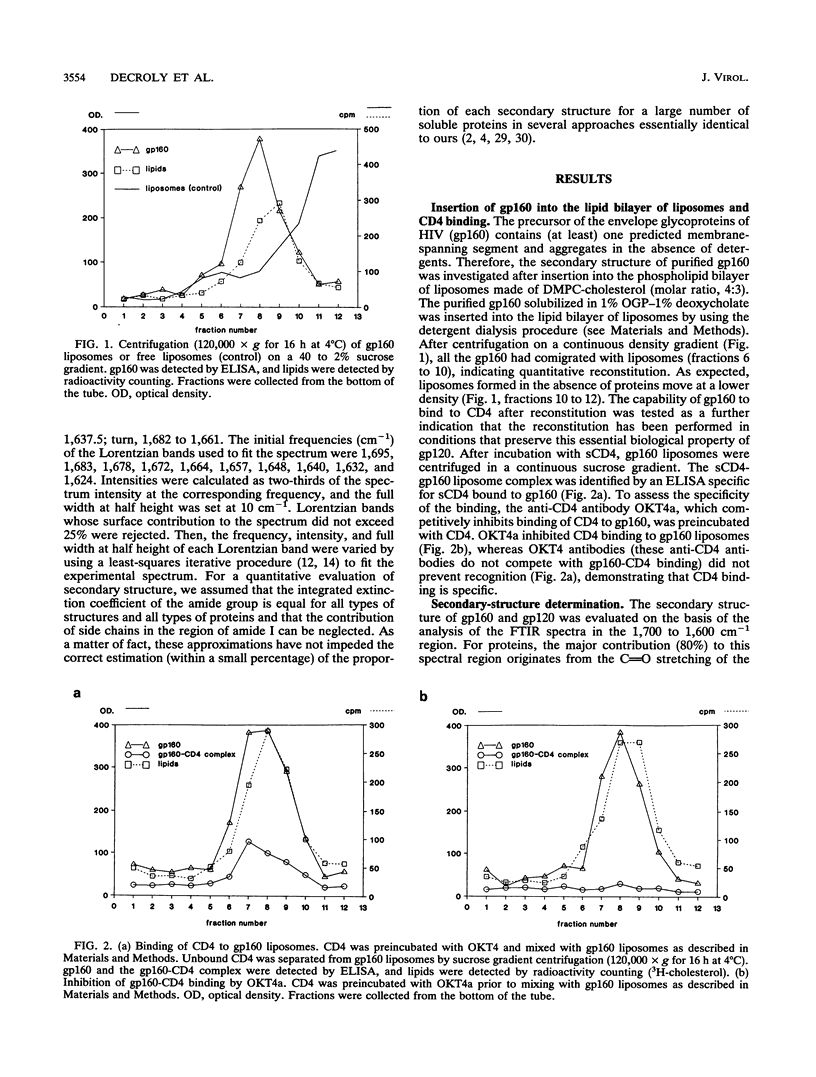
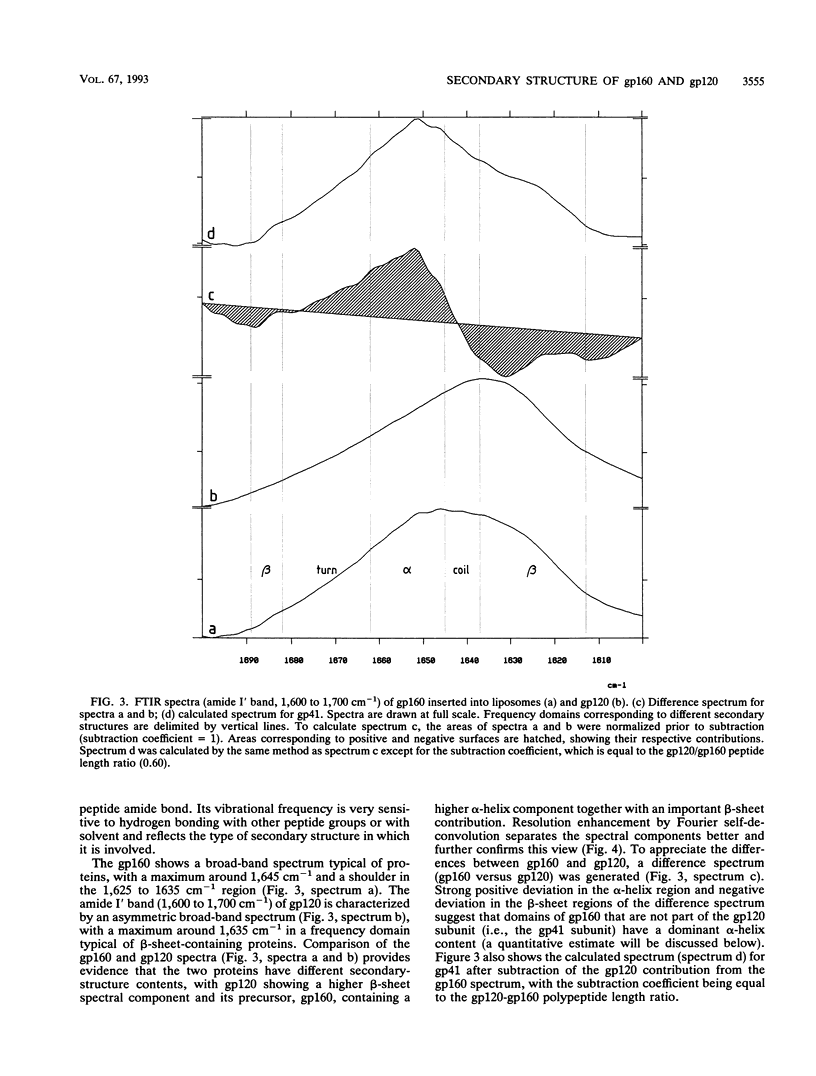
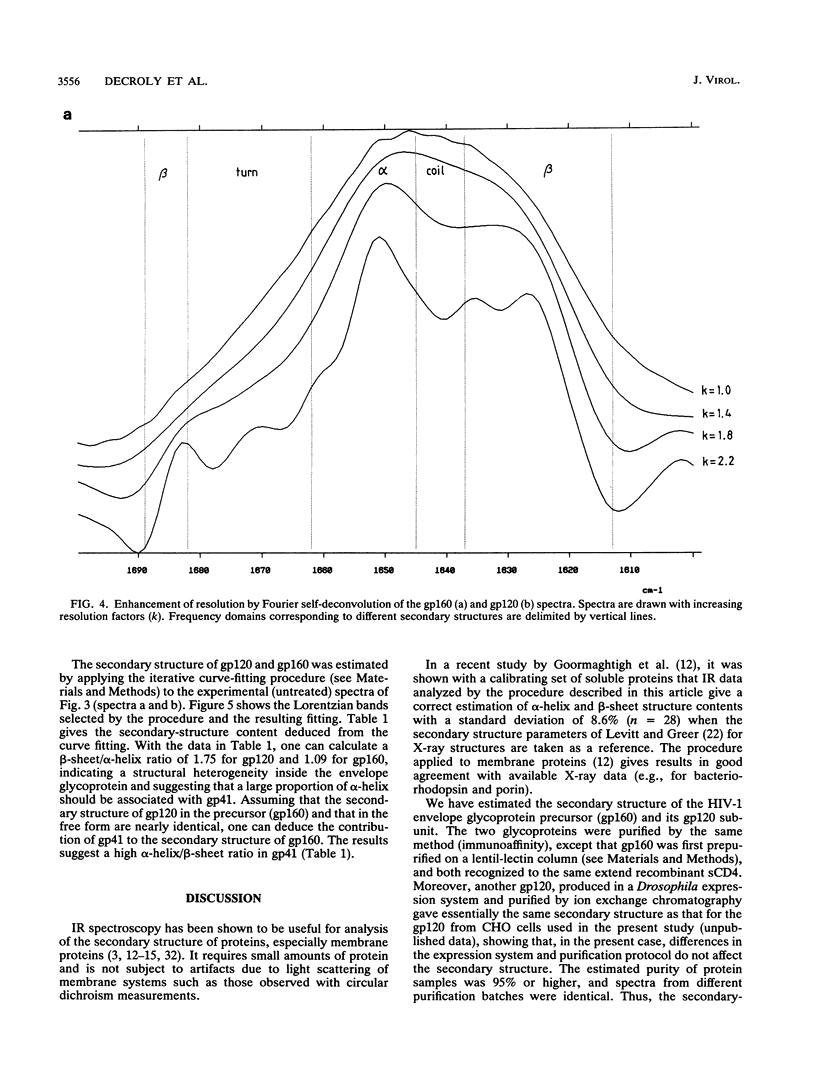
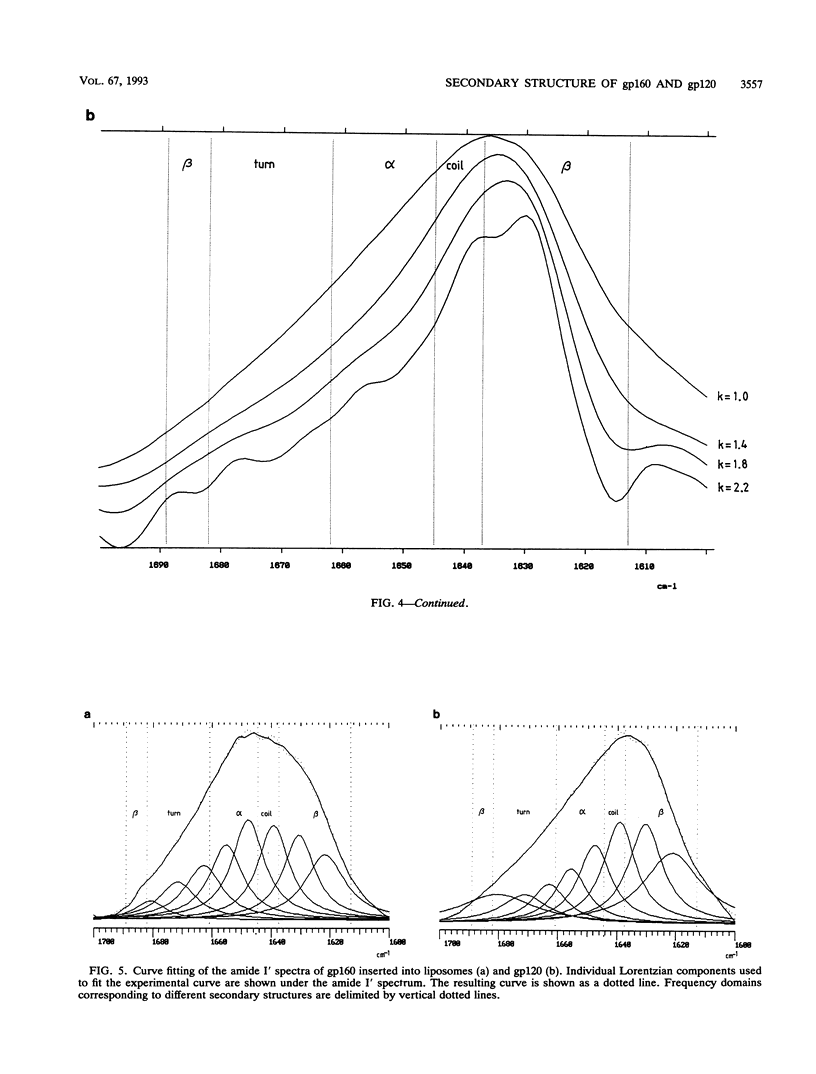
The secondary structure of the precursor (gp160) of the envelope protein of human immunodeficiency virus type 1 (BH10) and its receptor-binding subunit (gp120) was studied by Fourier-transformed attenuated total reflection spectroscopy. A higher alpha-helix/beta-sheet ratio in the gp120 subunit than in the precursor indicates a structural heterogeneity between the two subunits (gp120 and gp41), in agreement with classical secondary-structure predictions. The secondary structure of gp41 was estimated and compared with existing models. The high alpha-helical content in gp41 and the dominant beta-sheet content in gp120 resemble the distribution in influenza virus hemagglutinin subunits.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brauner J. W., Mendelsohn R., Prendergast F. G. Attenuated total reflectance Fourier transform infrared studies of the interaction of melittin, two fragments of melittin, and delta-hemolysin with phosphatidylcholines. Biochemistry. 1987 Dec 15;26(25):8151–8158. doi: 10.1021/bi00399a020. [DOI] [PubMed] [Google Scholar]
- Byler D. M., Susi H. Examination of the secondary structure of proteins by deconvolved FTIR spectra. Biopolymers. 1986 Mar;25(3):469–487. doi: 10.1002/bip.360250307. [DOI] [PubMed] [Google Scholar]
- Cabiaux V., Brasseur R., Wattiez R., Falmagne P., Ruysschaert J. M., Goormaghtigh E. Secondary structure of diphtheria toxin and its fragments interacting with acidic liposomes studied by polarized infrared spectroscopy. J Biol Chem. 1989 Mar 25;264(9):4928–4938. [PubMed] [Google Scholar]
- Castresana J., Muga A., Arrondo J. L. The structure of proteins in aqueous solutions: an assessment of triose phosphate isomerase structure by Fourier-transform infrared spectroscopy. Biochem Biophys Res Commun. 1988 Apr 15;152(1):69–75. doi: 10.1016/s0006-291x(88)80681-8. [DOI] [PubMed] [Google Scholar]
- Eisenberg D., Wesson M. The most highly amphiphilic alpha-helices include two amino acid segments in human immunodeficiency virus glycoprotein 41. Biopolymers. 1990 Jan;29(1):171–177. doi: 10.1002/bip.360290122. [DOI] [PubMed] [Google Scholar]
- Freed E. O., Myers D. J., Risser R. Characterization of the fusion domain of the human immunodeficiency virus type 1 envelope glycoprotein gp41. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4650–4654. doi: 10.1073/pnas.87.12.4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fringeli U. P., Günthard H. H. Infrared membrane spectroscopy. Mol Biol Biochem Biophys. 1981;31:270–332. doi: 10.1007/978-3-642-81537-9_6. [DOI] [PubMed] [Google Scholar]
- Gallaher W. R., Ball J. M., Garry R. F., Griffin M. C., Montelaro R. C. A general model for the transmembrane proteins of HIV and other retroviruses. AIDS Res Hum Retroviruses. 1989 Aug;5(4):431–440. doi: 10.1089/aid.1989.5.431. [DOI] [PubMed] [Google Scholar]
- Goormaghtigh E., Cabiaux V., Ruysschaert J. M. Secondary structure and dosage of soluble and membrane proteins by attenuated total reflection Fourier-transform infrared spectroscopy on hydrated films. Eur J Biochem. 1990 Oct 24;193(2):409–420. doi: 10.1111/j.1432-1033.1990.tb19354.x. [DOI] [PubMed] [Google Scholar]
- Goormaghtigh E., De Meutter J., Szoka F., Cabiaux V., Parente R. A., Ruysschaert J. M. Secondary structure and orientation of the amphipathic peptide GALA in lipid structures. An infrared-spectroscopic approach. Eur J Biochem. 1991 Jan 30;195(2):421–429. doi: 10.1111/j.1432-1033.1991.tb15721.x. [DOI] [PubMed] [Google Scholar]
- Goormaghtigh E., Vigneron L., Knibiehler M., Lazdunski C., Ruysschaert J. M. Secondary structure of the membrane-bound form of the pore-forming domain of colicin A. An attenuated total-reflection polarized Fourier-transform infrared spectroscopy study. Eur J Biochem. 1991 Dec 18;202(3):1299–1305. doi: 10.1111/j.1432-1033.1991.tb16503.x. [DOI] [PubMed] [Google Scholar]
- Gorga J. C., Dong A., Manning M. C., Woody R. W., Caughey W. S., Strominger J. L. Comparison of the secondary structures of human class I and class II major histocompatibility complex antigens by Fourier transform infrared and circular dichroism spectroscopy. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2321–2325. doi: 10.1073/pnas.86.7.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffar O. K., Dowbenko D. J., Berman P. W. Topogenic analysis of the human immunodeficiency virus type 1 envelope glycoprotein, gp160, in microsomal membranes. J Cell Biol. 1988 Nov;107(5):1677–1687. doi: 10.1083/jcb.107.5.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helseth E., Olshevsky U., Furman C., Sodroski J. Human immunodeficiency virus type 1 gp120 envelope glycoprotein regions important for association with the gp41 transmembrane glycoprotein. J Virol. 1991 Apr;65(4):2119–2123. doi: 10.1128/jvi.65.4.2119-2123.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski M., Potz J., Basiripour L., Dorfman T., Goh W. C., Terwilliger E., Dayton A., Rosen C., Haseltine W., Sodroski J. Functional regions of the envelope glycoprotein of human immunodeficiency virus type 1. Science. 1987 Sep 11;237(4820):1351–1355. doi: 10.1126/science.3629244. [DOI] [PubMed] [Google Scholar]
- Krimm S., Bandekar J. Vibrational spectroscopy and conformation of peptides, polypeptides, and proteins. Adv Protein Chem. 1986;38:181–364. doi: 10.1016/s0065-3233(08)60528-8. [DOI] [PubMed] [Google Scholar]
- Levitt M., Greer J. Automatic identification of secondary structure in globular proteins. J Mol Biol. 1977 Aug 5;114(2):181–239. doi: 10.1016/0022-2836(77)90207-8. [DOI] [PubMed] [Google Scholar]
- Maddon P. J., Molineaux S. M., Maddon D. E., Zimmerman K. A., Godfrey M., Alt F. W., Chess L., Axel R. Structure and expression of the human and mouse T4 genes. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9155–9159. doi: 10.1073/pnas.84.24.9155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin I., Defrise-Quertain F., Decroly E., Vandenbranden M., Brasseur R., Ruysschaert J. M. Orientation and structure of the NH2-terminal HIV-1 gp41 peptide in fused and aggregated liposomes. Biochim Biophys Acta. 1993 Jan 18;1145(1):124–133. doi: 10.1016/0005-2736(93)90389-h. [DOI] [PubMed] [Google Scholar]
- Miller M. A., Garry R. F., Jaynes J. M., Montelaro R. C. A structural correlation between lentivirus transmembrane proteins and natural cytolytic peptides. AIDS Res Hum Retroviruses. 1991 Jun;7(6):511–519. doi: 10.1089/aid.1991.7.511. [DOI] [PubMed] [Google Scholar]
- Olshevsky U., Helseth E., Furman C., Li J., Haseltine W., Sodroski J. Identification of individual human immunodeficiency virus type 1 gp120 amino acids important for CD4 receptor binding. J Virol. 1990 Dec;64(12):5701–5707. doi: 10.1128/jvi.64.12.5701-5707.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard S. R., Meier W., Chow P., Rosa J. J., Wiley D. C. CD4-binding regions of human immunodeficiency virus envelope glycoprotein gp120 defined by proteolytic digestion. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11320–11324. doi: 10.1073/pnas.88.24.11320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surewicz W. K., Mantsch H. H. New insight into protein secondary structure from resolution-enhanced infrared spectra. Biochim Biophys Acta. 1988 Jan 29;952(2):115–130. doi: 10.1016/0167-4838(88)90107-0. [DOI] [PubMed] [Google Scholar]
- Susi H., Byler D. M. Resolution-enhanced Fourier transform infrared spectroscopy of enzymes. Methods Enzymol. 1986;130:290–311. doi: 10.1016/0076-6879(86)30015-6. [DOI] [PubMed] [Google Scholar]
- Syu W. J., Huang J. H., Essex M., Lee T. H. The N-terminal region of the human immunodeficiency virus envelope glycoprotein gp120 contains potential binding sites for CD4. Proc Natl Acad Sci U S A. 1990 May;87(10):3695–3699. doi: 10.1073/pnas.87.10.3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venable R. M., Pastor R. W., Brooks B. R., Carson F. W. Theoretically determined three-dimensional structures for amphipathic segments of the HIV-1 gp41 envelope protein. AIDS Res Hum Retroviruses. 1989 Feb;5(1):7–22. doi: 10.1089/aid.1989.5.7. [DOI] [PubMed] [Google Scholar]
- Wilson I. A., Skehel J. J., Wiley D. C. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A resolution. Nature. 1981 Jan 29;289(5796):366–373. doi: 10.1038/289366a0. [DOI] [PubMed] [Google Scholar]
- Young J. A. HIV and HLA similarity. Nature. 1988 May 19;333(6170):215–215. doi: 10.1038/333215c0. [DOI] [PubMed] [Google Scholar]


